Abstract
Requirement for rocuronium upon surgery changes only minimally in patients with end-stage liver diseases. Our study consisted of both human and rat studies to explore the reason. The reduction rate of rocuronium infusion required to maintain neuromuscular blockade during the anhepatic phase (relative to paleohepatic phase) was examined in 16 children with congenital biliary atresia receiving orthotopic liver transplantation. Pharmacodynamics and pharmacokinetics of rocuronium were studied based on BDL rats. The role of increased Oatp2 and decrease Oatp1 expressions in renal compensation were explored. The reduction of rocuronium requirements significantly decreased in obstructively jaundiced children (24 ± 9 vs. 39 ± 11%). TOF50 in BDL rats was increased by functional removal of the kidneys but not the liver, and the percentage of rocuronium excretion through urine increased (20.3 ± 6.9 vs. 8.6 ± 1.8%), while that decreased through bile in 28d-BDL compared with control group. However, this enhanced renal secretion for rocuronium was eliminated by Oatp2 knock-down, rather than Oatp1 overexpression (28-d BDL vs. Oatp1-ShRNA or Oatp2-ShRNA, 20.3 ± 6.9 vs. 17.0 ± 6.6 or 9.3 ± 3.2%). Upon chronic/sub-chronic loss of bile excretion, rocuronium clearance via the kidneys is enhanced, by Oatp2 up-regulation.
Similar content being viewed by others
Introduction
Rocuronium is steroidal neuromuscular blocking agent with a rapid onset and intermediate duration of action1. Rocuronium is eliminated primarily as unchanged molecule in the bile, with only minor contribution of urinary elimination2,3,4.
However, two previous human studies reported that rocuronium infusion requirement was not significantly reduced in the anhepatic phase of liver transplantation5,6. Also, plasma clearance of rocuronium was not influenced by end-stage liver diseases7,8. Based on these observations, extra-hepatic clearance for rocuronium is reasonable to be inferred. Obviously, the kidneys are the most likely candidate for such alternative pathway6.
Organic anion transporting polypeptides (rodents: Oatps; human: OATPs) are a superfamily of transmembrane transporters that eliminates a wide variety of structurally unrelated amphipathic organic compounds, including rocuronium, bile salts and thyroid hormones9,10,11,12. Impaired bile secretion, such as upon cholestasis, alters the expression of Oatps in the kidneys and cholangiocytes13,14. We therefore examined the expression of Oatps in the liver and kidneys upon bile duct ligation (BDL) in the current study.
The current study consisted of a human study which examined the requirement of rocuronium to maintain neuromuscular blockade in anhepatic phase of orthotopic liver transplantation (OLT), and rat studies to determine the possible contribution of the kidneys to rocuronium clearance under the condition of impaired bile secretion. In human study, the reduction of rocuronium infusion requirement during the anhepatic phase (relative to paleohepatic phase) of OLT was examined in 16 children with congenital biliary atresia. The rat experiments were conducted on 7 or 28 days after ligating the bile duct. Potential contribution of the renal excretion vs. hepatic secretion on rocuronium clearance was examined by functionally removing the liver vs. kidneys (pharmacodynamics) and by concentration determination (pharmacokinetics). The underlying mechanisms focused on Oatp1 and Oatp2 in kidneys were explored by Oatp2 knock-down and Oatp1 overexpression via adeno-associated virus (AAV).
Results
Human studies
Sixteen children were included in this study. Demographic and clinical information including age, gender, weight, total bilirubin, serum albumin, PT and ascites, whether undergoing Kasai portoenterostomy, CTP score, was shown in Table 1
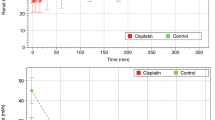
The onset time after rocuronium bolus injection (Fig. 1A) and no reaction period of TOF (time from T1 disappearing to appearing, Fig. 1B) in obstructively jaundiced (OJ) children were significantly larger than that in control group (93 ± 13 vs. 72 ± 10 s; 58 ± 10 vs. 43 ± 6.3 min, respectively). The requirement of rocuronium to maintain a stable neuromuscular blockade was significantly lower in obstructively jaundiced group during the anhepatic phase (2.4 ± 0.57 vs. 3.3 ± 0.97 μg•kg−1•min−1, Fig. 1D), while the reduction of rocuronium requirement during the anhepatic phase (relative to paleohepatic phase), which negatively correlated with the degree of extrahepatic compensation, significantly decreased in OJ group compared with that in control group (24 ± 9 vs. 39 ± 11%, Fig. 1E). The reduction of rocuronium negatively correlated with serum bilirubin (r2 = 0.27, p = 0.037, Fig. 1G).
