Abstract
Betatrophin/angiopoietin-like protein 8 (ANGPTL8) is a liver-secreted protein recently identified as a potent stimulator of beta cell proliferation in mice. However, it is unclear how betatrophin is regulated in humans with non-alcoholic fatty liver disease (NAFLD). We investigated the role of betatrophin in mice and in humans with and without NAFLD. Serum betatrophin levels were examined by ELISA in 164 subjects, including 96 patients with NAFLD. Levels were significantly elevated in subjects with NAFLD compared with controls (1.301 ± 0.617 vs. 0.900 ± 0.574 μg/L, P < 0.001), even after stratification by diabetic or obesity status. Circulating betatrophin positively correlated with obesity or glycemic indices, liver enzyme profiles, and NAFLD status, and was confirmed by multivariate regression analyses (β = 0.195, P = 0.040). However, when including insulin resistance index in the model, the significant association between betatrophin level and NAFLD was diminished due to a mediation effect of insulin resistance on this relationship. Palmitate or tunicamycin increased betatrophin expression in HepG2 cells, while a chemical chaperone blocked its induction. Hepatic expression of betatrophin was elevated in mice with NAFLD including db/db or ob/ob mice and mice with a high-fat or methionine-choline deficient diet. In conclusion, circulating betatrophin was increased in mice and humans with NAFLD and its expression was induced by endoplasmic reticulum stress in hepatocytes (Clinical trial no. NCT02285218).
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is emerging as a worldwide public health issue because the prevalence of obesity is increasing, populations are aging, and sedentary lifestyles prevail1. NAFLD is defined histologically as the accumulation of ectopic liver fat (mainly triglycerides) in more than 5% of hepatocytes with no evidence of significant alcohol intake or other secondary etiology of liver disease. It is implicated in an increased risk of mortality2 as well as serious comorbidities like diabetes and cardiovascular diseases3,4,5. Among subgroups of NAFLD, non-alcoholic steatohepatitis (NASH) is a progressive and serious condition which can further develop into cirrhosis or hepatocellular carcinoma6,7. Compared to simple steatosis, NASH has greater risks of liver-related or cardiometabolic complications5. Despite its high prevalence and consequences, NAFLD screening and detection primarily depends on expensive imaging devices, including ultrasonography, or blood assays of liver enzyme profiles, which are often inaccurate8. This conundrum demonstrates a critical need for reliable biomarkers to identify NAFLD.
Recently betatrophin, also termed angiopoietin-like protein 8 (ANGPTL8), lipasin, or Re-feeding Induced in Fat and Liver (RIFL), has been introduced as a novel protein that is secreted primarily from fat and the liver in mice and is likely to modulate glucose homeostasis and lipid metabolism9. Betatrophin knockout mice have decreased serum levels of triglycerides10,11, whereas betatrophin overexpression has been shown to markedly elevate serum triglycerides12,13. These findings are consistent with previous evidence of the inhibitory function of betatrophin on lipoprotein lipase activity10. Yi et al. showed that mice with severe insulin resistance have a dramatically increased level of betatrophin, which could induce pancreatic β-cell proliferation and lower blood glucose levels14.
Contrary to several animal studies showing that liver and brown/white fat are major betatrophin-producing tissues12,13,15, in humans betatrophin is highly expressed mainly in the liver12, suggesting a species-specific expression pattern. Recent human studies reported that serum betatrophin levels are increased in subjects with type 1 diabetes (T1D)16, obesity17, and type 2 diabetes (T2D)17,18,19, However, there is no report of the role of betatrophin in patients with fatty liver. Considering that obesity, T2D, and NAFLD share insulin resistance as a common pathophysiologic mechanism20 and that the dominant expression of betatrophin is in human liver12, we hypothesized that circulating betatrophin levels might be elevated in subjects with NAFLD.
Therefore, the present study investigates the circulating levels of betatrophin and the clinical parameters associated with betatrophin levels in subjects from a human cohort with or without NAFLD, which is further elucidated using in vivo and in vitro models.
Results
Baseline characteristics of the study population
Baseline characteristics of study participants according to NAFLD status are shown in Table 1. Among a total of 164 subjects (30 normal subjects, 25 subjects with impaired fasting glucose [IFG], and 109 subjects with T2D), 134 age- and sex-matched patients were examined by imaging devices to assess hepatic steatosis, which resulted in 96 (72%) of subjects classified as having NAFLD. Subjects with NAFLD had a significantly higher body mass index (BMI) and serum levels of fasting glucose, glycosylated hemoglobin (HbA1c), alanine aminotransferase (ALT), aspartate transaminase (AST), triglyceride (TG), fasting insulin, and homeostasis model assessment of insulin resistance (HOMA-IR), compared to those without NAFLD. A higher number of patients with T2D were included in the NAFLD group; there was no significant difference in other parameters, such as age, blood pressure, total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C), between the two groups.
