Abstract
Deficient emotion regulation has been proposed as a crucial pathological mechanism in bipolar disorder (BD). We therefore investigated emotion regulation impairments in BD, the related neural underpinnings and their etiological relevance for the disorder. Twenty-two euthymic patients with bipolar-I disorder and 17 unaffected first-degree relatives of BD-I patients, as well as two groups of healthy gender-, age- and education-matched controls (N=22/17, respectively) were included. Participants underwent functional magnetic resonance imaging while applying two different emotion regulation techniques, reappraisal and distraction, when presented with emotional images. BD patients and relatives showed impaired downregulation of amygdala activity during reappraisal, but not during distraction, when compared with controls. This deficit was correlated with the habitual use of reappraisal. The negative connectivity of amygdala and orbitofrontal cortex (OFC) observed during reappraisal in controls was reversed in BD patients and relatives. There were no significant differences between BD patients and relatives. As being observed in BD patients and unaffected relatives, deficits in emotion regulation through reappraisal may represent heritable neurobiological abnormalities underlying BD. The neural mechanisms include impaired control of amygdala reactivity to emotional stimuli and dysfunctional connectivity of the amygdala to regulatory control regions in the OFC. These are, thus, important aspects of the neurobiological basis of increased vulnerability for BD.
Similar content being viewed by others
Introduction
Bipolar disorder (BD) is a highly heritable, chronic disease characterized by increased affect lability and intensity,1 elevated emotional reactivity and presumably impaired emotion regulation.2, 3, 4 Even though impairments in the implicit regulation of emotion have been repeatedly observed,5, 6, 7, 8 there are only few reports on the voluntary regulation of emotion9 in BD.10, 11 The etiological relevance of deficits in emotion regulation has been suggested by many authors; however, empirically it is still unclear if these deficits emerge during the course of the disease or precede its development, potentially representing increased vulnerability. Furthermore, the neural correlates underlying the potential impairments are largely unknown. They are particularly interesting, as neural changes may precede behavioral manifestations in healthy high-risk populations.12, 13, 14, 15, 16, 17
The regulation of emotion entails implicit, automatic and more voluntary processes that may occur in parallel, but can also be separated experimentally.2 Both seem to be supported by the interactions of neural circuits underlying emotion generation, including the amygdala, with cognitive control networks, mainly in the prefrontal and anterior cingulate cortex.18, 19, 20 Voluntary emotion regulation, in particular, has been suggested to comprise a number of techniques that can be organized along a continuum ranging from attentional control (for example, the disengagement of attention from emotional stimuli through a distracting task) to cognitive change (for example, the top–down reappraisal of a certain stimulus).9 Direct comparisons of reappraisal and distraction in healthy individuals showed common effects in dorsolateral and medial prefrontal cortex and in the downregulation of amygdala activity, but also specifics including lateral orbitofrontal (OFC) involvement in reappraisal, which was related to amygdala downregulation.21, 22
Models of emotion processing in BD2, 4 propose abnormalities in both, those regions involved in early emotion reactivity and those involved more in the top–down regulation of emotion. A recent meta-analysis on functional magnetic resonance imaging (fMRI) studies in BD corroborates this with the finding of increased parahippocampal gyrus/amygdala activity in response to emotional stimuli.23 In contrast, BD patients show reduced activation and reduced gray matter in the dorsolateral and ventrolateral prefrontal cortex.23 Consequently, the hypoactivation of these structures might lead to a deficit in the voluntary downregulation of exaggerated emotional responses in bipolar patients. On a self-report level, BD patients and unaffected relatives show more frequent use of maladaptive emotion regulation strategies, such as rumination and self-blame, but less frequent use of adaptive strategies, such as putting into perspective.24, 25 Moreover, previous neuroimaging studies of emotion reactivity and regulation reported altered connectivity between the prefrontal and limbic structures, which might underlie the proposed deficit to cognitively regulate emotions in bipolar patients.5, 10, 11, 26, 27 Thus, the existing self-report and neuroimaging data strongly suggest emotion regulation deficits in bipolar patients. However, so far, no study experimentally compared different emotion regulation capacities and their neural underpinnings in bipolar patients and high-risk populations.
Investigations with healthy relatives of BD patients have shown alterations not only in self-reported emotion regulation skills,25 but also in neural responses to emotional stimuli28 as well as to cognitive challenges.29, 30 These studies have, therefore, given rise to the hypothesis that altered ventral prefrontal-limbic activity and connectivity, critical for the cognitive regulation of emotion, may be a precursor of the disorder.31, 32 Similarly, alterations in euthymic BD patients have been interpreted as representing a vulnerability trait marker, although healthy individuals at risk to develop BD have not been included in this particular study.33 The evidence, therefore, suggests impairments in voluntary emotion regulation in BD, which may already manifest in high-risk populations such as unaffected relatives of BD patients. Directly testing this question is both timely and important to identify vulnerability markers that enable early diagnosis and potentially preventive interventions, to refine etiological models and to develop more specific and targeted psychotherapy for BD.
