Abstract
The ability to control T cells engineered to permanently express chimeric antigen receptors (CARs) is a key feature to improve safety. Here, we describe the development of a new CAR architecture with an integrated switch-on system that permits to control the CAR T-cell function. This system offers the advantage of a transient CAR T-cell for safety while letting open the possibility of multiple cytotoxicity cycles using a small molecule drug.
Similar content being viewed by others
Introduction
Adoptive immunotherapy using engineered T-cells has emerged as a powerful approach to treat cancer. The potential of this approach relies on the ability to redirect the specificity of T cells through genetic engineering and transfer of chimeric antigen receptors (CARs) or engineered TCRs1. Numerous clinical studies have demonstrated the potential of adoptive transfer of CAR T cells for cancer therapy2,3,4,5 but also raised the risks associated with the cytokine-release syndrome (CRS) and the “on-target off-tumor” effect3,6,7,8. To date, few strategies have been developed to pharmacologically control CAR engineered T-cells and may rely on suicide mechanisms9,10,11,12,13,14. Such suicide strategies leading to a complete eradication of the engineered T-cells will result in the premature end of the treatment. Consequently, implementing non-lethal control of engineered CAR T-cells represents an important advancement to improve the CAR T-cell technology and its safety. Small molecule based approaches that rely on dimerizing partner proteins have already been used to study, inter alia, the mechanism of T-cell receptor triggering15. Very recently, Lim and colleagues have adapted this approach to control engineered T-cells through the use of a multichain receptor16.
Here, we describe a strategy to create a switchable engineered CAR T-cells. Our approach is based on engineering a system that is directly integrated in the hinge domain that separate the scFv from the cell membrane. In addition, we chose to implement this strategy in a novel CAR architecture that relies on the FceRI receptor scaffold. The particularity of this design reside in the possibility to split or combine different key functions of a CAR such as activation and costimulation within different chains of a receptor complex, mimicking the complexity of the TCR native architecture. In this report, we showed that the hinge engineering approaches allowed to turn a T-cell endowed with an engineered CAR from an off-state to an on-state. By controlling the scFv presentation at the cell surface upon addition of the small molecule, our system allowed to further induce the cytolytic properties of the engineered T-cell. Overall, this non-lethal system offers the advantage of a “transient CAR T-cell” for safety while letting open the possibility of multiple specific cytotoxicity cycles using a small molecule drug.
Results
Experimental setup and CAR architecture
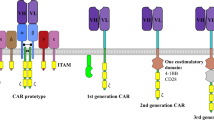
The CAR T-cell performance is intimately linked to an optimal interaction of the scFv to the targeted antigen. We thus conceived a system where controlled of the hinge that separates the scFv from the cell membrane could be obtained upon addition of a small molecule. As a first proof of concept, we focused on the well described and widely used macrolide rapamycin that binds with high affinity to the FKBP12 protein, creating a complex that subsequently interacts with a domain of mTOR (FKBP-rapamycin binding, FRB)17,18. In addition, we chose to implement this molecular switch strategy in a novel CAR architecture based on the FcεRI receptor scaffold, an oligomeric complex composed of three different polypeptide chains (alpha, beta and gamma)19,20. The native activation domains on the gamma and beta subunits were substituted by the intracytoplasmic signaling region of the ζ-chain of the CD3–T cell receptor and by the signaling domains from co-stimulatory 4-1BB (CD137) respectively. The extracellular domain of the alpha subunit was substituted by a single-chain variable fragment (scFv) targeting the well documented CD19 antigen fused to a hinge domain derived from the T-cell surface glycoprotein CD8 alpha chain (CD8a) (Fig. 1A)21,22. Our strategy was comforted by very recent studies that have reported such approach to create engineered mutichain receptors with novel possibilities to control and improve the efficiency of CAR T-cells16,23.