Elevated betatrophin levels in human subjects with non-alcoholic fatty liver disease
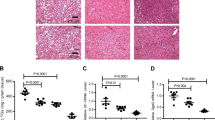
Serum betatrophin levels were significantly elevated in subjects with NAFLD compared with controls (1.301 ± 0.617 vs. 0.900 ± 0.574 μg/L, P < 0.001, Fig. 1A). Obese and overweight individuals also showed increased levels of betatrophin (1.271 ± 0.608 vs. 1.234 ± 0.686 vs. 0.828 ± 0.356 μg/L, P < 0.001, Fig. 1B). Furthermore, non-obese subjects with NAFLD had higher serum betatrophin concentrations (1.188 ± 0.467 vs. 0.849 ± 0.467 μg/L, P = 0.030, Fig. 1C), whereas obese subjects with NAFLD showed a marginal increase in betatrophin levels (1.367 ± 0.579 vs. 0.979 ± 0.799 μg/L, P = 0.061). Similar to recent reports showing that betatrophin levels were increased in subjects with T2D or insulin resistance17,18,19, serum betatrophin levels were mildly elevated, with no statistically significant difference between subjects with IFG or T2D compared to normal subjects (P = 0.065, Fig. 1D). After stratification by glycemic status, we demonstrated that serum betatrophin levels were significantly elevated in NAFLD subjects regardless of the presence of T2D (no T2D group: 1.197 ± 0.638 vs. 0.797 ± 0.506 μg/L, P = 0.041; T2D group: 1.328 ± 0.613 vs. 0.992 ± 0.627, P = 0.032; Fig. 1E).
(A) Serum betatrophin levels were elevated in NAFLD patients. (B) Serum betatrophin levels are increased in subjects who were overweight and obese. (C) Box-and-whisker plots of betatrophin levels in subjects with and without NAFLD, stratified by their obesity status. Box indicates first and third quartiles and median values with whiskers from minimum to maximum. (D) Comparison of circulating betatrophin levels among normal, IFG, and diabetic subjects. Data are expressed as mean ± SD. (E) Box-and-whisker plots of betatrophin levels in subjects with and without NAFLD, stratified by their glycemic condition. Box indicates first and third quartiles and median values with whiskers from minimum to maximum. *P < 0.05; **P < 0.005 and ***P < 0.001.
Association of betatrophin with clinical parameters
Next, we evaluated the association between serum betatrophin levels and various clinical and biochemical profiles (Table 2). In the Spearman correlation analysis, betatrophin levels had strong positive correlations with HbA1c (r = 0.439), NAFLD status (r = 0.330), ALT (r = 0.326), HOMA-IR (r = 0.320), and fasting glucose (r = 0.310), respectively. BMI (r = 0.281), fasting insulin (r = 0.255), AST (r = 0.226), and TG levels (r = 0.241) were also significantly related to betatrophin levels.
To adjust for age, sex and other covariates, which were significantly associated with betatrophin levels by Spearman correlation, multivariate regression analyses were conducted (Table 2). In model 1, in which AST and ALT levels were excluded, betatrophin levels were independently associated with HbA1c (STD β = 0.410, P = 0.008) and NAFLD (STD β = 0.204, P = 0.028). In model 2, after adding the liver enzyme profiles, these associations were not significantly altered. However, in model 3, which included HOMA-IR (fasting glucose was removed from the model due to collinearity), HbA1c remained statistically significantly associated with betatrophin levels (STD β = 0.300, P = 0.005), whereas the association between NAFLD and betatrophin levels was diminished in this model. This finding from model 3 can be explained by the mediation effect of HOMA-IR on the relationship between betatrophin and NAFLD (average causal mediation effect = 0.074, 95% confidence interval = 0.016–0.148, P = 0.040).
Figure 2 presents the receiver operating characteristic (ROC) curve of serum betatrophin levels to determine the utility of this biomarker for predicting NAFLD. The area under the ROC curve (AUROC) was 0.711 (95% confidence interval =0.613–0.810, P < 0.001). Use of betatrophin cutoff of 1.051 μg/L was associated with the highest value to predict NAFLD with regard to the following: the Youden Index 39; sensitivity 65.6%; specificity 73.7%.
Tunicamycin-induced ER stress increased the expression of betatrophin
Endoplasmic reticulum (ER) stress plays a primary role in NAFLD development and progression to non-alcoholic steatohepatitis21. Therefore, tunicamycin, an ER stress inducer, was used to investigate whether betatrophin expression is regulated by ER stress. Tunicamycin treatment increased betatrophin mRNA levels in HepG2 cells in both a dose- and time-dependent manner (Fig. 3A,B). Elevated betatrophin protein expression was also confirmed with immunoblots (Fig. 3C,D). Tunicamycin significantly activated ER stress markers such as eIF2α and ATF6, in HepG2 cells.
(A,B) Tunicamycin increased betatrophin mRNA expression in HepG2 cells in a dose- (A) and time (B)-dependent manner. (C,D) Tunicamycin increased betatrophin and ER stress marker protein expression in HepG2 cells in a dose- (C) and time- (D) dependent manner. Cells were treated with various concentrations of tunicamycin for 12 h prior to quantitative real-time PCR and 24 h prior to immunoblots. For the time-dependent experiment, 1 μg/ml of tunicamycin was added. Data are expressed as mean ± SD obtained from at least six individual experiments. *P < 0.05; **P < 0.01; ***P < 0.001 compared with controls.