The present study investigated two cognitive emotion regulation strategies (that is, reappraisal and distraction) in euthymic patients with BD-I, unaffected first-degree relatives of BD-I patients and, respectively, matched healthy controls. We used an established experimental paradigm that activates ventral-limbic brain areas related to emotional responses and a prefrontal–parietal network related to emotion regulation in healthy participants.21 Habitual use of maladaptive and adaptive regulation strategies was assessed using the Cognitive Emotion Regulation Questionnaire (CERQ).34
We hypothesized exaggerated emotional responses in BD patients and relatives in self-reports and limbic activity. Similarly, emotion regulation deficits are expected, reflected in the reduced downregulation of the amygdala in emotion regulation conditions. On the basis of the known functional disturbances in dorsolateral and ventrolateral prefrontal as well as parietal cortices in BD patients reduced activation in these brain areas and thus deficient emotion regulation should be observable for distraction as well as reappraisal. Furthermore, we expect that reduced amygdala downregulation in BD patients and high-risk individuals is mediated by disturbed functional connectivity between the prefrontal cortex and limbic brain regions.
Materials and methods
Participants and diagnostic assessment
All participants underwent the Structured Clinical Interview for DSM-IV (SCID-I/-II)35, 36, 37, 38 and screening for exclusion criteria (neurological disorders, head trauma with loss of consciousness, metal implants, tattoos, substance abuse or dependence, age <18 or >65 years). Interviews and observer rating scales for mania (Young Mania Rating Scale; YMRS)39 and depression (Hamilton Depression Rating Scale; HAMD)40 were conducted by senior clinical psychologists. Participants completed the Beck Depression Inventory (BDI)41, 42 and the CERQ.34 The study was approved by the ethics committee of the Medical Faculty Mannheim, Heidelberg University. All participants provided written informed consent before entering the study.
Sample 1: BD patients and healthy controls
Twenty-two euthymic patients with BD-I and 22 gender-, age- and education-matched healthy volunteers with no history of psychiatric disorders participated (Table 1). Patients were recruited at the Central Institute of Mental Health, Mannheim and through local psychiatrists, psychotherapists and patient support groups. None of the patients currently met the criteria for any DSM-IV disorder other than BD. A life chart assessing variables related to illness course was completed for all patients. Euthymia was defined as a HAMD score <5 and a YMRS score <7.43 We inquired about current medication and verified its stability during the past 6 months. To analyze the psychotropic medication effect, we calculated the total medication load according to a published algorithm44 reflecting both the dose and variety of different medication.45 The composite measure was generated by summing all individual medication codes for each medication category (2.32 (2.08)). We then checked for correlations of this index with the effects of interest in bipolar patients.
Sample 2: Relatives and healthy controls
Seventeen healthy first-degree relatives of BD-I patients and 17 gender-, age- and education-matched healthy controls participated (Table 1). None fulfilled the criteria for any mental disorder or took any psychotropic medication. The relatives were not related to those BD patients tested in this study. Five relatives were siblings, four were children and eight were parents of BD patients. Twelve relatives had one case in the family and five relatives had two or more cases in the family.
Experimental paradigm and procedure
The experimental paradigm (Figure 1a)21 confronted participants with 32 emotional, highly arousing (16 negative, 16 positive) and 16 neutral, low arousing images (from the International Affective Picture System, IAPS).46 They were required to simply view the pictures (view condition) or to downregulate the emotional response by reinterpreting the meaning of the stimuli (reappraisal condition) or by distraction through an arithmetic task (distraction condition). Each picture was presented once in each condition (except for neutral images, which were not presented in the reappraisal condition) yielding 128 pseudorandomly presented trials. Instructions regarding the condition were displayed after an initial emotion induction phase (1 s) as a semi-transparent overlay on the images. The regulation phase (6 s) was followed by a rating of participants’ current emotional state on a nine-point scale using the Self-Assessment Manikins ranging from unpleasant to pleasant (4 s). Participants were instructed and trained outside the scanner in the application of the emotion regulation strategies. Six additional training trials were presented inside the scanner. In case of any difficulties with the procedure, the practice block was repeated, which resolved all problems as reported by the participants. Participants completed a questionnaire after the experiment that asked for the applied regulation techniques to ensure correct application of the instructions.