(A) The onset time of rocuronium after bolus injection. (B) No reaction period of TOF (time from T1 disappearing to appearing. (C) Recovery index (time for T1 from 25% to 75%). (D and F) the infusion requirement of rocuronium to maintain a stable neuromuscular in the anhepatic (E) and neohepatic phase (F). (E) The reduction of rocuronium requirement in the anhepatic phase (relative to paleohepatic phase), which negatively correlated with the extra-hepatic compensation. (G) The negative correlation between the reduction of rocuronium and serum bilirubin. (H) Continuous recording of the twitch response of adductor pollicis to TOF stimulation. *Significantly (p < 0.05) different from control. **Significantly (p < 0.01) different from control.
Rat experiments
7d-BDL increased serum bilirubin (445 ± 163 vs. 155 ± 99μmol·L−1, p < 0.01). The majority of elevated bilirubin was the conjugated type. 7d-BDL alone potentially increased TOF50 (11 ± 8.3 vs. 4.5 ± 3.4 and 28 ± 15 vs. 13 ± 5.8 min for 1 and 5 mg•kg−1, respectively, Fig. 2A), although there was no significant difference. Removing the liver in 7d-BDL rats did not significantly affect TOF50 (no-liver vs. systemic metabollism, 13 ± 5.8 vs. 20 ± 9.9 min; p > 0.05; Fig. 2B). Removing the kidneys in 7d-BDL rats increased TOF50 (no-kidney vs. systemic metabolism, 73 ± 47 vs. 28 ± 15 min; p < 0.05; Fig. 2C).
(A) The TOF50 time after rocuronium bolus injection (1 or 5 mg·kg−1) in Sham and 7d-BDL groups. (B) Comparisons of TOF50 time after a single i.v. bolus injection of rocuronium of 1 mg·kg−1 in Sham and 7-d BDL rats with or without liver. (C) Comparisons of TOF50 time after a single i.v. bolus injection of rocuronium of 5 mg·kg−1 in Sham and 7-d BDL rats with or without kidneys. (D) Comparisons of excretion percentage through bile and urine between 28-d BDL rats and Sham rats. *Significantly (p < 0.05) different. **Significantly (p < 0.01) different.
After injection of rocuronium 5 mg·kg−1, The excretion percentage of the administered dose through the urine statistically increased in 28d-BDL group compared with control group in the first 4 hours after injection (20.3 ± 6.9 vs. 8.6 ± 1.8%, p < 0.05; Fig. 2D), while opposite result was observed in bile excretion (8.9 ± 3.0 vs. 28.4 ± 9.3%, p < 0.05; Fig. 2D).
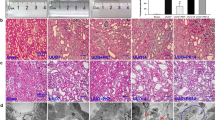
Previous studies have reported that the excretion of rocuronium is mediated by Oatp1, Oatp2 and Oatp3 in rodents15,16,17. Considering the evidence that the oatp3 mRNA is undetectable in kidneys18, we mainly focused on the Oatp1 and Oatp2 in kidneys. BDL increased Oatp2, mostly in the glomerulus on day 14 after the surgery and throughout the entire nephronay on day 28 (Fig. 3A and 4). The mean positivity for Otap2 in kidney was increased to 20.1 ± 2.2, 24.6 ± 2.7, 28.6 ± 3.1% on 7, 14 and 28 days after the surgery, respectively vs. 17.9 ± 1.9% in Sham group (p < 0.05, Fig. 3B). Western blot analysis also confirmed increased Oatp2 in the kidney of BDL rats (Fig. 3C). Meanwhile, BDL down-regulated the mean positivity of Oatp2 in the liver (18.2 ± 2.1, 17.1 ± 1.9 and 16.9 ± 1.8% on 7, 14 and 28 days after the BDL surgery respectively, vs. 20.0 ± 2.2% prior to BDL, p < 0.05, Figure S1). The mean positivity of Oatp1 in kidney decreased (28.2 ± 3.2, 26.9 ± 2.9 and 25.9 ± 2.8% on 7, 14 and 28 days after the BDL surgery, respectively vs. 29.8 ± 3.4% prior to BDL, p < 0.05, Figure S2).
