Abstract
Many previous studies have provided evidence that the ADIPOQ +45T>G polymorphism (rs2241766) might cause metabolic syndrome (MS). As a cardiovascular manifestation of MS, the incidence of stroke is associated with adiponectin; however, the results remain controversial and inconsistent. Systematic searches of relevant studies published up to Dec 2014 and Jan 2016 on the ADIPOQ +45T>G polymorphism and the risk of MS and adiponectin levels and the risk of stroke, respectively, were conducted in MEDLINE and EMBASE. The odds ratio (OR) or risk ratio (RR) and their 95% confidence interval (95% CI) were extracted. Sixteen studies containing 4,113 MS cases and 3,637 healthy controls indicated a weak positive association between ADIPOQ +45 T>G and MS in the dominant genetic model (OR = 1.30, 95% CI = 1.03–1.65), which was also validated by stratified subgroup analyses. Twelve studies including 26,213 participants and 4,246 stroke cases indicated that 5 μg/ml increments in adiponectin level were not relevant to stroke risk (RR = 1.05, 95% CI = 1.00–1.10, P = 0.069). This study suggested a weak positive association of ADIPOQ +45T>G with MS and a strong association with metabolic-related disease. Additionally, adiponectin level was not a causal factor of increasing stroke risk.
Similar content being viewed by others
Introduction
Metabolic syndrome (MS) constitutes a group of risk components including abdominal obesity, insulin resistance, hyperglycaemia, hyperlipidaemia, and hypertension1. This disorder is currently widely prevalent, present in 20–25% of the world’s adult population, and is particularly common in the industrialized societies of the world, where it is prevalent in epidemic proportions. Both genetic and environmental factors contribute to the development of MS2. Many studies have demonstrated the genetic mechanisms of MS and metabolic-related disease, but these mechanisms remain controversial and require further research.
Adiponectin is the most abundant adipose tissue-derived adipocytokine encoded by ADIPOQ (also known as APM1), which is located on chromosome 3q27, spans 17 kb, contains 3 exons and 2 introns3,4, and comprises 247 amino acids5. It is involved in the regulation of glucose and lipid metabolism, cardiovascular function, and amelioration of insulin resistance (IR) and has anti-inflammatory, anti-diabetic and anti-atherogenic properties; additionally, low circulating levels of adiponectin are associated with central obesity, insulin resistance, MS, and type 2 diabetes (T2DM)6,7. Great attention has paid to ADIPOQ, which is considered an important factor in MS and metabolic-related disease. Therefore, identifying the intrinsic genetic mechanisms and clarifying the similarities as well as partial distinctions of these mechanisms are issues that we pursue with interest in this study.
ADIPOQ +45T>G (rs2241766) is one of the most common variants of exon 2 on the ADIPOQ gene, and some studies have provided evidence that the ADIPOQ +45 T>G polymorphism is associated with serum levels of adiponectin3, MS components, insulin sensitivity, obesity and T2DM8. To our knowledge, the results to date have been discordant. There has been a meta-analysis conducted by GAO M et al. that differed from ours9, but that study was limited to the Chinese population, lacked a detailed stratified analysis, and used data that were collected before August 2012. Thus, we conducted an updated analysis on the relationship between ADIPOQ +45 T>G and MS risk.
In addition, as a common cardiovascular manifestation of MS, the association between the incidence of stroke and adiponectin has also been inconsistent10,11,12. Although Arregui M et al. conducted a meta-analysis including 9 studies on this topic; the overall RR estimate might have been inaccurate due to the significant publication bias and obvious heterogeneity, which both reduced the validity and accuracy of the conclusion.
Considering the lack of knowledge on the specific ADIPOQ genetic determinants of MS, the controversy regarding the association between adiponectin and stroke, and the need to update the latest data with stratified subgroup analyses to obtain more accurate conclusions, the objective of the present updated study was as follows: to investigate the latest data among the whole population and obtain greater statistical power to detect the association between the ADIPOQ +45 T>G polymorphism and MS; to further assess the relationship between ADIPOQ +45 T>G and metabolic-related disease in a published meta-analysis and beyond that, compare the similarities and differences in genetic background between MS and metabolic-related disease; and to examine the relationship between adiponectin and risk of stroke.
Methods
This study included two meta-analyses. The primary one assessed the relationship of the ADIPOQ +45 T>G polymorphism and MS risk, and the secondary one investigated the dose-response relationship of adiponectin and risk of stroke.
Literature Searches
This study was conducted according to the meta-analysis guidelines defined in the PRISMA statement13,14. The publication search was carried out in multiple electronic databases: PubMed, EMBASE, CNKI (China National Knowledge Infrastructure), and Wanfang databases.
For the primary analysis of the ADIPOQ +45 T>G polymorphism and MS risk, the following subject terms were used to conduct the search: “adiponectin” or “ADIPOQ” or “adiponectin gene polymorphism”, “AMP1”, and “metabolic syndrome” or “MS” or “metabolic syndrome X” or “syndrome X”. The relevant articles in English and Chinese published before Dec 31, 2014, were included.
For the secondary subject of adiponectin and stroke risk, the following subject terms were used for the search: “adiponectin” and “stroke”. The relevant articles in English published before Jan 26th, 2016, were included.
