Abstract
The presence of IL-17-positive cells is observed in a variety of inflammatory associated cancers and IL-17 has been found to be involved in angiogenesis. However, it remains unclear how IL-17 might contribute to tumor angiogenesis. In our study, IL-17 enhanced the formation of vessel-like tubes in HUVECs both directly (when HUVECs were incubated with IL-17) and indirectly (when HUVECs were incubated in conditioned cell media (CCM) from IL-17-treated cancer cells). Our results from experiments using siRNA-mediated knockdowns of STAT3 and GIV suggest that the effects of IL-17 were mediated by activating STAT3/GIV signaling in NSCLC cells and subsequently up-regulating its downstream target VEGF. Consistent with these findings, immunostaining experiments on human NSCLC tissues indicated that IL-17 and GIV expression were significantly and positively associated with increased tumor vascularity. The clinical significance of IL-17 was authenticated by our finding that the combination of intratumoral IL-17 + cells and GIV expression served as a better prognosticator for survival than either marker alone. Therefore, our finding highlights a novel aspect of STAT3/GIV pathway in the IL-17 promotes tumor angiogenesis of NSCLC.
Similar content being viewed by others
Introduction
Non-small-cell lung cancer (NSCLC) accounts for 80–85% of total lung malignancies1.The outcome of NSCLC is poor and the disease is rarely curable. The overall five-year survival rate is less than 15%2 and is largely due to lung cancer cell metastasis3,4. Angiogenesis is a critical hallmark of malignancy and can occur at different stages of the tumor progression5. Angiogenesis is regulated by a balance between pro- and anti- angiogenesis factors and the disruption of this balance contributes to the pathogenesis of numerous disorders including cancer6.
T helper 17 (Th17) cells are an important inflammatory component whose main physiological role is to promote host defense against infectious agents. Th17 cells are well known for their role in contributing to autoimmune diseases7. Recently, Th17 cells and their signature cytokine, interleukin-17 (IL-17), have been found to be present in increased frequencies within certain tumors8,9,10. Chang and colleagues has demonstrated a critical role for Th17 cell-mediated inflammation in lung tumorigenesis11. In our previous study, we found that serum IL-17 was elevated and the levels positively correlated with VEGF concentration in NSCLC patients12. Consistently, transfection of IL-17 into tumor cells augmented the progression of the disease in nude mice via effects on the vascular endothelium and increased neoangiogenesis13,14. However, IL-17’s mechanisms underlying its modulation of human NSCLC cell angiogenesis remain elusive.
Accumulating evidence is defining Signal transducer and activator of transcription 3 (STAT3) as an important pathway for signal transduction in cancer metastasis and angiogenesis15,16. GIV(Gα-Interacting Vesicle-associated protein, also known as Girdin) is a guanidine exchange factor (GEF) that modulates key signaling pathways during a diverse set of biological processes such as wound healing, macrophage chemotaxis, cancer invasion/metastasis and tumor angiogenesis. GIV is a direct target of the STAT3 in breast cancer cells17. Others have reported that GIV is expressed exclusively in colorectal carcinoma cells with high metastatic potential and is virtually undetectable in those with poor metastatic potential, implying the involvement of GIV in tumor metastasis18. Here, we speculate that GIV may play a role in the angiogenesis of cancer cells. In this study, we attempted to elucidate the exact role and associated molecular mechanism of IL-17 in NSCLC angiogenesis. The clinical relevance and prognostic significance of IL-17 in human NSCLC were also investigated.
Results
IL-17 is positively correlated with MVD in human NSCLC tissues and enhanced formation of vessel-like tubes in HUVECs
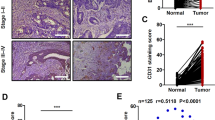
High densities of h17 cells infiltrating tumours have been associated with increased angiogenesis in studies from human gastric19, colorectal20, hepatocellular21 and pancreatic cancers22. In addition, the level of IL-17-producing cells has been positively correlated with MVD in a tumor-bearing mouse model23. To investigate the role of IL-17 in angiogenesis in patients with NSCLC, we stained consecutive sections in 67 NSCLC patients (Fig. 1a). We found that the majority of IL-17 staining was localized to the cytoplasm of mononuclear cells in NSCLC tissues. Our results indicated that patients with high IL-17 expression exhibited high MVD (p = 0.016, Fig. 1b). Moreover, a correlation analysis revealed a significant positive correlation between the density of IL-17-producing cells and MVD (r = 0.471; p < 0.001, Fig. 1c).