(A) represents expression of Oatp2 in Sham, 7d, 14d and 28d-BDL rats detected by IHC. (B) Represents statistical analysis of positive Oatp2 in the four groups. (C) Western blot of Oatp2 in kidney plasma membranes from Sham (lane 1) and 7-d BDL (lane 2) rats. The image has been cropped and 73KD represents Oatp2. *Significantly (p < 0.05) different from Sham. KD = kilodaltons.
To assess the efficiency of AAV vectors for gene transfer into renal cells, we used H9C2, 293 AAV and HER293 cell line, in which Oatp2 was over-expressed artificially, as an in vitro model. Figure S3A demonstrated the AAV vectors efficiently transduced into these cells. Meanwhile, the Oatp2 expression was also significantly inhibited in both mRNA (Figure S3B) and protein level (Figure S3C). The vector solutions were selectively injected into rat kidneys using the procedure as described in Methods. The over-expressed Oatp2 was obviously reversed by AAV vectors in 28d-BDL rats (Figure S3D and 5A). Figure S4 and 5A illustrated that the artificially upregulation of Oatp1 via AAV in kidneys was also successfully established. Oatp2 knock-down rather Oatp1 artificial upregulation in kidneys of 28d-BDL rats significantly prolonged TOF50 (blank vs. Oatp1-ShRNA, or Oatp2-ShRNA, 15 ± 7.8 vs. 18 ± 7.5, or 36 ± 22 min, Fig. 5b), which indicated us the excretion disorder of rocuronium after Oatp2 knock-down. The increased urinary excretion percentage of rocuronium induced by BDL was reversed after Oatp2 knock-down instead of Oatp1 artificial overexpression (28-d BDL vs. Sham, Oatp1-ShRNA or Oatp2-ShRNA, 20.3 ± 6.9 vs. 8.6 ± 1.8, 17.0 ± 6.6 or 9.3 ± 3.2%, Fig. 5C), while Oatp1overexpression and Oatp2 knock-down in kidneys had no effects on bile excretion in BDL rats (28-d BDL vs. Sham, Oatp1-ShRNA or Oatp2-ShRNA, 8.9 ± 3.0 vs. 28.4 ± 9.3, 15.0 ± 5.3 or 13.9 ± 7.7%, Fig. 5C).
(A) The renal Oatp1 and Oatp2 expressions in 28d-BDL rats administered with blank solution, AAV-rSlco1a1 or rSlco1a2 ShRNA. The image has been cropped and 90KD represents Oatp1 and Oatp2. (B) TOF50 time in 28d-BDL rats administered with blank solution, AAV-rSlco1a1 or rSlco1a2 ShRNA. (C) Comparisons of excretion percentage through bile acid and urine among Sham and 28-d BDL rats administered with blank solution, AAV-rSlco1a1 or rSlco1a2 ShRNA. *Significantly (p < 0.05) different.
Discussion
The results from the human experiments in the current study demonstrated the reduction of rocuronium requirement during the anhepatic phase (relative to paleohepatic phase) significantly decreased in OJ group compared with that in control group: subjects with OJ prior to the surgery tended to require relatively higher rate of rocuronium infusion in the anhepatic phase. Interestingly, the reduction of the rocuronium negatively correlated with serum bilirubin prior to OLT. In the experiments with BDL rats, TOF50 was increased significantly by removing the kidneys, but not the liver, which was consistent with another interesting finding that BDL induced alternation of excretion pathway for rocuronium (urine increased while bile decreased). Furthermore, the enhanced renal excretion was eliminated by Oatp2 knock-down rather Oatp1 upregulation in kidneys.
The current finding that children with OJ tend to require higher rocuronium infusion rate for muscular blockade in the anhepatic phase of the OLT clearly suggested the existence of compensatory mechanisms for rocuronium clearance upon compromised bile secretion. The results also suggested that the extent of the compensation was dependent on baseline serum bilirubin. Experiments in BDL rats clearly demonstrated that this compensation occurs in the kidneys. In contrast to significantly prolonged TOF50 by kidney removal, removing the blood supply to the liver in 7d-BDL rats did not significantly affect rocuronium clearance, and 28d-BDL rats tended to eliminate rocuronium via urinary excretion rather than bile acid pathway.