Engineered CAR T cells are responsive to addition of a small molecule
To design an integrated system to switch the scFv/antigen interaction between on/off states, we inserted either the FRB, the FKBP12, or fusion of the FRB and FKBP12 between the CD8a hinge and the scFv domains (Fig. 1B and Supplementary Table 1). As a starting experiment, we transfected primary T cell with mRNAs encoding each chain of the multichain CAR (mcCAR). Upon addition of rapamycin, we monitored changes in the detection of the extracellular hinge domain by tracking the Fab’2 domain of the CD19-targeting scFv (100 nM, 20 h). First in absence of the small molecule (rapamycin), we found that a high level of surface detection could only be achieved for the wild type mcCAR and the FKBP-mcCAR with above 90% of positive cells with an overall high MFI (Fig. 2A). For the FRB-mcCAR, the percentage of positive cells was also high (above 80%) but with a lower MFI (3–5 fold decreased when compared to the mcCAR or FKBP-CAR), which might have resulted from a destabilization of the chain due to the FRB being out of its native context (mTOR). The presence of both FKBP and FRB in the stalk region virtually abolished surface detection of the scFv, independently of their reciprocal position (below 40% of positive cells, with up to 40 fold decrease in MFI when compared to the mcCAR). Interestingly, while the addition of rapamycin barely affected the mcCAR, FRB-mcCAR and FKB-mcCAR constructs, when considering the percentage of positive cells or the MFI (Fig. 2A,B and Supplementary Fig. 1), it strongly improved (up to 15 fold when considering the MFI and 3 fold when considering the percentage of positive cell) the surface detection of the FKBP/FRB-mcCAR and FRB/FKBP-mcCAR constructs (Fig. 2A,B), turning the system from an off to an on state. This variation of detection upon addition of rapamycin may results from different factors, including stabilization of the CAR chain that is containing the switch on component. However it has to be noted that the small molecule was always required to efficiently turn-on the detection of the FKBP/FRB-CAR of the CAR at the surface of the T-cell.
Surface detection of the engineered CAR in response to small molecules.
(A) Percentages of live cells positive for surface detection of mcCAR in function of presence of vehicle (DMSO) or rapamycin. The detection of the Fab’2 region of the scFv is shown in a representative experiment. (B) Fold increase of the median fluorescence intensity (MFI) upon addition of the small molecule rapamycin as depicted in the whole live cell population. (C) The fold increase in the median fluorescence intensity (MFI) upon addition of the small molecule AP21967 is depicted using the T2098L mutant FKBP/FRB* construct. N = 2, error bars denote s.d.
As an alternative to the use rapamycin-resistant T-cells (rapamycin has immunosuppressive properties)24, we next tested the synthetic non-immunosupressing AP21967 rapamycin synthetic analog, which binds to the FKBP12 but does not promote the binding to the FRB domain of mTOR. Accordingly, we also include the T2098L mutation in the FRB domain (referred as FRB*) to allow the FKBP/AP21967/FRB* complex to be formed25. We focused only on the FKBP/FRB-CAR scaffold as it showed the strongest rapamycin-induced increase in detection (Fig. 2B). Consistent with our previous results, the new construct was only strongly detected on the T-cell surface upon addition of the AP21967 drug (Fig. 2C and Supplementary Fig. 2).
To evaluate the AP21967 usable dose range for the switch-on system we performed a dose response assay (Fig. 3A). The results we obtained indicated a maximum signal induction at 100 nM and an EC50 value of approximately 10 nM (8.2–10.1 nM, Fig. 3A) that was independent from the amount of transfected engineered CAR. To validate the portability of the switch-on approach, we engineered a CAR targeting CD123. As demonstrated by a similar EC50 value of 10 nM (7.3–8.7 nM, Supplementary Fig. 3), we found that the nature of the scFv did not influence the switch-on properties. Remarkably, the EC50s are in range with rapamycin concentrations reported in peripheral blood or tumor tissue of patients26, suggesting that the switch-on system may be sensitive to clinically relevant concentration.
Characterization of the small molecule switch-on system.
(A) Determination of the AP21967 EC50 with CD19 targeting engineered CAR. T-cells transfected with three doses (D, D1/2 and D1/4) of mRNA coding for the engineered CAR were treated with increasing amount of AP21967 rapamycin synthetic analog. The Fab’2 region of the scFv is detected. (B) Competition experiment between AP21967 (10 nM) and tacrolimus (0 to 500 nM). N = 2, error bars denote s.d.