We also conducted a manual search to find additional relevant data based on the references identified in the retrieved articles.
Inclusion and Exclusion Criteria
The inclusion criteria of the primary meta-analysis were as follows: the studies (1) evaluated the relationship between ADIPOQ +45T>G (rs2241766) and MS risk; (2) were case-control studies; (3) provided detailed genotype data to estimate odds ratios (ORs) and 95% confidence intervals (CIs).
The inclusion criteria of the secondary meta-analysis were as follows: the studies (1) used a prospective cohort to investigate the relationship of adiponectin and stroke risk; (2) included stroke as an endpoint; (3) had a follow-up duration of over 1 year; (4) used clear diagnostic criteria for stroke; and (5) provided data on the relative risk (RR) with 95% CI. In all identified studies, all subjects were free of stroke at baseline according to the stroke diagnostic criteria used and participated in the entire follow-up survey.
All the articles that met the above requirements were included, regardless of the sample size and the population of the studies. Unpublished conference papers were not included. For overlapping studies, the most recent publication or the largest sample size publication providing more information was selected.
Data Extraction
Two investigators (H.-P.Y. and L.S.) systematically extracted and reviewed all studies according to a standardized form and then independently entered this information into an electronic database. The following items were reviewed and extracted from each eligible study for the primary meta-analysis when possible: name of the first author, year of publication, ethnicity, country where the study conducted, mean age, total number of cases and controls, allele frequencies, genotyping method, diagnostic criteria of MS, and language of the article. Different ethnicity descents were categorized as East Asian, South Asian, and Southeast Asians.
The extracted information for the articles for the secondary meta-analysis included the name of the first author, publication year, name of the study, country, mean age, duration of follow-up, proportion of men, number of stroke cases and total participants, and adjusted variables. In addition, all of the multivariate adjusted RRs and 95% CIs based on a comparison of the highest adiponectin level category with the lowest adiponectin level category and the ORs/RRs with their 95% CIs were extracted. We abstracted the values for each category of adiponectin level, the median value of the adiponectin level for each category, the number of cases and participants/person-time for each category, and the most completely adjusted RRs with 95% CIs (odds, hazard or risk ratios were combined into RRs) for each category.
Quality assessment
A quality assessment of the primary meta-analysis was performed by two authors independently according to the Newcastle-Ottawa scale15 for non-randomized studies. The scale contains nine points: selection (4 points), comparability (2 points), and estimation of outcomes or exposures (3 points).
The qualities of the identified studies for the secondary meta-analysis were assessed by two authors independently according to a “methodological quality assessment scale” (Supplementary Table S3), which was modified from a previous publication16,17. Five items including the representativeness of the cases, source of controls, sample size, quality control of the genotyping methods, and Hardy–Weinberg equilibrium (HWE) were assessed in this scale. The quality score ranged from 0 to 10, with a high score indicating a good quality study. Any disagreements between the two investigators were resolved through discussion.
Statistical Analysis
The primary meta-analysis of the relationship between the ADIPOQ +45 T>G polymorphism and MS risk was conducted as follows. ORs and 95% CIs were calculated as estimates of the relative strength of the associations between the ADIPOQ +45 T>G polymorphism and MS risk. The pooled ORs were calculated from a weighted average of OR from each study. Due to the potential functional role of the ADIPOQ +45 T>G polymorphism, carriers with at least one risk allele will present the corresponding phenotype or traits. Moreover, to obtain all of the data included, the dominant model (GG + GT vs. TT) was adopted. Allele contrast (G vs. T) and recessive models (GG vs. GT + TT) were also performed in the meantime. After adjusting for the potential confounding factors that might have contributed to the heterogeneity, the dominant model was adopted for further investigation. Subgroup analyses were also conducted to explore the effects of the following confounding factors: language of the article, diagnostic criteria, and ethnicities.
The secondary meta-analysis of the relationship between adiponectin and risk of stroke was conducted as follows. Multivariable-adjusted RRs and 95% CIs were used to obtain the overall RR for the highest adiponectin level category compared with the lowest category using random-effects models. RRs and 95% CIs for each SD increase in log-transformed adiponectin were used to obtain the overall RR per SD in log μg/ml. The RR of each 5 μg/ml adiponectin level increment was calculated for studies that provided enough raw data to quantify the association between adiponectin level and risk of stroke using GLST (generalized least-squares trend estimation) and VWLS (variance-weighted least square regression model) for the dose-response analysis, as proposed by Greenland, Longnecker, Orsini et al. The detailed methods of the dose-response meta-analysis have been described before18.
The between-study heterogeneity was calculated using Cochran’s Q test (Mantel-Haenszel chi-squared test) and was considered significant if the P value was less than 0.119,20. Additionally, the I2 statistic was used to test for heterogeneity, with values of I2 less than 25%, 25–50%, and greater than 50% representing low, moderate and high degrees of inconsistency, respectively. When the P value was ≥0.1 or I2 ≤ 25%, a fixed effects model (Mantel–Haenszel method) was selected; otherwise, a random effects model (DerSimonian and Laird method) was selected. We then explored the sources of heterogeneity using meta-regression and stratified analysis. Sensitivity analyses were performed to evaluate the stability of the results. A leave-one-out method was used to evaluate each study, and a pooled estimate was calculated for the remaining studies. Begg’s funnel plots were conducted to evaluate the publication bias qualitatively; Begg’s test and Egger’s test were performed to quantitatively assess the publication bias. All P values were two-sided, with the statistically significant level defined as lower than 0.05. The statistical analyses were conducted using Stata (version 12.0; StataCorp LP, TX, USA).