Oatps are widely expressed in a variety of organs, including the kidneys, and seem to be responsible for extrahepetic clearance of rocuronium. Combining the evidence that the excretion of rocuronium is mediated by Oatp1, Oatp2 and Oatp3 in rodents15,16,17 and the oatp3 mRNA is undetectable in kidney18, we mainly focused on the Oatp1 and Oatp2 in kidney. The altered expressions of Oatp1 and Oatp2 in kidneys and liver are consistent with previous studies19,20 which might contribute to reduce the accumulation of biliary components. We speculated that the surge of bilirubin or bile acids upon BDL stimulated increased expression of Oatp2 in nephron units. In the kidneys, Oatp2 functions as an efflux transporter upon cholestasis21. Combined with our results that Oatp2 knock-down rather Oatp1 overexpression in kidneys eliminated the renal compensation for rocuronium excretion in BDL rats, we could conclude adaptive increase of Oatp2 in kidneys could reduce plasma bile acids and other organic anions, such as exogenously administered rocuronium
Oatp1 in renal tubules reabsorb a number of organic compounds that reached the renal tubles22. Oatp2 in the liver represents a high-affinity backup system for removal of certain cholephilic substances from portal blood23. Lower expression of Oatp1 in the kidneys and Oatp2 in liver of BDL rats discovered in the current study are consistent with increased renal excretion and decreased hepatic secretion upon hepatic damage, although the Oatp1 has been proved to play no role in renal enhanced excretion of rocuronium in the present study.
Pregnane X receptor (PXR) response elements could be detected on the Oatp2 gene and the Oatp2 was up-regulated in PXR knockout mice24,25. Bile acids as signaling molecules could activate the nuclear hormone receptors PXR. So, the up-regulation of Oatp2 in kidneys may attribute to the activated PXR. BDL is also associated with inflammation. Serum levels of pro-inflammatory cytokines, such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor Necrosis Factor alpha (TNF-α), are elevated in BDL rats26,27. Recombinant TNF-α, IL-1, and IL-6, are each capable of producing down-regulation of Oatp2 mRNA expression in mice28,29,30. However, the specific cellular and molecular mechanisms responsible for cytokines-related regulation of Oatp2 remains to be determined.
In a few previous studies5,6, reduction of rocuronium requirement in the anhepatic period (relative to that in the paleohepatic phase) is less prominent than that in the current study. Several possible explanations could account for the difference. First, we did not adopt the commonly used veno-venous bypass during the anhepatic period, since blocking the inferior vena cava could decrease blood flow to the kidneys6. As a result, renal clearance of rocuronium might have been reduced. Also, the different results may be caused by different subjects adopted which were children with congenital biliary atresia, other than adults with end-stage liver diseases.
Neither electromyography (EMG) nor mechanomyography was used in the current study. Despite of this limitation, we have been using the acceleration transducer to monitor neuromuscular function in similar study, with consistent results31,32,33,34. Specifically, our results correlated well with the results obtained using mechanomyography or EMG35. Also, TOF50 time was measured using a bioassay, representing a weakness of the study.
In conclusion, kidneys play a prominent role in the clearance of rocuronium when excretion through the bile is diminished, via increased expression of Oatp2 in kidneys. The results from the current study encourage the use of rocuronium in patients with cholestasis.
Methods
Clinical study
Patients and Anaesthesia
The protocol was approved by the Institutional Ethics Committee of the Renji Hospital and the Shanghai Jiao Tong University. The methods used in our article have been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiment involving human. All subjects’ guardians gave written informed consent prior to the study. The study is registered with www.chictr.org.cn, number ChiCTR-OOC-16008707.
The study included 16 children with congenital biliary atresia (10 underwent Kasai portoenterostomy) scheduled to receive OLT under general anaesthesia during a period from July to September 2016. The subjects were divided into two groups according to serum bilirubin (n = 8): obstructively jaundiced group (OJ) and control group. Patients who had one or more of the following conditions were not included: a neuromuscular disease such as one of multiple sclerosis, Huntington’s disease, Creutzfeldt-Jakob disease, myasthenia gravis or polymyositis ect. Chronic kidney diseases and acute renal failure. Anaesthesia was induced with ketamine (5–8 mg•kg−1, i.m.) and fentanyl (5 μg•kg−1, i.v.). After the induction, rocuronium (Esmeron®, 0.6 mg•kg−1) was immediately given as an intravenous bolus to facilitate tracheal intubation. Anaesthesia was maintained with propofol and rocuronium infusion. Patients were ventilated mechanically with oxygen and air to maintain end-tidal carbon dioxide in the range 30 to 40 mmHg. Blood potassium was maintained at >3.5 mmol•L−1. Blood calcium was maintained at >1.0 mmol•L−1. Urine output was measured hourly. During the anhepatic phase, portal vein and hepatic vein were completely blocked, instead of bypassing the veno-venous bypass from the lower limbs and portal vein to the jugular vein.