(a) Sequence of events in a trial. The example pictures resemble those in the experiment, but are not part of the IAPS. (b) Emotional state ratings during the experiment. The means of self-assessment-Manikin-valence-ratings are displayed for BD patients (left), healthy relatives (right) and their respective controls. BD, bipolar disorder; Con, control; IAPS, International Affective Picture System; Rel, relative; SAM, Self-Assessment-Manikin.
To validate the normative IAPS ratings of the pictures, all participants rated each image after the experiment in arousal and valence, again using Self-Assessment Manikins. These ratings were largely compatible with the normative data (Supplementary S1).
MRI data acquisition
MRI data were collected on a 3T Siemens Magnetom TIM Trio at the Central Institute of Mental Health, Mannheim. A high-resolution T1-weighted 3D image was acquired (slice thickness=1.1 mm, FOV=256 × 240 × 176 mm, matrix=256 × 240 × 160). Functional images were obtained from 40 gradient-echo T2*-weighted slices (slice thickness=2.3 mm). A single shot echo planar sequence with parallel imaging GRAPPA technique (acceleration factor 2) was used with TR=2700 ms, flip angle=90°, TE=27 ms, FOV=220 mm2, matrix=96 × 96, slice gap=0.7 mm.
fMRI data analysis
Image processing and statistical analyses were done with SPM8 (http://www.fil.ion.ucl.ac.uk/). Functional images were realigned, slice time corrected and spatially normalized using the Montreal Neurological Institute template. For normalization, the images were resampled every 3 mm using sinc interpolation. Images were smoothed using a 9 × 9 × 9 mm Gaussian kernel.
Individual participants’ data were analyzed using a General Linear Model for blood oxygen level-dependent signal changes. Movement parameters calculated during realignment were included as parameters of no interest. Individual statistical parametric maps were calculated to elucidate: (1) the emotional response per se (view emotional vs view neutral), (2) the distraction effect (distraction emotional vs view emotional) and (3) the reappraisal effect (reappraisal emotional vs view emotional).
Two types of second-level random-effects analyses were conducted: One-sample t-tests were calculated on the above-mentioned individual contrast images across patients, unaffected relatives and controls. To evaluate the differences between patients and matched controls, relatives and matched controls and patients and relatives, two-sample t-tests were computed for all the contrasts. For the analyses, we averaged across negative and positive stimuli as direct contrasts of these conditions yielded no differences in the emotion regulation networks and to enhance statistical power.21 As there were no gender differences, we also averaged across male and female participants.
To assess the functional connectivity of the amygdala during emotion regulation, we calculated a standard psychophysiological interaction analysis as implemented in SPM8.47 To this end, we extracted the deconvolved time series from a 5-mm spherical seed region around the peak activation (reappraisal vs view) in the anatomically defined amygdala regions of interest (ROI) as the first regressor. The second regressor represented the experimental condition (regulation vs view) and the regressor of interest was the interaction of the two. A second-level random-effects analysis with two-sample t-tests was calculated to compare the connectivity differences between the groups.
For all analyses, anatomically defined ROI from the WFU PickAtlas v2.0 (ref. 48) were used to examine activations in the amygdala and the regulation networks as observed in our previous investigations of reappraisal and distraction.21, 49 These included as separate, bilateral masks OFC, dorsolateral (dlPFC, middle frontal) and dorsomedial prefrontal (dmPFC, superior medial), anterior cingulate (ACC) and parietal cortex (inferior, superior). Activations were thresholded at a family-wise error-corrected P<0.05. In addition, we applied the Bonferroni–Holm method, which adjusts the P-values that were already corrected for family-wise error rates within each ROI according to the total number of ROIs used in the analyses50, 51 or accordingly for the number of seed regions in the psychophysiological interaction analysis analysis.52 Results that were significant at a whole-brain family-wise error-corrected P<0.05 level are also reported.
To allow for correlations of the observed activations with the questionnaire and clinical measures, we extracted individual % signal change from the significant cluster in a 5-mm radius sphere around the respective activation peak (if they fell into the respective anatomical ROI, for example, the amygdala).
Statistical analyses of behavioral data
Emotion ratings were analyzed with SPSS 20.0 (IBM, IBM Statistics, Armonk, NY, USA). First, to analyze the emotional responses to the pictures in the viewing condition, repeated-measures analyses of variance with emotion as within-subject factor and group as between-subject factor were calculated. Second, to detect the effects of regulation strategies on emotional state we conducted repeated-measures analysis of variance with emotion and condition as within-subject factors and group as between-subject factor. As there were no neutral pictures in the reappraisal condition, the neutral condition was neglected for the second analyses. For the CERQ data, we used t-tests to compare the groups.