IL-17 expression is associated with MVD and IL-17 promotes in vitro tube formation in HUVECs.
(a) IL-17-positive cells expression and MVD staining for CD34 in NSCLC tissues (magnification, 200×). (b) Quantification of stains of immunohistochemistry; 5 random high-powered fields per section were counted for number of CD34-stained vessels intensity and distribution; Date are expressed as means; Student′s t test; *p < 0.05. (c) Significant positive correlations were found between the IL-17 expression and MVD. Spearman′s rank correlation coefficient; r = 0.471; p < 0.001. (d) Representative photographs (left panel) and mean numbers of tube length (right panel) at ×100 magnification. HUVECs were seeded on Matrigel-coated plates incubated with IL-17 (10 ng/ml, 50 ng/ml or 100 ng/ml) or vehicle control at 37 °C for 8 h (n = 3). *p < 0.05.
Next, we examined this cytokine’s effect on tube formation by HUVECs. HUVECs were treated with recombinant human IL-17(rhIL-17) or a vehicle control. As expected, treatment with IL-17 promoted the formation of vessel-like tubes in a dose-dependent manner (Fig. 1d). Quantitative analysis of endothelial cell networks revealed that IL-17 significantly increased the tube length compared to control cultures. These findings demonstrated that IL-17 plays a potential role in promoting angiogenesis.
IL-17 activates STAT3 in NSCLC cells
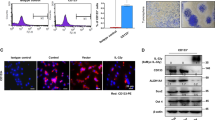
IL-17 is known for its effects on angiogenesis being reliant on the surrounding endothelial cells and fibroblasts. In our present study, we found expression of the IL-17 receptor (IL-17R) at the mRNA level in all detected NSCLC cell lines (Fig. 2a). Because STAT3 activation in tumor cells plays a critical role in tumor progression by augmenting tumor survival and tumor angiogenesis and by suppressing antitumor immunity24, we explored the possibility mechanism that IL-17 mediates tumor angiogenesis via activation of STAT3 in tumor cells. Here, we cultured A549 cells and H520 cells with or without recombinant human IL-17 and found that IL-17 stimulated increased phosphorylation of STAT3 in vitro as early as 6 h after IL-17 treatment. This effect lasted for 24 h (Fig. 2b). Furthermore, this increased phosphorylation was confirmed by immunofluorescence assays tumor cells that were cultured for 24 h in the presence or absence of IL-17. IL-17 treatment of NSCLC cells markedly increased p-STAT3 expression (Fig. 2c and Fig. S1).
IL-17 promotes NSCLC angiogenesis via STAT3 activation.
(a) mRNA expression of IL-17R in NSCLC cell lines. (b) Western blotting showed that phosphorylation of STAT3 were obviously increased as early as 6 h after IL-17 treatment and lasted for 24 h after IL-17 stimulation. A549 cells were incubated with IL-17 at the indicated concentrations for 24 h or at 100 ng/ml for the indicated time. (c) Immunofluorescence assays showed that recombinant human IL-17(100 ng/ml for 24 h) significantly elevated the expression of p-STAT3 in A549 cells. Photomicrographs were taken at ×200 magnification. Control, PBS. (d) A549 cells or A549-siRNA-STAT3 cells were treated with IL-17 at 100 ng/ml for 24 h, media was harvested, added to HUVECs plated on Matrigel. HUVEC were seeded in 96-well plates coated with matrigel and treated with CCM or CCM-IL-17 from A549 cells or A549-siRNA-STAT3 cells for 16 h. In this experiment, HUVEC incubated with 0.5% serum containing 1640 media served as a negative control. Tubular structures were photographed at 40× magnification and tube length was measured as described in ‘Materials and Methods’. Tube length data is presented as mean ± standard deviation of three samples for each treatment.