Modulation of the response to AP21967 with tacrolimus
The possibility to further modulate the system using alternative small molecule competitors offers additional control of the engineered CAR T-cells. To illustrate the possibility to tune the amount of CAR locked in an on-state at the cell surface, we used tacrolimus (FK506), a small molecule known to bind to the FKBP12 without enabling to form a complex with the FRB. AP21967 (or rapamycin) and tacrolimus have identical FKBP12 binding core and should compete for the same binding site within the FKBP moiety27,28. T-cells transfected with the engineered CAR were incubated with a fixed amount of AP21967 (10 nM) and increasing amount of tacrolimus (0 to 500 nM) and the surface labelling of the scFv was recorded. As expected, addition of increasing amounts of tacrolimus competed with AP21967 for the binding site on FKBP and decreased the surface detection of the CAR (Fig. 3B).
Engineered CAR T cells display antitumor cytotoxicity upon addition of AP21967
We next intended to illustrate that the cytotoxicity of a T-cell expressing such engineered CAR can be controlled in line with its surface presentation. Therefore, we evaluated in vitro the cytolytic capacities of CAR T-cells toward a CD19pos and a CD19neg model cell line, using flow-based assay29. Remarkably, the results we obtained showed that the engineered FKBP/FRB-CAR T-cell presented a significant cell lysis activity only in presence of the AP21967 (Fig. 4A and Supplementary Fig. 4). We further showed that the hinge engineering did not impair the specificity feature of the engineered T-cell as no cytotoxicity was observed on CD19neg target cells (Fig. 4A). Next we performed a dose response (0, 1, 5, 10, 33, 100 nM) of the AP21967 and measured the resulting cytolytic capacities of the engineered CAR T-cells. We found that the level of target cell killing correlated, as expected, with variation of the AP21967 (Fig. 4B). We futher calculated an EC50 of approximately 10 nM (12.7 nM), in range of the one determined using the surface detection. The level of targeted cell killing also correlated with the level of CAR detection (Supplementary Fig. 5).
Specific cytolytic properties of the engineered CAR T-cells.
(A) The effect of the AP21967 rapamycin synthetic analog on the cytolytic capacites of the of the CAR T cells toward model antigen presenting cell was assessed in a flow-based cytotoxicity assay. The CD19pos and a CD19neg target cell viability was measured after coculture with engineered CAR T-cells in presence or absence of AP21967. Effector/target ratios was set to 20:1. NT represents non-transfected T-cells, N = 2, error bars denote s.d. (B) The effect of the AP21967 rapamycin synthetic analog dose on the cytolytic capacites of the of the CAR T cells was measured in a flow-based cytotoxicity assay using the CD19pos cell line. The Effector/target ratio was set to 10:1. The experiment was done in triplicate, error bars denote s.d.
Altogether, the results presented here provide the proof of principle of engineering the hinge domain of a CAR molecule to create an integrated switch-on system for logic gating strategies. Beyond these first steps, additional work will have to be performed to asses the full therapeutic potential of this technology, including inter alia (i) the stable integration of the CAR in the cell genome and (ii) a complete characterization of the AP21967 rapamycin synthetic analogue that has not been used in human.
Discussion
The possibility to endow T cells with chimeric antigen receptors (CAR) has been described for more than two decades. Recent clinical implementation of adoptive cell transfer of CAR engineered T cells has proven a powerful and successful approach to cancer immunotherapy. The capability to control T cells endowed permanently with such molecules is a key feature to improve the safety of this technology. Within the conceptual framework of a CAR molecule, many alterations have been described to improve the capacity of engineered T cells to elicit antitumor functions in vitro and in vivo. Prior studies were in particular focused on optimizing the cytotoxic activity or proliferative capacities of CAR T-cells through engineering of the intracellular domain of the CARs, leading to the 2nd and 3rd generation of molecules30,31. However, the development of such highly active engineered T-cells have also raised concerns on the safety of this technology32. In particular, outcomes from clinical trial pointed out the cytokine-release syndrome (CRS) and the “on-target off-tumor” undesired targeting, both potentially leading to fatal issues. Therefore, increasing resources and effort have been allocated to better master the specificity and activation of engineered T-cell in a spatial and temporal manner using synthetic biology approaches33.