Results
Behavioral data
Online emotional state ratings
Analysis of the emotional state ratings (Figure 1b) after each trial yielded a significant main effect of emotion in the viewing condition (Sample 1: F(2,84)=191.5, P<0.001, Sample 2: F(2,64)=129.9, P<0.001). Planned comparisons revealed that negative and positive trials differed from each other and from neutral trials indicating successful emotion induction (all P<0.001). There were no group effects regarding BD patients and controls (all P>0.45), but regarding relatives and their controls there was a significant interaction of emotion and group (F(2,64)=3.3, P<0.05) indicating less positive ratings of positive stimuli in relatives (F(1,32)=7.8, P<0.01). Comparing BD patients with relatives showed the same pattern (F(2,74)=6.4, P<0.01; F(1,37)=5.8, P<0.05).
Regarding the effects of the different regulation strategies on emotional state, we found a significant main effect of emotion (Sample 1: F(1,42)=134.3, P<0.001, Sample 2: F(1,32)=130.3, P<0.001), a main effect of task (Sample 1: F(2,84)=5.8, P<0.01, Sample 2: F(2.64)=10.8, P<0.001), as well as a significant interaction of emotion and task (Sample 1: F(2,84)=48.1, P<0.001, Sample 2: F(2,64)=43.0, P<0.001). Repeated contrasts regarding the interaction revealed that emotional pictures were rated less negative or positive during distraction and reappraisal as compared with the view condition (all P<0.001). There were no group effects regarding BD patients and controls (all P>0.15), but regarding relatives and their controls there was a significant interaction of emotion and task with group (F(2,64)=4.8, P<0.05) indicating stronger downregulation of positive emotion during reappraisal in controls (F(1,32)=8.0, P<0.01). There were no differences between BD patients and relatives.
Habitual emotion regulation strategies (CERQ)
We observed significant group differences in maladaptive and adaptive emotion regulation strategies (Supplementary S2). BD patients reported more frequent use of rumination, self-blame and catastrophizing, but less frequent use of positive reappraisal. Relatives also reported less frequent use of positive reappraisal, but there were no differences in the other regulation strategies, including putting into perspective. Comparing BD patients and relatives showed higher scores for patients in rumination, self-blame and catastrophizing, but no differences in positive reappraisal.
fMRI data
Common effects for emotional responding, reappraisal and distraction
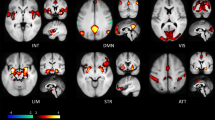
To assess whether the response to the emotional pictures per se and the two emotion regulation strategies activated the same networks identified previously,21, 49 we first averaged across all participants. This analysis yielded activation patterns that were largely compatible with our previous data and other reports in the literature (Supplementary S3). Amygdala activation (together with ventral temporal and occipital cortex and poster cingulate gyrus/precuneus) was increased in response to emotional stimuli during the view condition. In turn, amygdala activation was reduced in both emotion regulation conditions. The control network for reappraisal included bilateral OFC, dmPFC, dlPFC and inferior parietal cortices. Distraction also activated dlPFC, dmPFC extending into ACC, insula and superior parietal cortices.
Group differences
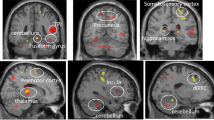
Sample 1 - BD patients vs controls When comparing the activation in BD patients and controls, we found no differences in responses to emotional compared with neutral stimuli in the view condition. However, for reappraisal, we observed less downregulation of left amygdala activity and right amygdala/parahippocampal activity in BD patients (Figure 2, Table 2). There were no differences between the groups in the distraction condition or in activation of the regulatory control networks.
Increased amygdala activation during reappraisal for BD patients (a) and relatives (b) compared with their respective controls as well as % signal change in the left amygdala. The difference in % signal change between the reappraisal and view conditions correlated negatively with habitual reappraisal use in the CERQ (c), which was also decreased in BD patients and relatives (d). BD, bipolar disorder; CERQ, Cognitive Emotion Regulation Questionnaire; Con, control; Rel, relative.
To elucidate the changes in amygdala connectivity during reappraisal, we conducted a psychophysiological interaction between-group analysis directly contrasting connectivity in the reappraisal vs view condition in bipolar patients vs healthy controls. Here we found significant differences in the connectivity between left amygdala (seed region) and right OFC (Figure 3, Table 2) and ACC, and right amygdala with right OFC. Connectivity during reappraisal between amygdala and OFC and ACC activity was reversed in BD patients compared with healthy controls. Although controls showed negative connectivity during reappraisal (that is, an activation increase in the OFC was associated with activation decrease in the amygdala), BD patients showed positive connectivity between these regions.