Inhibition of ER stress suppressed tunicamycin or palmitate-induced expression of betatrophin
To further confirm the role of ER stress in the expression of betatrophin, a chemical chaperone, 4-phenylbutyric acid (4-PBA), was used as an ER stress inhibitor. Tunicamycin-induced mRNA expression of betatrophin (Fig. 4A) was significantly attenuated by 4-PBA (3 mM), indicating involvement of ER stress in betatrophin expression regulation. Next we investigated whether the hepatic betatrophin expression is induced by palmitate, which aggravates NAFLD and is one of the most abundant saturated free fatty acids in plasma. Similar to tunicamycin, palmitate elevated betatrophin mRNA levels in HepG2 cells (Fig. 4B), which were also attenuated by pretreatment with 4-PBA. We confirmed that 4-PBA effectively inhibited tunicamycin- or palmitate-induced expression of ER stress markers such as eIF2α and ATF6 as well as betatrophin protein in HepG2 cells (Fig. 4C). To evaluate whether immune stress signaling is involved in betatrophin expression, HepG2 cells were treated with lipopolysaccharides (LPS) to stimulate toll-like receptors (TLRs). LPS alone did significantly increase the expression of betatrophin, while combined treatment with LPS and palmitate did not additionally increase betatrophin mRNA in HepG2 cells (Fig. 4D). Immunoblots confirmed that betatrophin and NF-κB were induced by treatment with LPS.
(A,B) Tunicamycin (A) and palmitate (B) increased betatrophin mRNA expression in HepG2 cells in a dose-dependent manner and its effect was suppressed by 4-PBA pretreatment. (C) Tunicamycin and palmitate induced betatrophin and ER stress marker protein expression in HepG2 cells. Pretreatment with 3 mM PBA significantly decreased betatrophin expression. (D) Betatrophin mRNA expression in HepG2 cells was increased with LPS treatment, but was not additionally induced by combined treatment with palmitate and LPS. Immunoblots revealed that betatrophin and NF-κB were induced by LPS treatment. Cell lysates were harvested after 12 or 24 h of treatment for betatrophin detection using quantitative real-time PCR or immunoblots, respectively. Data are expressed as mean ± SD obtained from at least six individual experiments. *P < 0.05 and **P < 0.01 compared with controls.
Betatrophin expression is increased in various animal models with fatty liver
To investigate whether betatrophin expression is increased in mice with fatty liver in vivo, we used db/db, ob/ob mice, and mice treated with an high fat diet (HFD) or methionine-choline deficient (MCD) diet. Changes in body weight and serum glucose levels were elevated in db/db, ob/ob, and HFD-induced obese mice, but were significantly decreased in MCD diet-fed mice compared with control C57BL6J mice (Fig. 5A,B). Hepatic TG contents were remarkably increased in db/db, ob/ob, HFD, and MCD diet-fed mice compared with controls (Fig. 5C). Hepatic lipid droplets were accumulated in these mouse models (Fig. 5D). The hepatic expression of betatrophin mRNA (Fig. 5E) and protein (Fig. 5F) was significantly elevated in db/db, ob/ob, HFD, or MCD diet-fed mice compared with controls. Increased liver levels of ER stress markers were also confirmed in these mice (Fig. 5F). Furthermore, expression of inflammation and fibrosis-related genes was also increased in these models (Fig. 5G). Notably, the strongest expression of TNFα, type Iα collagen (COL1A1), and TGFβ in MCD mice correlated with the highest expression of betatrophin.
(A,B) Changes in body weights (A) and serum glucose levels (B) in control, db/db and ob/ob mice, and mice fed with high-fat diet (HFD) and methionine-choline-deficient (MCD) diet. The x axis represents 2, 4, 6, and 8 weeks for the HFD group. Black circles = control mice; black triangles = db/db mice; white circles = ob/ob mice; black squares = mice fed with HFD; white diamonds = mice fed with MCD. (C,D) Hepatic TG contents (C) and liver histology ((D,H,E) stain) in control, high-fat diet (HFD)-fed, db/db, ob/ob, and MCD-fed mice. Magnification, 200×. (E) The hepatic betatrophin mRNA expression in the various animal models. (F) Hepatic betatrophin and ER stress marker protein expression in various animal models. (G) mRNA expression of inflammation and fibrosis-related genes in various animal models. Data are expressed as mean ± SD (A,B,C) or SEM (E,G) obtained from at least six individual experiments. *P < 0.05; **P < 0.01; ***P < 0.001 compared with control mice.
Tunicamycin-treated mice were used to confirm the role of ER stress in the regulation of betatrophin expression in vivo. Tunicamycin markedly increased hepatic expression levels of betatrophin as well as ER stress markers as compared to the control group (Fig. 6A,B). Pre-treatment with ER stress inhibitor, 4-PBA significantly decreased betatrophin expression in the liver (Fig. 6C).
Liver lysates were harvested after 24 h of treatment with tunicamycin for protein or mRNA detection using immunoblots (A,B) or quantitative real-time PCR (C), respectively. (B) Densitometric graph of the optical density-based data of the immunoblots. Data are expressed as mean ± SD. **P < 0.01 and ***P < 0.001.