Rocuronium Infusion
Rocuronium infusion was commenced when the twitch response in the adductor pollicis muscle to train-of-four (TOF) stimulation fully recovered from the initial bolus injection (0.6 mg•kg−1, as described above). Rocuronium was infused at an initial rate of 0.3 μg•kg−1•min−1, and adjusted to maintain 85–95% neuromuscular paralysis. The infusion rate must be stable for at least 20 min to be included in the analysis of rocuronium requirement.
Neuromuscular function monitoring
The level of neuromuscular paralysis was evaluated by measuring the acceleration of the adductor pollicis twitch response to asupramaximal stimulation (0.2msec duration single stimulus at a frequency of 0.1 Hz) to the ulnar nerve at the wrist, via the TOF-Watch®SX.
Rat experiments
All animal experiments were conducted in accordance with the Institutional Guidelines on the Use of Live Animals for Research, and approved by the Animal Careand Use Committee of the Second Military Medical University.
Adult male Sprague-Dawley (SD) rats (250–350 g) were housed under a 12 h light/12 h dark cycle at 22 °C, with free access to food and water until 8 hours prior to pentobarbital anaesthesia (50 mg•kg−1; i.p.) after atropine injection (0.25 mg•kg−1). Rats were intubated and ventilated (frequency: 90/minute; pressure: 0.015–0.02 Mpa) using a TKR-200 respirator. The end-tidal PCO2 was maintained at 35–45 mmHg. Twenty-one rats received BDL. Briefly, the common bile duct was securely ligated close to the hilus and cut between the two ligatures. A group of rats (n = 18) receiving Sham operation (all the procedures with the exception of BDL) was included as a healthy control.
On day 7 after BDL (17 BDL rats, 15 Sham operation rats), the liver was exposed and the liver artery was ligated. The portal vein was blocked with a vascular clamp at its distal end. The left hepatic lobe was partly excised to expose the portal vein along its inferior margin. A heparinized silicone catheter (diameter: 0.2 cm) was inserted into the portal vein. The other end of the silicone catheter was fixed in the left hepatic vein. The vascular clamp was then opened to allow blood flow to the left hepatic vein from the portal vein. For kidneys removal, both renal arteries were ligated at the pedicles.
The right hindlimb of the rat was immobilized and the sciatic nerve was isolated surgically in the popliteal space. A bipolar platinum electrode was attached to the peripheral portion of the nerve. The distal tendon of the right tibial was isolated from the surrounding tissue and connected to a force transducer (TB-611, Nihon Kohden, Tokyo, Japan) at a resting tension of 30 g. TOF stimulation (TOF-Watch® SX), consisting of four supramaximal pulses (0.2msec duration; 2 Hz), was applied to the sciatic nerve via the electrodes every 15 sec. The T1 block was maintained at 95-105% for at least 5 min before rocuronium administration (i.v. bolus injection of rocuronium of 1 mg•kg−1 or 5 mg•kg−1). Neuromuscular block was monitored by measuring the time needed for TOF resumption to 50% (TOF50).
In vivo rSlco1a1 or rSlco1a2 -ShRNA Transduction into the Kidneys
To achieve efficient rAAV-DJ-ZsGreen-rSlco1a1 or rSlco1a1 ShRNA delivery into the kidney in vivo, we used a catheter-based gene delivery system in rats. The flexible Solo-Cath catheter (2Fr; Solo-mon Scientific, Plymouth Meeting, Pa., USA) was inserted via the left iliac artery and the abdominal aorta, and the tip was placed beneath the left artery. After the aorta was clamped just above the left renal artery, 1 ml of AAV vector solution was injected after 1-2 ml of saline to wash out blood via the catheter. The left renal vein then was clamped for 10 min. Catheter and clip were removed. Meanwhile the BDL procedures were carried out, and rats were sacrificed on day 28.