Results
Characteristics of the Included Studies
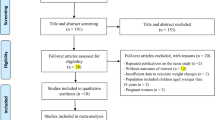
The characteristics of each study included in these two meta-analyses are summarized in Tables 1 and 2.
Supplementary Fig. S1A shows the detailed procedure by which articles are identified and assessed for inclusion in the primary meta-analysis. Table 1 illustrates the characteristics of all the included studies in this first meta-analysis. The 16 studies9,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 contained 4,113 MS cases and 3,637 healthy controls. The distributions of the genotype frequencies of the controls were all consistent with the HWE (all P > 0.05). The serum adiponectin levels presented in Table 1 shows that the concentration of adiponectin is lower in MS patients and higher in controls (P = 0.0016).
Supplementary Fig. S1B shows the detailed procedure by which articles are identified and reviewed for inclusion in the secondary meta-analysis. The 12 studies11,36,37,38,39,40,41,42,43,44,45,46 in this analysis contained 26,213 participants and 4,246 stroke cases. Seven studies11,36,37,40,41,43,44 (8 data points due to separate data for men and women) were included to compare the highest adiponectin level category with the lowest category. Two studies37,45 (3 data points due to separate data for men and women) were included to estimate the RR per SD increase in log-transformed adiponectin level. Finally, 11 studies11,36,37,38,39,40,41,42,43,44,46 (13 data points due to separate data for men and women) were included to estimate the dose-response relationship between adiponectin level and stroke.
Quality assessment
Quality assessments were performed for the included studies both in the primary and the secondary meta-analyses. All studies had acceptable qualities according to the methodological quality assessment (Table 1 and Supplementary Table S3) and the Newcastle-Ottawa scale (Supplementary Table S4).
ADIPOQ +45 T>G polymorphism and MS risk
A weak positive association was visible in the relationship between ADIPOQ +45 T>G and MS under the dominant genetic model (OR = 1.30, 95% CI = 1.03–1.65) (Fig. 1A). Results of allele contrast and recessive models were consistent with the dominant model.
(A) The relationship between ADIPOQ +45T>G and the risk of metabolic syndrome in the dominant model. The odds ratio (OR) and 95% confidence intervals (CIs) are presented graphically by a square box and horizontal line, respectively. Box sizes are proportional to inverse-variance weights. The diamond represents the overall OR with its 95%CI using a random effects model. Leu HB-1 and Leu HB-2 represent two separate populations. CHEN F-1: MS; CHEN F-2: MS with CHD. (B) The relationship between adiponectin level and the risk of stroke under a random effects model. The risk ratio (RR) and 95% CIs are presented graphically by a square box and horizontal line, respectively. Box sizes are proportional to inverse-variance weights.
Adiponectin levels and stroke risk
The combined RR of stroke was 1.11 (95% CI = 0.87–1.41, P = 0.389) for the highest adiponectin level category compared to the lowest adiponectin level category (Supplementary Fig. S2). Two studies (3 points) that provided the RRs of stroke per SD in log-transferred adiponectin level demonstrated that the overall RR was 1.20 (95% CI = 0.99–1.44, P = 0.059). Additionally, 11 studies with 13 data points that provided sufficient raw data for the dose-response analysis indicated that the RR of stroke for each 5 μg/ml adiponectin level increment was 1.05 (95% CI = 1.00–1.10, P = 0.069) (Table 3 and Fig. 1B). After excluding two papers by Prugger C et al. and Gardener H et al., the RR of stroke for each 5 μg/ml adiponectin level increment was 1.02 (95% CI = 0.99–1.05, P = 0.261; I2 = 27.8%). Therefore, there was no association between adiponectin levels and stroke risk.
Stratified Analysis and Meta-Regression
Considering the significant heterogeneity in both meta-analyses, a stratified analysis and meta-regression were performed successively to investigate the potential sources of heterogeneity.