Discussion
The present study demonstrated for the first time, that serum betatrophin levels were significantly elevated in patients with NAFLD compared with controls. This association was maintained even after controlling for diabetic status. In addition, serum betatrophin concentration was positively correlated with glycemic and liver-related laboratory parameters as well as TG or HOMA-IR levels. Furthermore, the human study results were supported by animal experiments in vivo. These experiments showed that various fatty liver mouse models had markedly increased hepatic betatrophin expression. Both pharmacologically- and nutritionally-activated ER stress and TLR signaling induced betatrophin expression in hepatocytes in vitro.
Betatrophin/ANGPTL8 is a recently identified, novel protein secreted from hepatic or adipose tissues. It has been proposed to play a dual role in TG metabolism and pancreatic β-cell proliferation by independent research groups9,12,13,22. Quagliarini et al. suggested that ANGPTL8 regulates postprandial TG and fatty acid levels by inhibiting the lipoprotein lipase through interaction with ANGPTL322. A mouse model of severe insulin resistance showed a markedly increased betatrophin level, which acted as a potent mitogen for insulin-producing β-cells14. However, a subsequent study reported contradictory results, demonstrating no impairment in β-cell expansion in betatrophin knockout mice23. These conflicting results raised controversy regarding the function of betatrophin in relation to glucose metabolism. In addition, Wang et al. clearly showed that betatrophin knockout mice manifested lower levels of serum TG with reduced fat mass, but no alteration in glucose homeostasis10, indicating that betatrophin may be required for lipid metabolism regulation. A recent study proposed that betatrophin modulates the autophagic process, which leads to regulation of hepatic lipid turnover24.
With regard to the tissue expression pattern of betatrophin, betatrophin mRNA was mainly expressed in the liver and in brown and white adipose tissues in mice12,13,14,15. However, in humans it is predominantly enriched in the liver12, suggesting that betatrophin might serve as a hepatokine for which the function remains to be fully established. Based on this finding, we focused on betatrophin expression regulation in a human hepatocyte cell line. To date, there is no report regarding betatrophin expression distribution in other human tissues.
With regard to betatrophin expression regulation, nutritional status (fasting and feeding) can effectively modulate mRNA levels in mice livers13 as well as in brown and white fat tissues12,15. In 3T3-L1 adipocytes, betatrophin expression was markedly increased during adipogenesis and was induced by insulin treatment13. However, tumor necrosis factor α or peroxisome proliferator-activated receptor γ knockdown significantly suppressed the betatrophin expression in 3T3-L1 adipocytes13. The present study showed that hepatic betatrophin expression was significantly elevated in mouse models with fatty liver, such as db/db, ob/ob, and HFD or MCD diet-fed mice. Consistent with our findings, both epididymal fat and liver showed increased betatrophin mRNA levels in ob/ob mice13, In addition, hepatic betatrophin expression was upregulated in sterol regulatory element-binding protein (SREBP)-1a or SREBP-2 transgenic mice and mice treated with a liver X receptor agonist22, all of which displayed hepatic steatosis25,26. The association between betatrophin and NAFLD is also supported by the recent study showing that hepatic lipid droplets were surrounded by intracellular betatrophin in hepatocytes24.
Notably, our study demonstrated that palmitate- or tunicamycin-mediated ER stress induced betatrophin expression in hepatocytes. It is well established that hyperlipidemia or lipotoxicity can activate ER stress, which then contributes to the development of insulin resistance, T2D, and NAFLD27,28. Our result may explain the underlying mechanism for increased betatrophin levels in obese or T2D subjects17,18,19,29 who are likely to have activated ER stress. Among the three essential pathways for unfolded protein response induced by ER stress, PERK-eIF2α-activating transcription factor 4 (ATF4) signaling regulates lipogenesis and hepatic steatosis by activating lipogenic genes, such as SREBP-1c27. We also confirmed that palmitate- or tunicamycin-treated HepG2 cells and the livers of various NAFLD mouse models had increased p-eIF2α and ATG6 levels, indicating the activation of ER stress. In addition, TLR signaling was involved in the induction of betatrophin expression in hepatocytes.
To date, all human studies have focused on investigating the relationship between serum betatrophin levels and diabetes, obesity, or dyslipidemia. An additional novel finding of this study is that we observed significantly increased betatrophin concentrations in subjects with NAFLD independent of diabetic status. However, this significant association between NAFLD and betatrophin levels was diminished when HOMA-IR was added in the final regression model, implying that insulin resistance was involved as a mediator in this association. Higher serum betatrophin levels were found in subjects with T1D16 and T2D17,18,19,30,31 whereas conflicting results were also reported in T2D32. Consistent with our data, several studies also reported that betatrophin levels were positively correlated to BMI17,29,31, fasting glucose17,19,31, HbA1c18,19, and estimated glomerular filtration rate (eGFR)31, and were negatively correlated to age31. Some studies showed a correlation between betatrophin concentration and TC33, LDL-C33, HDL-C32, and TG levels32. These discrepancies in the association of betatrophin levels with metabolic parameters in human studies may be explained by differences in patient populations. Further studies are required to elucidate this issue.