Sample 2 - Relatives vs controls The results in unaffected relatives were largely comparable to those in BD patients. We found no differences in responses to emotional compared with neutral stimuli in the view and distraction conditions, but during reappraisal, relatives showed less downregulation of amygdala activity than controls (Figure 2, Table 2). There were no differences between the groups in the distraction condition or in activation of the regulatory control networks.
The psychophysiological interaction analysis in sample 2 showed that the connectivity of left amygdala with bilateral OFC (Figure 3) and of right amygdala with right OFC was reversed in relatives compared with controls. As in sample 1, the controls showed negative connectivity during reappraisal, whereas relatives showed positive connectivity between these regions.
BD patients vs relatives There were no differences in the activation or connectivity of the amygdala between BD patients and unaffected relatives.
Correlation analysis
When correlating individual reappraisal use with amygdala activity in the reappraisal vs view condition, we found a significant negative correlation (Figure 2; r=−0.37; P<0.01) indicating that participants high in habitual reappraisal use are more successful in downregulating amygdala activity in the experimental setting. This pattern was consistent when calculating correlations in the two samples separately (Sample 1: r=−0.35;<0.05, Sample 2: r=−0.47; P<0.01).
We found no significant correlations of amygdala activity with any of the measures of current symptoms including BDI, HAMD and YMRS (all P's>0.10). In the patient group only, we also tested for correlations with clinical characteristics. There were no correlations with the number of previous episodes, time since last episode, number of hospitalizations or age at first hospitalization, age at onset of the disease or medication load (all P's>0.20).
Discussion
We investigated cognitive emotion regulation and its neural correlates in BD-I patients and unaffected relatives, which revealed several important results. First, BD patients showed an emotion regulation deficit with respect to amygdala downregulation during the reappraisal condition, mediated by reduced altered connectivity between OFC and amygdala. Interestingly, this regulation deficit was only present in the reappraisal condition and not during distraction. Second, these results were also observed in unaffected relatives, with increased genetic risk to develop BD in the future, which suggests their interpretation as a vulnerability marker for BD. As the relatives are not affected by previous disorder episodes, the deficit does not seem to be a consequence of but a predisposition for the development of the illness. As the relatives are unmedicated, the observed impairments are not an artifact of medication in BD patients. Third, the deficient downregulation of amygdala activity is paralleled by the self-report of impaired habitual reappraisal, which gives an indication of the ecological validity of the experimental effect.
The pattern of limbic hyperactivation and altered connectivity with frontal regions is in line with the suggestion of impaired prefrontal control over emotion generating regions like the amygdala in BD.2 However, it further characterizes the conditions of this impairment. We observed altered amygdala activity in patients and relatives only in the reappraisal condition, not during simple viewing of emotional stimuli. This contrasts other reports of increased amygdala responses to mildly sad faces8 and facial affect matching.7 However, there are also reports of lacking amygdala group differences or even blunted amygdala responding to emotional stimuli in depressed BD patients.53, 54 Such inconsistencies have been found during euthymia as well.55, 56 As these studies did not explicitly instruct participants on how to treat arising emotions, it is possible that the patients and controls applied regulation differently, potentially in line with their habitual use of emotion regulation.25 By explicitly instructing the use of certain regulation strategies, the present study allowed studying the specific effects of strategies, which could offer an explanation for the discrepant previous results.
The present data also differentiate between the regulation strategies. The observed deficit was only present in the reappraisal and not in the distraction condition. This is in line with the recent evidence in unipolar depression, where amygdala regulation was also selectively impaired during reappraisal.49 Together with the data from healthy participants, where the effect of distraction on amygdala activity is stronger and more extended,21, 22 this suggests that the regulatory effects of distraction are more robust and less prone to impairment than reappraisal. A possible reason could be that although distraction shifts attention away from the emotional content of a stimulus, reappraisal requires focusing on these aspects in order to reinterpret their meaning. Furthermore, even though the difficulty of the conditions did not differ between the groups (Supplementary S4), the reappraisal task is by definition less specified and directive than the distraction condition as there are more reappraisal options than solutions for the arithmetic problems. This seems to be a particular challenge for BD patients and relatives.