For the primary meta-analysis, first of all, we conducted a stratified analysis based on language and diagnostic criteria of the overall data and found that they were not the sources of heterogeneity (I2 was largely unchanged, data were shown in Supplementary Fig. S3A,B). In addition, the results of the sensitivity analysis and publication bias analysis showed that the data from SHEN J et al. and CHEN F-2 et al. deviated substantially from most studies. After removal of the two studies, a weak positive association was still visible in the relationship between ADIPOQ +45 T>G and MS under the dominant genetic model (OR = 1.13, 95% CI = 1.03–1.24). Furthermore, I2 was reduced from 82.5% to 40.1%, with P = 0.050 (Supplementary Fig. S3A,B). Based on these findings, as well as similar results observed in the meta-regression (Tau-square significantly reduced), we excluded the SHEN J et al. and CHEN F-2 et al. studies from further analysis. In the subsequent stratified analysis based on language, we arrived at the conclusion that under the dominant model, a weak positive association was found in the relationship between the +45T>G polymorphism and MS susceptibility (in Chinese, OR: 1.12, 95% CI: 0.97–1.28; in English, OR: 1.14, 95% CI: 1.00–1.30) (Supplementary Fig. S3A). Further stratified analyses based on the diagnostic criteria suggested the same results (WHO: OR = 1.10, 95%CI = 0.78–1.55; IDF: OR = 1.05, 95%CI = 0.89–1.25; CDS: OR = 1.55, 95%CI = 1.22–1.97; NCEP ATP III: OR = 1.07, 95%CI = 0.92–1.23) (Supplementary Fig. S3B). As we can see from Supplementary Fig. S3A, data from the studies in English and from those that used the CDS diagnostic criteria seems to be more consistent (English: I2 = 40.8%, P = 0.134; CDS: I2 = 0.1%, P = 0.974). Finally, considering the differences in the genetic background of the population and that the risk allele frequency of +45 T>G polymorphism differed greatly among ethnicities, we further performed a subgroup analysis on East Asians. Asian Indians (South Asians) and individuals from Thailand (Southeast Asians) were excluded in this analysis. Data from East Asians leads to the same conclusion (OR = 1.17, 95%CI = 1.01–1.35, P = 0.035) (Supplementary Fig. S3C).
For the secondary meta-analysis, the stratified analysis was performed based on study design, country, mean age, sex, follow-up duration, and measure of association (data were shown in Supplementary Table S1). There was no relationship between the risk of stroke and elevated adiponectin levels and it remained for all factors we proposed (i.e., all RR values were around 1). We found that these factors were not the sources of heterogeneity (I2 was largely unchanged). The meta-regression showed that the association did not substantially differ due to the effect of these potential confounding factors (i.e., all P were >1).
Sensitivity Analysis
As shown in Supplementary Fig. S4A, one-way sensitivity analyses for the primary analysis were performed by excluding one study at a time in each genetic model. The results of the dominant genetic models indicated that the overall results were dependable. As we can see, the two papers by SHEN J et al. and CHEN F-2 et al. were far from the centre line, which might have influenced the overall tendency. For the secondary analysis, two papers by Prugger C et al. and Gardener H et al. appeared far from the centre line, which might have influenced the overall tendency (Supplementary Fig. S4C). After removing these studies, the tau-squared decreased for the primary and secondary analysis (from 0.1354 to 0.0194 for the primary analysis and from 0.0038 to 0.0011 for the secondary analysis) and explained 85.67% and 71.1% of the sources of heterogeneity, respectively.
Summary of Meta-Analysis Related to ADIPOQ +45T>G
Considering the close relationship between ADIPOQ +45 T > G and the typical components of MS as well as the many resulting potential disease risks such as obesity, cancer, and hypertension, we summarized data from the published meta-analyses as well as the primary meta-analysis in our study (Fig. 2, Supplementary Table S2). The results showed that ADIPOQ +45 T>G appeared to be a strong risk factor for MS components, obesity, non-alcoholic fatty liver disease (NAFLD), T2DM, hypertension, cancer, diabetic nephropathy (DN), cardiovascular disease (CAD), coronary heart disease (CHD) and polycystic ovary syndrome (POVS).
Test of Heterogeneity
For the primary analysis, we performed a heterogeneity test based on the dominant model in our meta-analysis and found strong heterogeneity among the included studies (I2 = 82.5%, P < 0.001) (Fig. 1A). The allele contrast and recessive genetic models also showed substantial heterogeneity (data not shown). It was thus very important to identify the sources of heterogeneity and weaken their influence.
For the secondary analysis, clear heterogeneity was observed for both the RR of the highest adiponectin level category compared with the lowest category and the RR of each 5μg/ml increment in adiponectin level (I2 = 54.6%, P = 0.009) (Fig. 1B and Supplementary Fig. S2).
Due to the high heterogeneity in the two meta-analyses, stratified analyses, meta-regression, and sensitivity analyses were conducted. Based on the results above, we found that although some studies were far from the central line in the sensitivity analyses and might have been the sources of the heterogeneity, the conclusions regarding the associations in the two meta-analyses did not change when these studies were excluded. Therefore, the results of the two meta-analyses were reliable and stable.
Publication Bias Analysis
As shown in Supplementary Fig. S4B, we found no obvious asymmetry in the dominant genetic model in the primary analysis, and this result was statistically supported by the Begg’s and Egger’s test (PBegg’s = 0.325, PEgger’s = 0.118). Additionally, the studies by SHEN J et al. and CHEN F-2 et al. were found to have deviated far from the others. For the secondary analysis, there was no obvious publication bias based on the funnel plot, Begg’s test, and Egger’s test (PBegg’s = 0.631, PEgger’s = 0.378) (Supplementary Fig. S4D).