The current study has several strengths. Foremost, this translational research demonstrated that betatrophin levels were significantly increased in patients with NAFLD and in various fatty liver mouse models, implying that betatrophin may be a novel biomarker for NAFLD, similar to fetuin-A34 or selenoprotein P35. Additionally, we exposed one of the regulatory mechanisms involved in betatrophin expression in hepatocytes. Our study also has limitations, which can be addressed with further research. First, we measured serum betatrophin levels in a rather small number of subjects. Despite the relatively small sample size, the difference in betatrophin levels in the presence of NAFLD was significant, indicating a prominent association. Second, the cross-sectional study design does not permit unequivocal conclusions regarding causal relationships between betatrophin and NAFLD. Additional studies with genetically engineered mouse models, such as betatrophin knockout or betatrophin-expressing transgenic animals, should be conducted to elucidate the putative role of betatrophin in NAFLD. Tissue-level betatrophin expression and its relationship with hepatic lipid contents were not confirmed in the human liver specimens due to a lack of tissue samples. Finally, despite the clinical importance of discriminating NASH from NAFLD, we were unable to assess betatrophin’s ability to detect NASH as we lacked data regarding liver biopsies.
In conclusion, regardless of diabetic and obesity status, NAFLD subjects had significantly elevated circulating levels of betatrophin. The expression of betatrophin was highly induced by palmitate- or chemical-mediated ER stress in hepatocytes as well as in various fatty liver mouse models. This result indicates that betatrophin may be a candidate for use as a novel biomarker for NAFLD detection. Further research is warranted to investigate the functional role of betatrophin in NAFLD development and its mechanism of regulation.
Methods
Human subjects
We analyzed baseline clinical data and serum samples from subjects with NAFLD and T2D in the Severance metabOlic syndrome & NAFLD cohort (SONA cohort), an ongoing prospective cohort study supported by the Korea Health Industry Development Institute (KHIDI), for the cross-sectional study on betatrophin. A total of 164 subjects (30 normal glucose tolerant control subjects, 25 patients with IFG, and 109 patients with T2D) were recruited by the Diabetes Center at the tertiary-level, university-affiliated Severance Hospital, Yonsei University College of Medicine, from April 2014 to June 2015. Among them, 134 age- and sex-matched subjects were screened for having fatty liver by either abdominal ultrasound (n = 117, 87%) or computed tomography (CT, n = 17, 13%). Abdominal ultrasonography was performed with a 3.5-MHz transducer by trained radiologists who were blinded to the patients’ clinical and laboratory data, and fatty liver was defined based on standard criteria36. The liver attenuation index, derived from the difference between mean hepatic and splenic attenuation assessed from non-contrast CT images, was calculated for the diagnosis of fatty liver37. Control subjects were recruited after proper laboratory examination from individuals who visited the clinic for evaluating their health status due to their concern of having diabetes or other benign endocrinologic disorders such as benign thyroid nodules and osteopenia. The diagnoses of IFG and T2D were determined based on the American Diabetes Association guidelines38. Subjects who met the following criteria were excluded based on our protocol: (1) normal glucose tolerant subjects who took medications known to affect glucose or lipid metabolism; (2) types of diabetes other than T2D; (3) alcohol consumption >140 g/week for men and 70 g/week for women; (4) positive serologic markers for hepatitis B or hepatitis C virus; (5) presence of liver cirrhosis; and (6) (eGFR) <60 mL/min per 1.73 m2. Written informed consent was secured from all of the participants, and the protocol of this study was approved by the Institutional Review Board at Severance Hospital. All experiments were performed in accordance with relevant guidelines and regulations.
Clinical and laboratory measurements
Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters. Alcohol consumption was examined by a detailed interview and analyzed as previously described39. Following overnight fasting >8 h, blood samples were drawn from the antecubital vein, serum was separated from blood specimens within 60 min of acquisition, and serum was stored at −70 °C until analysis at a later date. Serum levels of fasting and postprandial glucose, TC, TG, HDL-C, AST, and ALT were measured in a certified laboratory by standard methods. HbA1c was measured by high-performance liquid chromatography with a Variant II Turbo chromatograph (Bio-Rad Laboratories, Hercules, CA). Fasting serum insulin levels were determined by immunoradiometric assay (Beckman Coulter, Fullerton, CA). LDL-C was calculated using the Friedewald formula: LDL-C (mg/dL) = TC (mg/dL)−[HDL-C (mg/dL) + TG (mg/dL) /5]. The HOMA-IR40 was calculated as previously described.
Serum betatrophin levels were measured in duplicate using a commercially available human ELISA kit (Phoenix Pharmaceuticals, Burlingame, CA) according to the manufacturers’ instructions, as previously described in a detailed published report17,31. The sensitivity of the betatrophin ELISA was 0.01 μg/L, and inter- and intra-assay coefficients of variation were <15% and <10%, respectively. Immunoblotting was used to confirm whether the antibody used in the ELISA kit precisely detected betatrophin at the expected size of ~22.5 kDa from human serum samples and betatrophin protein.