The reappraisal deficit in BD patients and relatives was also present in the self-report of habitual emotion regulation use (CERQ). In addition, dysfunctional regulation strategies, such as rumination, catastrophizing and self-blame, were reported more frequently in BD patients (but not in unaffected relatives), which is in line with a recent study using the same questionnaire.25 This study did, however, not find a decrease in reappraisal use as reported here. It is possible that this lack of a reappraisal difference is due to age differences between patients and controls in that study, as age has been shown to influence emotion regulation and also reappraisal specifically.57 It is intriguing that the habitual use of reappraisal correlates with the amygdala downregulation effect during experimental reappraisal, which also corroborates the ecological validity of our experimental procedure.
Surprisingly and in contrast to the neural activation patterns, subjective affect ratings during the experiment were not affected in BD. In contrast, the relatives of BD patients showed a smaller reduction in positive affect during reappraisal than controls. This effect is most likely due to the reduced potential of the positive stimuli to induce emotion in this group, as shown in relatives’ lower valence ratings for positive stimuli during the viewing condition. Interestingly, an opposite effect was observed for post hoc ratings of the images after the experiment, where relatives rated pictures more positively. This suggests some volatility in positive affect in the relatives, who may be more sensitive to external factors like the scanner environment, whereas currently euthymic BD patients have a more stable subjective evaluation of their current affect, potentially due to previous treatments. A dissociation between preserved behavioral performance and altered neural activation during cognitive–emotional tasks has been observed before in euthymic BD patients.13 Euthymia might thus indeed be a recovered state where measures like fMRI are more sensitive than behavioral ones to pick up altered emotional processing.
There are limitations to the present study. Patients with differing medical status were tested. The lack of a significant correlation with medication load44 suggests that medication does not have an important role for the effects, which is further supported by the results in the unmedicated healthy relatives. It has to be interpreted with great care nevertheless as the load score was originally designed for the evaluation of treatment adequacy and resistance. However, the use of this type of composite measure of total psychotropic medication load has been recommended for neuroimaging studies in BD45 and this particular score has been used previously.50, 58 Larger sample sizes could allow delineating the exact effects of different medications in future studies. Including symptomatic patients in future studies would have the additional potential to elucidate if emotion regulation varies with the symptomatic status of bipolar patients in the sense of mood-congruent valence effects. We focused on a priori-defined ROI based on previous investigations regarding the neural networks involved in emotion regulation,21, 49 future studies with larger sample sizes should also test for whole-brain differences between the tested groups. Although the presence of alterations in healthy individuals at increased risk to develop a disorder has consistently been interpreted as an indication of vulnerability,59, 60, 61 future longitudinal studies could allow much stronger conclusions regarding the etiological relevance of the observed deficits.62
To conclude, we found an emotion regulation deficit in euthymic BD patients and unaffected relatives when applying a reappraisal, but not a distraction strategy, indicated by the impaired downregulation of amygdala activity and altered connectivity with OFC. That healthy individuals at an increased genetic risk of developing bipolar disorder do show the deficit indicates that it may represent a vulnerability marker. The presence of the impairment during remission also highlights it as a crucial treatment target, which should also be assessed with sensitive neuroimaging methods.
References
Henry C, Van den Bulke D, Bellivier F, Roy I, Swendsen J, M'Bailara K et al. Affective lability and affect intensity as core dimensions of bipolar disorders during euthymic period. Psychiatry Res 2008; 159: 1–6.
Phillips ML, Ladouceur CD, Drevets WC . A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 2008; 13: 829, 833-857.
Wessa M, Kanske P, Linke J . Bipolar disorder: a neural network perspective on a disorder of emotion and motivation. Restor Neurol Neurosci 2014; 32: 51–62.
Wessa M, Linke J . Emotional processing in bipolar disorder: behavioural and neuroimaging findings. Int Rev Psychiatry 2009; 21: 357–367.
Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ et al. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry 2009; 66: 451–459.
Strakowski SM, Eliassen JC, Lamy M, Cerullo MA, Allendorfer JB, Madore M et al. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry 2011; 69: 381–388.
Altshuler L, Bookheimer S, Proenza MA, Townsend J, Sabb F, Firestine A et al. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry 2005; 162: 1211–1213.
Almeida JR, Versace A, Hassel S, Kupfer DJ, Phillips ML . Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biol Psychiatry 2010; 67: 414–421.
Ochsner KN, Gross JJ . The cognitive control of emotion. Trends Cogn Sci 2005; 9: 242–249.
Morris RW, Sparks A, Mitchell PB, Weickert CS, Green MJ . Lack of cortico-limbic coupling in bipolar disorder and schizophrenia during emotion regulation. Transl Psychiatry 2012; 2: e90.
Townsend JD, Torrisi SJ, Lieberman MD, Sugar CA, Bookheimer SY, Altshuler LL . Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry 2013; 73: 127–135.
Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ . Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol Psychiatry 2004; 55: 1163–1170.