The transient surface expression of a CAR through RNA electroporation of T cells has already been demonstrated to be an alternative to permanently modified T-cells for improved safety34. However, such RNA-modified CAR T-cells head toward a permanent “off state” (RNA degradation and cell division). The all-in-one system presented in this report, with the switch incorporated in the CAR, possesses the advantages to be transient-like and to keep the cells alive. Therefore, it open the possibility to spatiotemporally induce multiple cytotoxicity cycles to control potential adverse effects. In addition, this switch-on CAR system is perfectly compatible with novel approaches that promote targeted control of drugs or engineered CAR-T cells delivery35,36. Finally, one could imagine developing similar strategies of CAR engineering to promote a small molecule off-switch that perturb optimal presentation of the antigen targeting moiety. In conclusion, while this work provides the basic framework to use alternative multi chain CARs, we believe that the easy-to-implement strategy described in this report is paving the way toward the development of novel more controlled and potentially safer engineered CAR T-cells.
Methods
All individual chains of the CAR architecture were amplified by PCR using oligo pairs α-chain-F/α-chain-R, β-chain-F/β-chain-R and γ-chain-F/γ-chain-R prior to mRNA synthesis (Supplementary table 2). mRNA encoding the α-chain, β-chain or γ-chain were in vitro transcribed from the PCR product and polyadenylated using the mMessage mMachine T7 Ultra kit (Life technologies) following the manufacturer’s instructions. RNAs were purified with RNeasy columns (Qiagen), eluted in cytoporation medium T and quantified by measuring absorbance at 260 nm using a Nanodrop ND-1000 spectrophotometer. Quality of the RNA was verified on a denaturing formaldehyde/MOPS agarose gel. Sequences of FKBP and/or FRB domains are given in Supplementary table 1.
Transfection
T lymphocytes were transfected by electrotransfer of messenger RNA using an AgilePulse MAX system (Harvard Apparatus) 3 to 6 days after activation. Following removal of activation beads, cells were pelleted, resuspended in cytoporation medium T at 28 × 106 cells/ml. 5 × 106 cells were mixed with the previously synthetized mRNA into a 0.4 cm cuvette. This particular setup allowed maintaining an identical stoichiometry (1 α/1 β/2 γ) of each chain for all constructs. Dose D corresponded to a total amount of mRNA of 33.5 μg (16 μg alpha chain, 7.5 μg beta chain, 10 μg gamma chain).
The electroporation consisted of two 0.1 ms pulses at 1200 V followed by four 0.2 ms pulses at 130 V. Following electroporation, cells were diluted into 2 mL culture medium and incubated at 37 °C/5% CO2. Vehicle (DMSO or ethanol, Sigma-Aldrich or Carlo Erba), Rapamycin (Sigma-Aldrich) or AP21697 (Clontech) was added 2 hours after mRNA electrotransfer and T-cell were incubated for 20 hours at 37 °C, CO2 5%.
For competition experiments, AP21697 (10 nM) was added simultaneously with various amounts (0, 1, 10, 33, 100 or 500 nM) of tacrolimus (Sigma-Aldrich) and T-cell were incubated for 20 hours at 37 °C/5% CO2.
Flow cytometry
Primary labelling for the detection of the CD19 targeting α-chain was performed with anti-Fab’2-Biotin (goat anti-mouse IgG, Fab’2 fragment specific, Jackson Immunoresearch) in PBS FBS 2%, EDTA 2 mM, azide 0.1% for 20 min at 4 °C followed by a two washing steps with PBS FBS 2% EDTA 2 mM azide 0.1%. Secondary labelling was performed with Streptavidin-APC (BD Pharmingen) in PBS FBS2% EDTA 2 mM azide 0.1% for 20 min at 4 °C followed by a washing step in PBS FBS2% EDTA 2 mM azide 0.1% and a washing step in PBS.