Cell culture and materials
HepG2 cells were maintained in high glucose Dulbecco’s modified Eagle’s medium (GE Healthcare Hyclone, Sungnam, Korea), supplemented with 10% fetal bovine serum, 100 U penicillin, and 100 μg streptomycin at 37 °C in a humidified incubator containing 5% CO2 and 95% air. Cells were treated with various concentrations of tunicamycin (ranging from 0.1 to 3 μg/ml; #654380, Calbiochem, La Jolla, CA) for 12 h prior to performing quantitative real-time polymerase chain reaction (PCR) and for 24 h prior to performing immunoblots. For the time-dependent experiment (from 0 to 24 h), 1 μg/ml of tunicamycin was added to the HepG2 cells. Palmitate (cat#P9767, Sigma-Aldrich, St. Louis, MO) was used after conjugation with bovine serum albumin (5%) and 4-PBA, 3 mM, chemical chaperone, cat#SML0309, Sigma-Aldrich) was pretreated for 1 h prior to adding either tunicamycin or palmitate. LPS (cat#L6529, Sigma-Aldrich) was used as indicated.
RNA isolation and real-time PCR
Total RNA was extracted using TRIzol® reagent (Invitrogen) according to the manufacturer’s instructions and subjected to reverse transcription using the High Capacity cDNA Transcription kit (Applied Biosystems, Foster City, CA) followed by quantitative real-time PCR using an ABI 7500 sequence detection system (Applied Biosystems). PCR was performed using the following primers (for SYBR Green): human betatrophin, forward (5′-gag act cag atg gag gag ga-3′) and reverse (5′-atg ctg ctg tgc cac cat ct-3′); mouse betatrophin, forward (5′-gct tta cac ctt cga gct ga-3′) and reverse (5′-atc cag gta gtc tca ggc tg-3′); TNFα, forward (5′-ctg tag ccc acg tcg tag c-3′) and reverse (5′-ttg aga tcc atg ccg ttg-3′); COL1A1, forward (5′-cct ggt aaa gat ggt gcc-3′) and reverse (5′-cac cag gtt cac ctt tcg cac c-3′); TGFβ, forward (5′-tga cgt cac tgg agt tgt acg g-3′) and reverse (5′-ggt tca tgt cat gga tgg tgc-3′); GAPDH, forward (5′-aac ttt ggc att gtg gaa gg-3′) and reverse (5′-tgt tcc tac ccc caa tgt gt-3′); β-actin, forward (5′-gga ctt cga gca aga gat gg-3′) and reverse (5′-agc act gtg ttg gcg tac ag-3′). Quantitative analyses were conducted using the ΔΔcycle threshold method and StepOne Software version 2.2.2.
Protein extraction and immunoblotting
Mouse livers and HepG2 cells were lysed in RIPA buffer (Cell Signaling Technology, Danvers, MA), and the protein extract was measured using the Bradford assay (Bio-Rad, 162-0115, Hercules, CA). Equal amounts of proteins (50 μg) were heat-denatured in 4× sample buffer (2% sodium dodecyl sulfate, 62.5 mM Tris (pH 6.8), 0.01% bromophenol blue, 1.43 mM mercaptoethanol, and 0.1% glycerol), separated on 10% or 12% sodium dodecyl sulfate-polyacrylamide gels, and electroblotted onto a nitrocellulose membrane (Bio-Rad). Membranes were subsequently treated with the appropriate antibodies against the following proteins: betatrophin (cat#TA326696, OriGene Technologies, Rockville, MD), phosphorylated eukaryotic translation initiation factor 2α (p-eIF2α; cat#ab32157, Abcam, Cambridge, MA), Activating transcription factor 6 (ATF6; cat# MAB0082, Abnova, Taipei city, Taiwan), NF-κB (p65, cat#sc-372, Santa Cruz Biotechnology, Santa Cruz, CA) and β-actin (cat#a5441, Sigma-Aldrich).
Animals
Eight-week-old db/db male mice (n = 6, C57BL/6J background), ob/ob male mice (n = 6, C57BL/6J background, Jackson Laboratory, 000632), and normal C57BL/6J mice (n = 24, Jackson Laboratory, 000664), purchased from Orient Bio (Sungnam, Korea), were maintained at ambient temperature (22 ± 1 °C) with 12-h light-dark cycles and free access to water and food. Db/db and ob/ob mice were fed a normal chow diet (Dyets, Inc., Bethlehem, PA) for 4 weeks. For the HFD-induced obesity and methionine-choline deficient (MCD) diet-induced fatty liver models, C57BL/6J mice were randomly divided into three groups: a control group was fed a chow diet for 4 weeks (n = 6) and 8 weeks (n = 6), an HFD group (n = 6) was fed a HFD (60% kcal from fat, Research diet, Bethlehem, PA) for 8 weeks, and an MCD group (n = 6) was fed an MCD diet (Research diet) for 4 weeks. Mouse body weight, blood glucose levels, and food intake were measured three times a week over the experimental period. Animals were sacrificed after a 6-h fast. Livers were isolated, immediately freeze-clamped in liquid nitrogen, and stored at −80 °C until assays were conducted. For in vivo experiments with tunicamycin, C57BL/6J mice (n = 4 to 6 per each group) were given a single 1 mg/kg body weight intraperitoneal injection of tunicamycin and then were sacrificed after 24 h. One hundred mg/kg 4-PBA was pre-treated in mice by oral gavage before injection of tunicamycin. All experimental procedures performed in this study followed ethical guidelines for animal studies and were approved by the Institutional Animal Care and Use Committee of Yonsei University College of Medicine (IACUC No. 2013-0147-1). All experiments were performed in accordance with relevant guidelines and regulations.