Discussion
Adiponectin is a plasma glycoprotein of adipose tissue origin47. Adiponectin levels were reported to be lower in subjects with the MS phenotype, including obesity, T2DM, dyslipidemia, and hypertension48. Studies also confirmed that the ADIPOQ gene (+45T>G polymorphism) is associated with adiponectin levels and resulting MS9,49,50. However, the findings are still conflicting. Therefore, we conducted an updated meta-analysis to derive a more precise evaluation of the association of the ADIPOQ +45 T>G polymorphism and MS risk. Furthermore, genetic factors like the ADIPOQ +45T>G polymorphism is related to serum adiponectin levels51,52. Thus, in addition to the ADIPOQ +45T>G contribution to MS, adiponectin levels might also contribute to stroke risk, which is considered a cardiovascular manifestation of MS12,53. Due to the inconsistent conclusions regarding the association between adiponectin levels and stroke risk, 12 studies on this topic were also included in our secondary meta-analysis.
In the primary analysis, due to the strong heterogeneity, a sensitivity analysis, stratified analysis and meta-regression were conducted in the dominant genetic model. Diversity in ethnicity, diagnostic criteria, and distinction between published English and Chinese language studies might be the common source of heterogeneity54,55,56, thus we tried to clarify the sources of heterogeneity through subgroup analysis by the above three factors. The stratified analysis and meta-regression indicated that language and diagnostic criteria were not sources of heterogeneity (Tau-squared value was not decreased). Meta-analysis is often affected by the potential “outlier points” among the involved studies, which might be correlated to the data reliability, small sample size, publication bias and clinical confounding. In the current meta-analysis, both of sensitivity analysis and publication bias analysis indicated 2 articles from SHEN J et al. and CHEN F-2 et al. deviated potentially from other studies (Supplementary Fig. S4A,B). The articles from SHEN J et al. and CHEN F-2 et al. contributed inappropriate data to the overall assessment and were thus removed. After their exclusion, the heterogeneity greatly improved (I2 reduced to 40.1%) and the Tau-squared value reduced accordingly whereas this positive association still existed. Therefore, we excluded the 2 studies for the further stratified analysis and meta-regression. Taken together, our study indicated that a weak positive association was visible in the relationship between ADIPOQ +45T>G and MS in the dominant genetic model (OR = 1.30, 95% CI = 1.03–1.65).
The first meta-analysis focused on the relationship between the ADIPOQ +45T>G polymorphism and the risk of MS in the whole population and had several limitations. First, the number of subjects in the included studies was still small, and only two studies used data from outside the Chinese population; thus, more large-scale studies are needed to assess the association between ADIPOQ +45T>G and MS. Second, environmental factors such as smoking, drinking, physical activity and family income might have a substantial influence on the progression of MS, but the lack of raw data makes it impossible for us to account for gene-environment interactions. Third, our analysis only focuses on ADIPOQ +45T>G; however, the development of MS is not due to a single gene, and there should be cross-talk between ADIPOQ +45T>G and other gene polymorphisms that should be considered27.
Given the complexity of the MS and the lack of clarity surrounding its definition, most reports to date have been restricted to the examination of the relationship between variant polymorphisms and the individual criteria of MS, rather than with the syndrome as a whole. For example, individuals carrying ADIPOQ +45T>G polymorphism were found to be associated with type 2 diabetes mellitus in Chinese, however, conflicting results have been reported in Asian and European subjects57,58. To our knowledge, this prospective study is the first to date to demonstrate the findings of studies about the association of ADIPOQ +45T>G with MS in the whole population together with a stratified analysis. There was only one prior meta-analysis, conducted by GAO M et al., that showed a significant relationship between ADIPOQ +45T>G and MS that differed from ours9. There might be several potential reasons for this difference. First, GAO M et al. performed their meta-analysis specifically in the Chinese population. In the present study, we focused on studies throughout the world that were up to date, and we presented a more comprehensive conclusion rather than studying a single ethnic group. Additionally, considering the distinct genetic backgrounds in the population, we further performed a subgroup analysis on East Asians. Second, GAO M et al.’s study only included 13 studies on ADIPOQ +45T>G in the Chinese population, whereas our study assessed 16 studies including Asian Indian and Thai populations. Furthermore, data from LI YP et al. were updated to the 2010 version. The larger sample size contributed to having greater statistical power to detect associations. Third, we also conducted a stratified analysis for different languages and diagnostic criteria as well as a subgroup analysis in East Asians to derive an accurate evaluation and validated our conclusions. Finally, and most importantly, we summarized the previous meta-analysis with ours and evaluated the pivotal role of ADIPOQ +45T>G in all potential metabolic-related disease risks including MS and metabolic-related disease. A clear risk of ADIPOQ +45T>G for obesity, cancer, NAFLD, T2DM, DN, hypertension, CAD, CHD and POVS was found. These disorders usually co-exist with each other59,60. This noteworthy finding led to the possible hypothesis that ADIPOQ +45T>G might be a common genetic factor behind these diseases and could explain the molecular mechanism of comorbidity in some patients.