Urine, bile acid and serum from 28d-BDL rats (n = 5) and Sham rats (n = 4) were collected 4 h after rocuronium administration (i.v. bolus injection 5 mg•kg−1). Concentrations of rocuronium were measured by HPLC-MS (Agilent Technologies, CA, USA; Applied Biosystems Sciex, MA, USA).
Immunohistochemistry
Paraffin sections (5μm) were deparaffinized and rehydrated. After incubation with 2% bovineserum albumin in Tris-buffered saline (TBS; pH 7.6) at room temperature for 30 min, sections were incubated overnight at 4 °C with either an anti-oatp1 (OATP21-A, 1:200, ADI, Samford, Connecticut, America) or anti-oatp2 (OATP21-B, 1:200, ADI, Samford, Connecticut, America). After extensive washing, the sections were incubated with a polymer enhancer and a polymerized anti-rabbit or anti-mouse IgG (dilution1:200, Jingmei, Shanghai, China) labeled with horseradish peroxidase. The staining was visualized with a DAB kit (Maixin Biologic Technology, Fujian, China), and appeared as buffy granules in cytoplasm.
Immunofluorescence
Tissue sections were prepared as described previously36. Nonspecific bindings were blocked with 2% bovine serum albumin (BSA) in TBS pH 7.6 at room temperature for 30 min. Then, the tissue sections were incubated overnight at 4 °C with the anti-Oatps antibody. Washing was then performed at room temperature using TBS pH7.6. And then, the tissues were incubated with a cyber green-conjugated goat anti-rabbit IgG antibody (dilution1:200, Jingmei, Shanghai, China) in 2% BSA-TBS at 1:400 for 3 h. The sections were analyzed using a confocal fluorescencemicroscope (Nikon UFX, Tokyo, Japan).
Western blotting analysis
Crude plasma membrane (CPM) was prepared as described previously37,38, and separated using a 7.5% polyacrylamide gel. The proteins were transferred to a polyvinylidene fluoride (PVDF) membrane, and then blocked with 5% non-fat dry milkin TBST buffer containing 0.05% Tween-20 at room temperature for 1 h. The membrane was then incubated overnight at 4 °C with an anti-oatp2 antibody (OATP21-B, 1:200, ADI, Samford, Connecticut, America). After appropriate washing, the membrane was incubated for 1 h with an anti-rabbit IgG antibody (dilution1:200, Jingmei, Shanghai, China) for detection of Oatp2 using ECL Plus (Amersham Pharmacia, Piscataway,NJ) for visualization.
Statistical Analysis
Quantitative data were expressed as the mean ± SD. A Student’s t-test was used to compare the measurement data of two groups. The reduction of rocuronium requirement in the anhepatic phase (relative to that in the paleohepatic phase) vs. serum bilirubin prior to the surgery was analyzed using a linear regression. Data from three or more samples were analyzed using one-way analysis of variance (ANOVA), followed by Dunnett’s t-test (the mean positivity of Oatps in tissue samples the Sham and 7, 14, 28-day BDL groups; the TOF50 time among blank, Oatp1-ShRNA and Oatp2-ShRNA groups) and Tukey’s multiple comparisons test (the TOF50 time from Sham + systemic metabolism, Sham + no-liver or no-kidney, BDL + systemic metabolism, BDL + no-liver or no-kidney; the excretion percentages of rocuronium though urine and bile). The level of statistical significance was set at p < 0.05. All statistical analyses were performed with PASW Statistics version 18.
Additional Information
How to cite this article: Wang, L. et al. Increased Renal Clearance of Rocuronium Compensates for Chronic Loss of Bile Excretion, via upregulation of Oatp2. Sci. Rep. 7, 40438; doi: 10.1038/srep40438 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Wierda, J. M., Kleef, U. W., Lambalk, L. M., Kloppenburg, W. D. & Agoston, S. The pharmacodynamics and pharmacokinetics of Org 9426, a new non-depolarizing neuromuscular blocking agent, in patients anaesthetized with nitrous oxide, halothane and fentanyl. Canadian journal of anaesthesia = Journal canadien d’anesthesie 38, 430–435, doi: 10.1007/bf03007578 (1991).
Khuenl-Brady, K. et al. The neuromuscular blocking effects and pharmacokinetics of ORG 9426 and ORG 9616 in the cat. Anesthesiology 72, 669–674 (1990).
van Miert, M. M., Eastwood, N. B., Boyd, A. H., Parker, C. J. & Hunter, J. M. The pharmacokinetics and pharmacodynamics of rocuronium in patients with hepatic cirrhosis. British journal of clinical pharmacology 44, 139–144 (1997).