Hepatic triglyceride measurement
Following liver homogenization, TG content in liver tissues was measured with the Triglycerides Quantification Kit (Biovision, K622, Milipitas, CA) according to the manufacturer’s instructions.
Statistical analysis
Data were compared using the Student’s t-test, analysis of variance, or Pearson’s χ2 test as appropriate followed by posthoc Bonferroni tests, using the SPSS 20.0 program (SPSS Institute, Chicago, IL). Mediation effect of HOMA-IR was examined by Baron Kenny Procedure41 using R version 3.2.2 (The R Foundation for Statistical Computing, Vienna, Austria)42. Graphs were plotted using PRISM (Version 5.0, GraphPad Software Inc, San Diego, CA). For the human study, data were presented as mean ± SD or N (%). Fasting glucose, AST, ALT, TG, fasting insulin, and HOMA-IR levels were log transformed for the analyses, because the value distributions were skewed based on the Kolmogorov-Smirnov test. We conducted Spearman correlation analyses to examine the relationship between serum betatrophin levels and clinical and biochemical variables, followed by multivariate regression analyses with adjustment for various covariates. Model 1 included variables that showed statistical significance in the simple correlation analysis except AST, ALT, and insulin resistance-related indices. Model 2 adjusted for Model 1 + AST and ALT, and Model 3 adjusted for Model 2 + HOMA-IR (fasting glucose was removed due to collinearity with HOMA-IR). The ability of serum betatrophin levels to predict NAFLD was evaluated by ROC curves and the AUROC was calculated. A P-value of <0.05 was considered statistically significant. All results are presented as representative data from six to nine experiments.
Additional Information
How to cite this article: Lee, Y.-h. et al. Association between betatrophin/ANGPTL8 and non-alcoholic fatty liver disease: animal and human studies. Sci. Rep. 6, 24013; doi: 10.1038/srep24013 (2016).
References
Loomba, R. & Sanyal, A. J. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 10, 686–690 (2013).
Blachier, M. et al. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol 58, 593–608 (2013).
Targher, G., Day, C. P. & Bonora, E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 363, 1341–1350 (2010).
Stefan, N., Kantartzis, K. & Haring, H. U. Causes and metabolic consequences of Fatty liver. Endocr Rev 29, 939–960 (2008).
Anstee, Q. M., Targher, G. & Day, C. P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 10, 330–344 (2013).
Ekstedt, M. et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44, 865–873 (2006).
Soderberg, C. et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology 51, 595–602 (2010).
Fracanzani, A. L. et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 48, 792–798 (2008).
Zhang, R. & Abou-Samra, A. B. A dual role of lipasin (betatrophin) in lipid metabolism and glucose homeostasis: consensus and controversy. Cardiovasc Diabetol 13, 133 (2014).
Wang, Y. et al. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc Natl Acad Sci USA 110, 16109–16114 (2013).
Tang, T. et al. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol 28, 749–755 (2010).
Zhang, R. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem Biophys Res Commun 424, 786–792 (2012).
Ren, G., Kim, J. Y. & Smas, C. M. Identification of RIFL, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism. Am J Physiol Endocrinol Metab 303, E334–351 (2012).
Yi, P., Park, J. S. & Melton, D. A. Betatrophin: a hormone that controls pancreatic beta cell proliferation. Cell 153, 747–758 (2013).
Fu, Z., Yao, F., Abou-Samra, A. B. & Zhang, R. Lipasin, thermoregulated in brown fat, is a novel but atypical member of the angiopoietin-like protein family. Biochem Biophys Res Commun 430, 1126–1131 (2013).
Espes, D., Lau, J. & Carlsson, P. O. Increased circulating levels of betatrophin in individuals with long-standing type 1 diabetes. Diabetologia 57, 50–53 (2014).
Fu, Z. et al. Elevated circulating lipasin/betatrophin in human type 2 diabetes and obesity. Sci Rep 4, 5013 (2014).
Espes, D., Martinell, M. & Carlsson, P. O. Increased circulating betatrophin concentrations in patients with type 2 diabetes. Int J Endocrinol 2014, 323407 (2014).
Hu, H. et al. Increased circulating levels of betatrophin in newly diagnosed type 2 diabetic patients. Diabetes Care 37, 2718–2722 (2014).
Utzschneider, K. M. & Kahn, S. E. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab 91, 4753–4761 (2006).
Imrie, D. & Sadler, K. C. Stress management: How the unfolded protein response impacts fatty liver disease. J Hepatol 57, 1147–1151 (2012).