Therefore, there exist some shared and distinct mechanisms that need to be clarified regarding MS, metabolic-related disease and their comorbidities. The answer might be somewhat as follows. First, the populations in the existing studies on MS and metabolic-related disease were so different that the confounding factors could not be controlled properly. Second, there must be a key individual characteristic on top of general factors (ADIPOQ +45T>G) that is present in MS and metabolic-related disease. According to the forest plot of our summarized analysis, although the ADIPOQ gene might be the “common soil” of individual MS components and other potential metabolic-related diseases, its contribution to MS as a whole appeared quite differently61. A genome wide association study (GWAS) suggested plasma adiponectin did not relate to genetic loci associated with MS parameters, indicating that MS is not modulated directly by the genetically determined adiponectin29,62. In fact, previous clinical observations categorized MS into two major components: one cluster was glucose/obesity/lipids, and the other was blood pressure29. These two groups significantly contributed to the genetic background of MS, indicating the heterogeneous nature of MS and the possibly distinct pathogenesis between MS with and without each cluster. In addition, metabolic-related diseases and MS might partially overlap with each other as specific clinical sub-phenotypes with a common genetic background. This could explain the inconsistent relationship found between ADIPOQ and individual MS parameters. Third, a well-designed follow-up cohort study including patients with both MS and metabolic-related disease that considers confounding factors and assesses a larger sample size with a focus on ADIPOQ +45T>G is required in the future.
In the secondary meta-analysis, various analytical methods demonstrated that there was no association between 5 μg/ml increments in adiponectin level and stroke risk (RR = 1.05, 95% CI = 1.00–1.10, P = 0.069). The conflicting results of the studies published previously may have existed for several reasons. First, the standards of measuring adiponectin level increments were not uniform in the included prospective and nested case-control cohort studies, which suggest it would be inappropriate to combine these measures in the overall RR. In our analysis, we stratified the analysis by the different measurement standards and evaluated the RR of stroke in 3 ways, as follows: stroke risk for the highest adiponectin level category compared with the lowest category; the overall RR per SD in log μg/ml of adiponectin; and the quantified RR of each 5 μg/ml increment in adiponectin level. Therefore, the conclusions we drew from these three analyses would be more accurate and reasonable. Second, serum adiponectin levels are related to various lipoproteins63. A limitation of the secondary analysis might be the limited data on HMW-adiponectin and the lack of data on other adiponectin fractions, which could have resulted in the influence of specific molecular-weight fractions of adiponectin on risk of stroke; this influence remains unclear.
In conclusion, our analysis suggested a weak positive association of ADIPOQ +45T>G with MS as well as a strong association with metabolic-related disease. As a result, the ADIPOQ gene could be treated as a promising physiological and pharmacological target in the prevention and treatment of disease with either a single component or comorbidity. Second, our analysis suggested that an elevated adiponectin level was not a causal factor contributing to an increase in the risk of stroke. Further large-scale studies in a rigorously defined cohort should be carried out to validate our findings.
Additional Information
How to cite this article: Yuan, H.-P. et al. Association of Adiponectin Polymorphism with Metabolic Syndrome Risk and Adiponectin Level with Stroke Risk: A Meta-Analysis. Sci. Rep. 6, 31945; doi: 10.1038/srep31945 (2016).
References
Kaur, J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 943162 (2014).
Taylor, J. Y. et al. An overview of the genomics of metabolic syndrome. J Nurs Scholarsh 45(1), 52–59 (2013).
Gu, H. F. et al. Single nucleotide polymorphisms in the proximal promoter region of the adiponectin (APM1) gene are associated with type 2 diabetes in Swedish caucasians. Diabetes 53 Suppl 1, S31–S35 (2004).
Stumvoll, M. et al. Association of the T-G polymorphism in adiponectin (exon 2) with obesity and insulin sensitivity: interaction with family history of type 2 diabetes. Diabetes 51(1), 37–41 (2002).
Henneman, P. et al. Genetic architecture of plasma adiponectin overlaps with the genetics of metabolic syndrome-related traits. Diabetes Care 33(4), 908–913 (2010).
Ruan, H. & Dong, L. Q. Adiponectin signaling and function in insulin target tissues. J Mol Cell Biol. [Epub ahead of print] (2016).
Chu, H. et al. AdipoQ polymorphisms are associated with type 2 diabetes mellitus: a meta-analysis study. Diabetes Metab Res Rev 29(7), 532–545 (2013).
Melistas, L. et al. Association of the +45T>G and +276G > T polymorphisms in the adiponectin gene with insulin resistance in nondiabetic Greek women. Eur J Endocrinol 161(6), 845–852 (2009).
Gao, M. et al. Association of genetic variants in the adiponectin gene with metabolic syndrome: a case-control study and a systematic meta-analysis in the Chinese population. PLoS One 8(4), e58412 (2013).
Kanhai, D. A. et al. Adiponectin and incident coronary heart disease and stroke. A systematic review and meta-analysis of prospective studies. Obes Rev 14(7), 555–567 (2013).
Arregui, M. et al. Adiponectin and risk of stroke: prospective study and meta-analysis. Stroke 45(1), 10–17 (2014).
Hao, G. et al. Serum total adiponectin level and the risk of cardiovascular disease in general population: a meta-analysis of 17 prospective studies. Atherosclerosis 228(1), 29–35 (2013).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7), e1000097 (2009).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6(7), e1000100 (2009).
Wells, G. A., D O’Connell, B. S., Peterson, J., Welch, V., Losos, M. & Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (Accessed: 1st May 2016) (2011).
Li, K. et al. Association of two polymorphisms rs2910164 in miRNA-146a and rs3746444 in miRNA-499 with rheumatoid arthritis: a meta-analysis. Hum Immunol 75(7), 602–608 (2014).