Wessa M, Houenou J, Paillere-Martinot ML, Berthoz S, Artiges E, Leboyer M et al. Fronto-striatal overactivation in euthymic bipolar patients during an emotional go/nogo task. Am J Psychiatry 2007; 164: 638–646.
Heissler J, Kanske P, Schonfelder S, Wessa M . Inefficiency of emotion regulation as vulnerability marker for bipolar disorder: Evidence from healthy individuals with hypomanic personality. J Affect Disord 2013; 152-154: 83–90.
Bertocci MA, Bebko GM, Mullin BC, Langenecker SA, Ladouceur CD, Almeida JR et al. Abnormal anterior cingulate cortical activity during emotional n-back task performance distinguishes bipolar from unipolar depressed females. Psychol Med 2012; 42: 1417–1428.
Deckersbach T, Rauch SL, Buhlmann U, Ostacher MJ, Beucke JC, Nierenberg AA et al. An fMRI investigation of working memory and sadness in females with bipolar disorder: a brief report. Bipolar Disord 2008; 10: 928–942.
Kanske P, Heissler J, Schonfelder S, Forneck J, Wessa M . Neural correlates of emotional distractibility in bipolar disorder patients, unaffected relatives, and individuals with hypomanic personality. Am J Psychiatry 2013; 170: 1487–1496.
Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J . Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 2006; 51: 871–882.
Gyurak A, Gross JJ, Etkin A . Explicit and implicit emotion regulation: a dual-process framework. Cogn Emot 2011; 25: 400–412.
Ochsner KN, Silvers JA, Buhle JT . Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 2012; 1251: E1–24.
Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M . How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex 2011; 21: 1379–1388.
McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN . The neural bases of distraction and reappraisal. J Cogn Neurosci 2010; 22: 248–262.
Houenou J, Frommberger J, Carde S, Glasbrenner M, Diener C, Leboyer M et al. Neuroimaging-based markers of bipolar disorder: evidence from two meta-analyses. J Affect Disord 2011; 132: 344–355.
Wolkenstein L, Zwick JC, Hautzinger M, Joormann J . Cognitive emotion regulation in euthymic bipolar disorder. J Affect Disord 2014; 160: 92–97.
Green MJ, Lino BJ, Hwang EJ, Sparks A, James C, Mitchell PB . Cognitive regulation of emotion in bipolar I disorder and unaffected biological relatives. Acta Psychiatr Scand 2011; 124: 307–316.
Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM . Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res 2008; 162: 27–37.
Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry 2009; 66: 516–521.
Surguladze SA, Marshall N, Schulze K, Hall MH, Walshe M, Bramon E et al. Exaggerated neural response to emotional faces in patients with bipolar disorder and their first-degree relatives. NeuroImage 2010; 53: 58–64.
Thermenos HW, Goldstein JM, Milanovic SM, Whitfield-Gabrieli S, Makris N, Laviolette P et al. An fMRI study of working memory in persons with bipolar disorder or at genetic risk for bipolar disorder. Am J Med Genet 2010; 153B: 120–131.
Whalley HC, Sussmann JE, Chakirova G, Mukerjee P, Peel A, McKirdy J et al. The neural basis of familial risk and temperamental variation in individuals at high risk of bipolar disorder. Biol Psychiatry 2011; 70: 343–349.
Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD et al. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord 2012; 14: 313–325.
Strakowski SM, Delbello MP, Adler CM . The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry 2005; 10: 105–116.
Versace A, Thompson WK, Zhou D, Almeida JR, Hassel S, Klein CR et al. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry 2010; 67: 422–431.
Garnefski N, Kraaij V . The Cognitive Emotion Regulation Questionnaire: Psychometric features and prospective relationships with depression and anxiety in adults. Eur J Psychol Assess 2007; 23: 141–149.
First MB, Gibbon M, Spitzer RL, Williams JBW . Structured Clinical Interview for DSM IV Axis I Disorders (SCID-I). American Psychiatric Publishing Inc.: Arlington, 1997.
Wittchen H-U, Zaudig M, Fydrich T . SKID-I. Strukturiertes Klinisches Interview für DSM-IV [Structural Clinical Interview for DSM-IV Axis I Disorders]. Hogrefe: Göttingen, 1997.
First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin L . Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II). American Psychiatric Publishing Inc.: Arlington, 1997.
Wittchen H-U, Zaudig M, Fydrich T . SKID-II. Strukturiertes Klinisches Interview für DSM-IV [Structural Clinical Interview for DSM-IV Axis II Disorders]. Hogrefe: Göttingen, 1997.
Young RC, Biggs JT, Ziegler VE, Meyer DA . A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133: 429–435.