Quagliarini, F. et al. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc Natl Acad Sci USA 109, 19751–19756 (2012).
Gusarova, V. et al. ANGPTL8/betatrophin does not control pancreatic beta cell expansion. Cell 159, 691–696 (2014).
Tseng, Y. H. et al. Chromosome 19 open reading frame 80 is upregulated by thyroid hormone and modulates autophagy and lipid metabolism. Autophagy 10, 20–31 (2014).
Horton, J. D. et al. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J Clin Invest 101, 2331–2339 (1998).
Gao, M. & Liu, D. The liver X receptor agonist T0901317 protects mice from high fat diet-induced obesity and insulin resistance. AAPS J 15, 258–266 (2013).
Flamment, M., Hajduch, E., Ferre, P. & Foufelle, F. New insights into ER stress-induced insulin resistance. Trends Endocrinol Metab 23, 381–390 (2012).
Yoon, H. J. & Cha, B. S. Pathogenesis and therapeutic approaches for non-alcoholic fatty liver disease. World J Hepatol 6, 800–811 (2014).
Fu, Z., Abou-Samra, A. B. & Zhang, R. An explanation for recent discrepancies in levels of human circulating betatrophin. Diabetologia 57, 2232–2234 (2014).
Chen, X. et al. Circulating betatrophin levels are increased in patients with type 2 diabetes and associated with insulin resistance. J Clin Endocrinol Metab 100, E96–E100 (2015).
Ebert, T. et al. Circulating Angiopoietin-like Protein 8 Is Independently Associated With Fasting Plasma Glucose and Type 2 Diabetes Mellitus. J Clin Endocrinol Metab 99, E2510–2517 (2014).
Gomez-Ambrosi, J. et al. Circulating betatrophin concentrations are decreased in human obesity and type 2 diabetes. J Clin Endocrinol Metab 99, E2004–2009 (2014).
Fenzl, A. et al. Circulating betatrophin correlates with atherogenic lipid profiles but not with glucose and insulin levels in insulin-resistant individuals. Diabetologia 57, 1204–1208 (2014).
Haukeland, J. W. et al. Fetuin A in nonalcoholic fatty liver disease: in vivo and in vitro studies. Eur J Endocrinol 166, 503–510 (2012).
Choi, H. Y. et al. Increased selenoprotein p levels in subjects with visceral obesity and nonalcoholic Fatty liver disease. Diabetes Metab J 37, 63–71 (2013).
Saadeh, S. et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 123, 745–750 (2002).
Limanond, P. et al. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology 230, 276–280 (2004).
American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care 37 Suppl 1, S14–80 (2014).
Lee, Y. H. et al. A simple screening score for diabetes for the Korean population: development, validation, and comparison with other scores. Diabetes Care 35, 1723–1730 (2012).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Baron, R. M. & Kenny, D. A. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51, 1173–1182 (1986).
R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/. (2013).
Acknowledgements
The authors thank Ms. Sunhee Lee, Severance Hospital, for her contributions to patient enrollment and administrative service. This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (No. HI14C2476) and by a grant (L.J.H. 2008) from the Korean Diabetes Association. The authors would like to thank Dong-Su Jang, MFA, (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea) for his help with the illustrations and Yun Ho Roh and Ji Eun Moon, PhD (Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, Korea) for statistical consultation.
Author information
Authors and Affiliations
Contributions
Conception and design: Y.-h.L. and B.-S.C.; Analysis and interpretation of the data: Y.-h.L., S.-G.L., C.J.L., J.H.L., E.S.K. and H.C.L.; Conducting the experiments: Y.-h.L., C.J.L., S.H.K., Y.-M.S., M.R.Y. and B.H.J. Drafting of the article: Y.-h.L., S.-G.L., B.-W.L. and B.-S.C.; Critical revision of the article for important intellectual content: M.R.Y., J.H.L., B.-W.L., E.S.K. and H.C.L.; Administrative, technical, or logistic support: B.-W.L., E.S.K., H.C.L. and B.-S.C.; Collection and assembly of data: Y.-h.L., S.-G.L., C.J.L. and S.H.K. All authors reviewed the manuscript. B.-S.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lee, Yh., Lee, SG., Lee, C. et al. Association between betatrophin/ANGPTL8 and non-alcoholic fatty liver disease: animal and human studies. Sci Rep 6, 24013 (2016). https://doi.org/10.1038/srep24013
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep24013
This article is cited by
-
Genetic predisposition to nonalcoholic fatty liver disease: insights from ANGPTL8 gene variants in Iranian adults
Lipids in Health and Disease (2023)
-
Urolithin C reveals anti-NAFLD potential via AMPK-ferroptosis axis and modulating gut microbiota
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
LILRB2/PirB mediates macrophage recruitment in fibrogenesis of nonalcoholic steatohepatitis
Nature Communications (2023)
-
Circulating angiopoietin-like proteins in metabolic-associated fatty liver disease: a systematic review and meta-analysis
Lipids in Health and Disease (2021)
-
Circulating ANGPTL8 levels and risk of kidney function decline: Results from the 4C Study
Cardiovascular Diabetology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.