Primary labelling for the detection of the CD123 targeting α-chain was performed with Fc-tagged recombinant CD123 (Lake Pharma) in PBS FBS2%, EDTA 2 mM, azide 0.1% for 20 min at 4 °C followed by a two washing steps with PBS FBS2% EDTA 2 mM azide 0.1%. Secondary labelling was performed with PE labeled Goat Anti-Mouse IgG (subclasses 1 + 2a + 2b + 3), Fcγ Fragment Specific (Jackson Immunoresearch) in PBS FBS2% EDTA 2 mM azide 0.1% for 20 min at 4 °C followed by a washing step in PBS FBS2% EDTA 2 mM azide 0.1% and a washing step in PBS. Following the extracellular labelling, the cell viability was monitored using the efluor450 or efluor780 (ebioscience) in PBS for 20 min 4 °C, followed by a washing step with PBS FBS2% EDTA 2 mM azide 0.1% and fixed in PFA 2%. Flow cytometry was performed using the MACSQUANT (Miltenyi Biotec) and data analysis was performed with the FlowJo software.
Cytotoxicity assay
The cytolytic activity of engineered T-cells endowed with the different CARs (transfected with dose D1/4), was assessed using a flow cytometry-based cytotoxicity assay. In this assay target cells presenting the CAR target antigen (Daudi, CD19pos) are labelled with CellTraceTM CFSE (Life Technologies) and control cells not presenting the CAR target antigen (K562, CD19neg) with CellTraceTM violet (Life Technologies). The target cell populations were co-incubate at 37 °C at various ratio of engineered effector CAR T cells (Effector/Target ratio of 10:1, 20:1 and 30:1) in a final volume of X-Vivo-15 media of 100 uL, for 5 h in presence of vehicle (Ethanol) or AP21967 (100 nM).
The whole cell population was recovered, washed in PBS and labeled with eFluor780 viability marker before being fixed by 2% PFA. Fixed cells were analyzed by flow cytometry to determine their viability.
Statistical analysis
EC50 values of dose-response assays on rapamycin synthetic analog were computed using the R library drc and a four parameter log-logistic function which has the following formula:

To evaluate the significance of the difference between rapamycin synthetic analog treated and untreated cells for CD19pos cell lysis by CAR T cells expressing the FKBP/FRB*, a standard paired t-test was used on the different transfections.
Additional Information
How to cite this article: Juillerat, A. et al. Design of chimeric antigen receptors with integrated controllable transient functions. Sci. Rep. 6, 18950; doi: 10.1038/srep18950 (2016).
References
Sharpe, M. & Mount, N. Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech 8, 337–350 (2015).
Brentjens, R. J. et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 5, 177ra138 (2013).
Grupp, S. A. et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368, 1509–1518 (2013).
Kalos, M. et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 3, 95ra73 (2011).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365, 725–733 (2011).
Brentjens, R. J. et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 118, 4817–4828 (2011).
Davila, M. L. et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6, 224ra225 (2014).
Morgan, R. A. et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 18, 843–851 (2010).
Budde, L. E. et al. Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS One 8, e82742 (2013).
Chen, Y. Y., Jensen, M. C. & Smolke, C. D. Genetic control of mammalian T-cell proliferation with synthetic RNA regulatory systems. Proc Natl Acad Sci USA 107, 8531–8536 (2010).
Narayanan, P. et al. A composite MyD88/CD40 switch synergistically activates mouse and human dendritic cells for enhanced antitumor efficacy. J Clin Invest 121, 1524–1534 (2011).
Marin, V. et al. Comparison of different suicide-gene strategies for the safety improvement of genetically manipulated T cells. Hum Gene Ther Methods 23, 376–386 (2012).
Philip, B. et al. A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood 124, 1277–1287 (2014).
Straathof, K. C. et al. An inducible caspase 9 safety switch for T-cell therapy. Blood 105, 4247–4254 (2005).
James, J. R. & Vale, R. D. Biophysical mechanism of T-cell receptor triggering in a reconstituted system. Nature 487, 64–69 (2012).
Wu, C. Y., Roybal, K. T., Puchner, E. M., Onuffer, J. & Lim, W. A. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science (2015).
Banaszynski, L. A., Liu, C. W. & Wandless, T. J. Characterization of the FKBP.rapamycin.FRB ternary complex. J Am Chem Soc 127, 4715–4721 (2005).