Qiu, M. T. et al. Hsa-miR-499 rs3746444 polymorphism contributes to cancer risk: a meta-analysis of 12 studies. PLoS One 7(12), e50887 (2012).
Yuan, H. et al. Serum Uric Acid Levels and Risk of Metabolic Syndrome: A Dose-Response Meta-Analysis of Prospective Studies. J Clin Endocrinol Metab 100(11), 4198–4207 (2015).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21(11), 1539–1558 (2002).
Higgins JPT, G.S.e., Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, Available at: www.cochrane-handbook.org. (Accessed: 1st May 2016) (2011).
Wang, S. J., Jia, W. P., Bao, Y. Q. & JQ, L. Association of adiponectin gene polymorphism with metabolic syndrome. J Chinese Practical Diagnosis and Therapy 26(7), 659–661 (2012).
Yao, M. Diploma Thesis, Chinese Academy of Medical Science & Peking Union Medical College, 2004.
Liu, D. X., Hua, Q. i., Guo, J. C. & Liu, R. K. Difference of adiponectin gene exon 2 Glyl5Gly genotype and allele frequency between patients with metabolic syndrome and normal persons. Chin J Clin Rehabilitation 10(6), 4–6 (2006).
Li, Y. P. et al. Adiponectin gene SNP11377 and SNP-4522 haplotypes are related to metabolic syndrome. Basic Clin Med 30(12), 1288–1292 (2010).
Zhu, X. W. et al. Studies on adiponectin gene polymorphisms in patients with metabolic syndrome. Shandong Med J 50(29), 10–12 (2010).
Cai, Q., Liu, H. J., Chen, T. & MC, Z. Mononucleotide polymorphism at +45 and +276 site of adiponectin gene in aircrew with metabolic syndrome. Clin J Med Officers 38(5), 813–815 (2010).
Ranjith, N., Pegoraro, R. J. & Shanmugam, R. Obesity-associated genetic variants in young Asian Indians with the metabolic syndrome and myocardial infarction. Cardiovasc J Afr 22(1), 25–30 (2011).
Bu, R. F. et al. The association of adiponectin gene polymorphism and serum adiponectin level with metabolic syndrome in older. Chin J Gerontology 31, 4112–4114 (2011).
Leu, H. B. et al. Adiponectin gene polymorphism is selectively associated with the concomitant presence of metabolic syndrome and essential hypertension. PLoS One 6(5), e19999 (2011).
Huang, F. Z., Liu, J., Pang, D. & PL, B. The association of adiponectin gene SNP +276 and SNP +45 with metabolic syndrome. Chinese J of Endocrinology and Metabolism 28(11), 911–913 (2012).
Li, X. T. et al. Association of the adiponectin gene (ADIPOQ) +45 T. G polymorphism with the metabolic syndrome among Han Chinese in Sichuan province of China. Asia Pac J Clin Nutr 21(2), 296–301 (2012).
Chen, F. et al. Study on the correlation of adiponectin gene polymorphism and coronary heart disease in the patients with metabolic syndrome in Han nationlity population in Ningxia. Clin J Crit Care Med 32(1), 24–29 (2012).
Shen, J. et al. Relation of metabolic syndrome with plasma adiponectin level and gene polymorphism in Ningxia Hui subjects. Chin J Geriatr Heart Brain Vessel Dis 15(1), 7–10 (2013).
Xu, J. et al. Association of adiponectin gene polymorphrism with metabolic syndrome in older Han adults from major cities in China. Journal of Hygiene Research 42(3), 353–359 (2013).
Suriyaprom, K., Phonrat, B. & Tungtrongchitr, R. Association of adiponectin gene -11377C > G polymorphism with adiponectin levels and the metabolic syndrome in Thais. Asia Pac J Clin Nutr 23(1), 167–173 (2014).
Soderberg, S. et al. Leptin, but not adiponectin, predicts stroke in males. J Intern Med 256(2), 128–136 (2004).
Matsumoto, M., Ishikawa, S. & Kajii, E. Association of adiponectin with cerebrovascular disease: a nested case-control study. Stroke 39(2), 323–328 (2008).
Stott, D. J. et al. Adipocytokines and risk of stroke in older people: a nested case-control study. Int J Epidemiol 38(1), 253–261 (2009).
Ogorodnikova, A. D. et al. High-molecular-weight adiponectin and incident ischemic stroke in postmenopausal women: a Women’s Health Initiative Study. Stroke 41(7), 1376–1381 (2010).
Khalili, P. et al. Total adiponectin does not predict cardiovascular events in middle-aged men in a prospective, long-term follow-up study. Diabetes Metab 36(2), 137–143 (2010).
Rajpathak, S. N. et al. Resistin, but not adiponectin and leptin, is associated with the risk of ischemic stroke among postmenopausal women: results from the Women’s Health Initiative. Stroke 42(7), 1813–1820 (2011).
Prugger, C. et al. Adipocytokines and the risk of ischemic stroke: the PRIME Study. Ann Neurol 71(4), 478–486 (2012).
Wannamethee, S. G. et al. Adiposity, adipokines, and risk of incident stroke in older men. Stroke 44(1), 3–8 (2013).
Gardener, H. et al. Adiponectin and risk of vascular events in the Northern Manhattan study. Atherosclerosis 226(2), 483–489 (2013).