Hamilton M . A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62.
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J . An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–571.
Hautzinger M, Keller F, Kühner C . BDI-II. Beck-Depressions-Inventar. Revision. Pearson Assessment: Frankfurt, 2009.
Mercer L, Becerra R . A unique emotional processing profile of euthymic bipolar disorder? A critical review. J Affect Disord 2013; 146: 295–309.
Sackeim HA . The definition and meaning of treatment-resistant depression. J Clin Psychiatry 2001; 62 (Suppl 16): 10–17.
Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ . Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry 2008; 165: 313–320.
Lang PJ, Bradley MM, Cuthbert BN . International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical report A-6. Center for Research in Psychophysiology, University of Florida: Gainesville, Florida, 2005.
Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ . Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 1997; 6: 218–229.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15: 273–289.
Kanske P, Heissler J, Schonfelder S, Wessa M . Neural correlates of emotion regulation deficits in remitted depression: the influence of regulation strategy, habitual regulation use, and emotional valence. Neuroimage 2012; 61: 686–963.
Linke J, King AV, Rietschel M, Strohmaier J, Hennerici M, Gass A et al. Increased Medial Orbitofrontal and Amygdala Activation: Evidence for a Systems-Level Endophenotype of Bipolar I Disorder. Am J Psychiatry 2012; 169: 316–325.
Ogawa K, Inui T . Neural representation of observed actions in the parietal and premotor cortex. Neuroimage 2011; 56: 728–735.
Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry 2013; 74: 898–907.
Altshuler L, Bookheimer S, Townsend J, Proenza MA, Sabb F, Mintz J et al. Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disord 2008; 10: 708–717.
Malhi GS, Lagopoulos J, Ward PB, Kumari V, Mitchell PB, Parker GB et al. Cognitive generation of affect in bipolar depression: an fMRI study. Eur J Neurosci 2004; 19: 741–754.
Foland-Ross LC, Bookheimer SY, Lieberman MD, Sugar CA, Townsend JD, Fischer J et al. Normal amygdala activation but deficient ventrolateral prefrontal activation in adults with bipolar disorder during euthymia. NeuroImage 2012; 59: 738–744.
Lagopoulos J, Malhi GS . A functional magnetic resonance imaging study of emotional Stroop in euthymic bipolar disorder. Neuroreport 2007; 18: 1583–1587.
Urry HL, Gross JJ . Emotion regulation in older age. Curr Dir Psychol Sci 2010; 19: 352–357.
Almeida JR, Akkal D, Hassel S, Travis MJ, Banihashemi L, Kerr N et al. Reduced gray matter volume in ventral prefrontal cortex but not amygdala in bipolar disorder: significant effects of gender and trait anxiety. Psychiatry Res 2009; 171: 54–68.
Seidman LJ, Faraone SV, Goldstein JM, Kremen WS, Horton NJ, Makris N et al. Left hippocampal volume as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Arch Gen Psychiatry 2002; 59: 839–849.
Frangou S . Risk and resilience in bipolar disorder: rationale and design of the Vulnerability to Bipolar Disorders Study (VIBES). Biochem Soc Trans 2009; 37: 1085–1089.
Durston S, Mulder M, Casey BJ, Ziermans T, van Engeland H . Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biol Psychiatry 2006; 60: 1062–1070.
Phillips ML, Swartz HA . A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry 2014; 171: 829–843.
Acknowledgements
This research was funded by a grant from the Deutsche Forschungsgemeinschaft (We3638/3-1). We thank Heike Schmidt and Janine Heissler for their help with the data acquisition. We also thank six anonymous reviewers for their helpful comments on a previous version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Kanske, P., Schönfelder, S., Forneck, J. et al. Impaired regulation of emotion: neural correlates of reappraisal and distraction in bipolar disorder and unaffected relatives. Transl Psychiatry 5, e497 (2015). https://doi.org/10.1038/tp.2014.137
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2014.137
This article is cited by
-
Emotion regulation in bipolar disorder type-I: multivariate analysis of fMRI data
International Journal of Bipolar Disorders (2023)
-
Childhood maltreatment and emotion regulation in everyday life: an experience sampling study
Scientific Reports (2023)
-
Deficits in explicit emotion regulation in bipolar disorder: a systematic review
International Journal of Bipolar Disorders (2021)
-
Neural mechanisms of affective matching across faces and scenes
Scientific Reports (2019)
-
Is Neural Processing of Negative Stimuli Altered in Addiction Independent of Drug Effects? Findings From Drug-Naïve Youth with Internet Gaming Disorder
Neuropsychopharmacology (2018)