Chen, J., Zheng, X. F., Brown, E. J. & Schreiber, S. L. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci USA 92, 4947–4951 (1995).
Kinet, J. P. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol 17, 931–972 (1999).
Kraft, S. & Kinet, J. P. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev Immunol 7, 365–378 (2007).
Kugler, M. et al. Stabilization and humanization of a single-chain Fv antibody fragment specific for human lymphocyte antigen CD19 by designed point mutations and CDR-grafting onto a human framework. Protein Eng Des Sel 22, 135–147 (2009).
Poirot, L. et al. Multiplex genome edited T-cell manufacturing platform for “off-the-shelf” adoptive T-cell immunotherapies. Cancer Res (2015).
Wang, E. et al. Generation of Potent T-cell Immunotherapy for Cancer Using DAP12-Based, Multichain, Chimeric Immunoreceptors. Cancer Immunol Res 3, 815–826 (2015).
Huye, L. E. et al. Combining mTor inhibitors with rapamycin-resistant T cells: a two-pronged approach to tumor elimination. Mol Ther 19, 2239–2248 (2011).
Bayle, J. H. et al. Rapamycin analogs with differential binding specificity permit orthogonal control of protein activity. Chem Biol 13, 99–107 (2006).
Cloughesy, T. F. et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med 5, e8 (2008).
Wilson, K. P. et al. Comparative X-ray structures of the major binding protein for the immunosuppressant FK506 (tacrolimus) in unliganded form and in complex with FK506 and rapamycin. Acta Crystallogr D Biol Crystallogr 51, 511–521 (1995).
Cabantous, S. et al. A new protein-protein interaction sensor based on tripartite split-GFP association. Sci Rep 3, 2854 (2013).
Valton, J. et al. A Multidrug-resistant Engineered CAR T Cell for Allogeneic Combination Immunotherapy. Mol Ther (2015).
Dotti, G., Gottschalk, S., Savoldo, B. & Brenner, M. K. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev 257, 107–126 (2014).
Sadelain, M., Brentjens, R. & Riviere, I. The basic principles of chimeric antigen receptor design. Cancer Discov 3, 388–398 (2013).
Minagawa, K., Zhou, X., Mineishi, S. & Di Stasi, A. Seatbelts in CAR therapy: How Safe Are CARS? Pharmaceuticals (Basel) 8, 230–249 (2015).
Chakravarti, D. & Wong, W. W. Synthetic biology in cell-based cancer immunotherapy. Trends Biotechnol 33, 449–461 (2015).
Beatty, G. L. et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res 2, 112–120 (2014).
Adusumilli, P. S. et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med 6, 261ra151 (2014).
Stephan, S. B. et al. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat Biotechnol 33, 97–101 (2015).
Author information
Authors and Affiliations
Contributions
A.J., A.M., L.P. and P.D. conceived the study and designed experiments. A.J., A.M. and J.M.F. performed experiments. J.V. provided conceptual advices and technical support. A.J., A.M., A.D. and L.P. analyzed experiments. A.J., L.P. and P.D. wrote the manuscript with support from all authors.
Ethics declarations
Competing interests
All co-authors are present Cellectis employees.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Juillerat, A., Marechal, A., Filhol, JM. et al. Design of chimeric antigen receptors with integrated controllable transient functions. Sci Rep 6, 18950 (2016). https://doi.org/10.1038/srep18950
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep18950
This article is cited by
-
Tuning CARs: recent advances in modulating chimeric antigen receptor (CAR) T cell activity for improved safety, efficacy, and flexibility
Journal of Translational Medicine (2023)
-
Hurdles to breakthrough in CAR T cell therapy of solid tumors
Stem Cell Research & Therapy (2022)
-
A deep insight into CRISPR/Cas9 application in CAR-T cell-based tumor immunotherapies
Stem Cell Research & Therapy (2021)
-
Programmable and multi-targeted CARs: a new breakthrough in cancer CAR-T cell therapy
Clinical and Translational Oncology (2021)
-
Engineering AvidCARs for combinatorial antigen recognition and reversible control of CAR function
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.