Bidulescu, A. et al. Associations of adiponectin and leptin with incident coronary heart disease and ischemic stroke in african americans: the jackson heart study. Front Public Health 1, 16 (2013).
Kizer, J. R. et al. Total and high-molecular-weight adiponectin and risk of coronary heart disease and ischemic stroke in older adults. J Clin Endocrinol Metab 98(1), 255–263 (2013).
Scherer, P. E. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 55(6), 1537–1545 (2006).
Santaniemi, M., Kesaniemi, Y. A. & Ukkola, O. Low plasma adiponectin concentration is an indicator of the metabolic syndrome. Eur J Endocrinol 155(5), 745–750 (2006).
Vasseur, F. et al. Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the APM1 gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians. Hum Mol Genet 11(21), 2607–2614 (2002).
Prakash, J., Mittal, B., Awasthi, S. & Srivastava, N., Association of adiponectin gene polymorphism with adiponectin levels and risk for insulin resistance syndrome. Int J Prev Med 6, 31 (2015).
Guzman-Ornelas, M. O. et al. Association of ADIPOQ +45T >G polymorphism with body fat mass and blood levels of soluble adiponectin and inflammation markers in a Mexican-Mestizo population. Diabetes Metab Syndr Obes 5, 369–378 (2012).
Wu, J., Liu, Z., Meng, K. & Zhang, L. Association of adiponectin gene (ADIPOQ) rs2241766 polymorphism with obesity in adults: a meta-analysis. PLoS One 9(4), e95270 (2014).
BC, Z., WJ, L., WL, C. & YW, X. Serum total adiponectin level and risk of cardiovascular disease in Han Chinese populations: a meta-analysis of 17 case-control studies. Clin Endocrinol (Oxf) 77(3), 370–378 (2012).
Thompson, S. G. Why sources of heterogeneity in meta-analysis should be investigated. BMJ 309(6965), 1351–1355 (1994).
Munafo, M. R. & Flint, J. Meta-analysis of genetic association studies. Trends Genet 20(9), 439–444 (2004).
Fan, Y. et al. Association between ADIPOQ +45T>G polymorphism and type 2 diabetes: a systematic review and meta-analysis. Int J Mol Sci 16(1), 704–723 (2015).
Vimaleswaran, K. S. et al. A novel association of a polymorphism in the first intron of adiponectin gene with type 2 diabetes, obesity and hypoadiponectinemia in Asian Indians. Hum Genet 123(6), 599–605 (2008).
Fan, Y. et al. Association between ADIPOQ +45T>G polymorphism and type 2 diabetes: a systematic review and meta-analysis. Int J Mol Sci 16(1), 704–723 (2014).
Valderas, J. M. et al. Defining comorbidity: implications for understanding health and health services. Ann Fam Med 7(4), 357–363 (2009).
Jakovljevic, M. & Ostojic, L. Comorbidity and multimorbidity in medicine today: challenges and opportunities for bringing separated branches of medicine closer to each other. Psychiatr Danub 25 Suppl 1, 18–28 (2013).
Stern, M. P. Diabetes and cardiovascular disease. The “common soil” hypothesis. Diabetes 44(4), 369–374 (1995).
Heid, I. M. et al. Clear detection of ADIPOQ locus as the major gene for plasma adiponectin: results of genome-wide association analyses including 4659 European individuals. Atherosclerosis 208(2), 412–420 (2010).
Cnop, M. et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 46(4), 459–469 (2003).
Acknowledgements
We would like to thank the authors of the original studies included in this meta-analysis. This work was supported by the Natural Science Foundation of China (81400790, 81061120527, 81370445, 81472408, 81571385), Beijing Nova program (Z121107002512058), National Department Public Benefit Research Foundation by Ministry of Health P. R. China (201302008), 12th 5 Year National Program from the Ministry of Scientific Technology (2012BAI10B01).
Author information
Authors and Affiliations
Contributions
H.-P.Y. and Z.Y. designed this study; H.-P.Y. and L.S. searched databases and collected full-text papers; H.-P.Y., X.-H.L. and F.-G.C. extracted and analyzed data; H.-P.Y., L.S., X.-Q.Z., F.Y., J.H. and C.-Y.J. wrote the manuscript; all authors reviewed this manuscript and approved the final draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yuan, HP., Sun, L., Li, XH. et al. Association of Adiponectin Polymorphism with Metabolic Syndrome Risk and Adiponectin Level with Stroke Risk: A Meta-Analysis. Sci Rep 6, 31945 (2016). https://doi.org/10.1038/srep31945
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep31945
This article is cited by
-
ADIPOQ gene (T45G and G276T) single nucleotide polymorphisms and their association with gestational diabetes mellitus in a Filipino population
BMC Endocrine Disorders (2023)
-
The association between serum adiponectin and 3-month outcome after ischemic stroke
Cardiovascular Diabetology (2019)
-
Allele-specific methylation contributed by CpG-SNP is associated with regulation of ALOX5AP gene expression in ischemic stroke
Neurological Sciences (2018)
-
The SG13S114 polymorphism of the ALOX5AP gene is associated with ischemic stroke in Europeans: a meta-analysis of 8062 subjects
Neurological Sciences (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.