Szenohradszky, J. et al. Pharmacokinetics of rocuronium bromide (ORG 9426) in patients with normal renal function or patients undergoing cadaver renal transplantation. Anesthesiology 77, 899–904 (1992).
Fisher, D. M. et al. Pharmacokinetics of rocuronium during the three stages of liver transplantation. Anesthesiology 86, 1306–1316 (1997).
Gao, L., Ramzan, I. & Baker, B. Rocuronium infusion requirements and plasma concentrations at constant levels of neuromuscular paralysis during three phases of liver transplantation. Journal of clinical anesthesia 15, 257–266 (2003).
Khalil, M. et al. Pharmacokinetics and pharmacodynamics of rocuronium in patients with cirrhosis. Anesthesiology 80, 1241–1247 (1994).
Magorian, T. et al. The pharmacokinetics and neuromuscular effects of rocuronium bromide in patients with liver disease. Anesthesia and analgesia 80, 754–759 (1995).
Kullak-Ublick, G. A. et al. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology 120, 525–533 (2001).
Kullak-Ublick, G. A., Stieger, B., Hagenbuch, B. & Meier, P. J. Hepatic transport of bile salts. Seminars in liver disease 20, 273–292, doi: 10.1055/s-2000-9426 (2000).
Klaassen, C. D. & Aleksunes, L. M. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacological reviews 62, 1–96, doi: 10.1124/pr.109.002014 (2010).
Svoboda, M., Riha, J., Wlcek, K., Jaeger, W. & Thalhammer, T. Organic anion transporting polypeptides (OATPs): regulation of expression and function. Current drug metabolism 12, 139–153 (2011).
Lee, J. et al. Adaptive regulation of bile salt transporters in kidney and liver in obstructive cholestasis in the rat. Gastroenterology 121, 1473–1484 (2001).
Denk, G. U. et al. Multidrug resistance-associated protein 4 is up-regulated in liver but down-regulated in kidney in obstructive cholestasis in the rat. Journal of hepatology 40, 585–591, doi: 10.1016/j.jhep.2003.12.001 (2004).
van Montfoort, J. E. et al. Drug uptake systems in liver and kidney. Current drug metabolism 4, 185–211 (2003).
Cattori, V. et al. Localization of organic anion transporting polypeptide 4 (Oatp4) in rat liver and comparison of its substrate specificity with Oatp1, Oatp2 and Oatp3. Pflugers Archiv : European journal of physiology 443, 188–195, doi: 10.1007/s004240100697 (2001).
van Montfoort, J. E. et al. Polyspecific organic anion transporting polypeptides mediate hepatic uptake of amphipathic type II organic cations. The Journal of pharmacology and experimental therapeutics 291, 147–152 (1999).
Walters, H. C., Craddock, A. L., Fusegawa, H., Willingham, M. C. & Dawson, P. A. Expression, transport properties, and chromosomal location of organic anion transporter subtype 3. American journal of physiology. Gastrointestinal and liver physiology 279, G1188–1200 (2000).
Geier, A. et al. Regulation of basolateral organic anion transporters in ethinylestradiol-induced cholestasis in the rat. Biochimica et biophysica acta 1609, 87–94 (2003).
Zollner, G. et al. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. Journal of hepatology 38, 717–727 (2003).
Kullak-Ublick, G. A. Regulation of organic anion and drug transporters of the sinusoidal membrane. Journal of hepatology 31, 563–573 (1999).
Brandoni, A., Di Giusto, G., Franca, R., Passamonti, S. & Torres, A. M. Expression of kidney and liver bilitranslocase in response to acute biliary obstruction. Nephron. Physiology 114, p35–40, doi: 10.1159/000276588 (2010).
Reichel, C. et al. Localization and function of the organic anion-transporting polypeptide Oatp2 in rat liver. Gastroenterology 117, 688–695 (1999).
Staudinger, J. L. et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proceedings of the National Academy of Sciences of the United States of America 98, 3369–3374, doi: 10.1073/pnas.051551698 (2001).
Guo, G. L., Staudinger, J., Ogura, K. & Klaassen, C. D. Induction of rat organic anion transporting polypeptide 2 by pregnenolone-16alpha-carbonitrile is via interaction with pregnane X receptor. Molecular pharmacology 61, 832–839 (2002).
Bohan, A., Chen, W. S., Denson, L. A., Held, M. A. & Boyer, J. L. Tumor necrosis factor alpha-dependent up-regulation of Lrh-1 and Mrp3(Abcc3) reduces liver injury in obstructive cholestasis. The Journal of biological chemistry 278, 36688–36698, doi: 10.1074/jbc.M304011200 (2003).
Tu, B. et al. Role of NF-kB in multiple organ dysfunction during acute obstructive cholangitis. World journal of gastroenterology : WJG 9, 179–183 (2003).
Hartmann, G., Cheung, A. K. & Piquette-Miller, M. Inflammatory cytokines, but not bile acids, regulate expression of murine hepatic anion transporters in endotoxemia. The Journal of pharmacology and experimental therapeutics 303, 273–281, doi: 10.1124/jpet.102.039404 (2002).
Siewert, E. et al. Interleukin-6 regulates hepatic transporters during acute-phase response. Biochemical and biophysical research communications 322, 232–238, doi: 10.1016/j.bbrc.2004.07.102 (2004).
Geier, A. et al. Cytokine-dependent regulation of hepatic organic anion transporter gene transactivators in mouse liver. American journal of physiology. Gastrointestinal and liver physiology 289, G831–841, doi: 10.1152/ajpgi.00307.2004 (2005).
Skinner, H. J., Girling, K. J., Whitehurst, A. & Nathanson, M. H. Influence of metoclopramide on plasma cholinesterase and duration of action of mivacurium. British journal of anaesthesia 82, 542–545 (1999).
Fu, W. et al. Rapacuronium recovery characteristics and infusion requirements during inhalation versus propofol-based anaesthesia. British journal of anaesthesia 85, 302–305 (2000).
Hernandez-Palazon, J., Tortosa, J. A., Martinez-Lage, J. F. & Perez-Ayala, M. Rocuronium-induced neuromuscular blockade is affected by chronic phenytoin therapy. Journal of neurosurgical anesthesiology 13, 79–82 (2001).
Wulf, H., Ledowski, T., Linstedt, U., Proppe, D. & Sitzlack, D. Neuromuscular blocking effects of rocuronium during desflurane, isoflurane, and sevoflurane anaesthesia. Canadian journal of anaesthesia = Journal canadien d’anesthesie 45, 526–532, doi: 10.1007/bf03012702 (1998).
McCluskey, A., Meakin, G., Hopkinson, J. M. & Baker, R. D. A comparison of acceleromyography and mechanomyography for determination of the dose-response curve of rocuronium in children. Anaesthesia 52, 345–349 (1997).
Pei, Q. L. et al. Increased expression of multidrug resistance-associated protein 1 (mrp1) in hepatocyte basolateral membrane and renal tubular epithelia after bile duct ligation in rats. Hepatology research : the official journal of the Japan Society of Hepatology 22, 58–64 (2002).
Meier, P. J. & Boyer, J. L. Preparation of basolateral (sinusoidal) and canalicular plasma membrane vesicles for the study of hepatic transport processes. Methods in enzymology 192, 534–545 (1990).
Oude Elferink, R. P. et al. Hepatobiliary secretion of organic compounds; molecular mechanisms of membrane transport. Biochimica et biophysica acta 1241, 215–268 (1995).
Acknowledgements
This work is supported by grant No. 81170427 from the National Natural Science Foundation, Beijing, China.
Author information
Authors and Affiliations
Contributions
Long Wang did the rat experiments. Mai-Tao Zhou and Wen Yin were responsible for human study. Chi-Wai Cheung revised this paper. Cai-Yang Chen wrote this paper. Li-Qun Yang gave specific guidance and analyzed the data. Da-Xiang Wen was the designer of the human study. Wei-Feng Yu was the designer and leader of our group.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, L., Zhou, MT., Chen, CY. et al. Increased Renal Clearance of Rocuronium Compensates for Chronic Loss of Bile Excretion, via upregulation of Oatp2. Sci Rep 7, 40438 (2017). https://doi.org/10.1038/srep40438
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep40438
This article is cited by
-
Influence of the Pringle maneuver during partial hepatectomy on the neuromuscular block induced by intermittent and continuous dosing of rocuronium
Journal of Anesthesia (2023)
-
miR-23a-3p is involved in drug resistance by directly targeting the influx drug transporter organic anion-transporting polypeptide 2
Child's Nervous System (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.