To explore the potential role of STAT3 in IL-17-mediated effects on NSCLC angiogenesis, STAT3 expression was reduced by small interfering RNA (siRNA) (Fig. S2). A549 and H520 tumor cells were exposed to STAT3 siRNA (A549-siRNA-STAT3 or H520-siRNA-STAT3) or empty vector and then treated with rhIL-17 at 100 ng/ml for 24 h. Conditioned cell media (CCM) from these cells were harvested and then added to HUVECs plated on Matrigel. We observed that CCM from rhIL-17-treated A549 cells strongly enhanced tube formation by HUVECs. On the other hand, in HUVECs plated with CCM from rhIL-17-treated A549-siRNA-STAT3 cells, the ability of IL-17 to promote tube formation was diminished, as evidenced by decreased tube length (Fig. 2d). These results suggest an important contribution from STAT3. We observed similar results regarding tube formation in experiments with CCM collected from H520 cells and H520-siRNA-STAT3 cells.
IL-17 promotes NSCLC angiogenesis via the STAT3/GIV signaling pathway
GIV is widely expressed in a variety of cancer cell lines, including MDA-MB-231 (estrogen-independent breast carcinoma cell line), Ls-174T (colon cancer cell line), A431 (skin squamous cell carcinoma cell line), HeLa (uterine cervical carcinoma cell line) and HT-1080 (fibrosarcoma cell line)25,26. Some experiments have shown that GIV is positively correlated with increasing tumor aggressiveness and angiogenesis27. In our study, western blot analysis of GIV expression in NSCLC cell lines was used to define the role of GIV in NSCLC progression (Fig. S3). IL-17 treatment of tumor cells markedly increased p-STAT3 expression (Fig. 3a) as well as GIV expression (Fig. 3a,b). Ying Dunkel et al. demonstrated that GIV expression is upregulated due to increased transcription mediated directly by STAT317. Consistent with this result, IL-17 significantly elevated the expression of GIV in A549 and H520 cells. However, depletion of STAT3 markedly reversed IL-17-mediated GIV expression changes at both the protein and mRNA levels in A549 and H520 cells (Fig. 3c). Furthermore, immunofluorescence assays showed that IL-17 treatment of cells clearly upregulated the expression of GIV (Fig. 3d and Fig. S4). Conversely, depletion of endogenous STAT3 resulted in decreased GIV expression. To further investigate the role of GIV on IL-17-mediated tumor angiogenesis, we depleted GIV in A549 and H520 cells (Fig. S5). In HUVEC tube formation assays, CCM from A549 cells treated with IL-17 significantly enhanced tube formation. GIV-siRNA significantly reversed IL-17-stimulated tube formation in A549 cells (Fig. 3e). These findings demonstrate that STAT3 positively regulates GIV expression and that IL-17 promotes NSCLC angiogenesis via STAT3/GIV activation.
IL-17 induces the expression of GIV and GIV participated IL-17 mediated angiogenesis in NSCLC cells.
(a) A549, H520, H1792 and H460 cells were treated with IL-17(100 ng/ml for 24 h) and expression levels of GIV, p-STAT3 and STAT3 were examined by Western blotting. (b) mRNA level of GIV were examined in tumor cells treated with IL-17 by real-time PCR. (c) A549 and H520 cells were cultured for 24 h with IL-17 or PBS. The protein and mRNA levels were measured by western blotting and qPCR. Depletion of STAT3 markedly reversed IL-17-mediated GIV expression increased both protein and mRNA levels. (d) Immunofluorescence assays showed that recombinant human IL-17(100 ng/ml for 24 h) significantly elevated the expression of GIV in A549 cells. However, the expression of GIV decreased if depleted of endogenous STAT3. The photomicrographs were taken at ×200 magnification. (e) HUVECs were seeded in 96-well plates coated with matrigel and treated with CCM or CCM-IL-17 from A549 cells or A549-siRNA-GIV cells for 16 h. Tubular structures were photographed at 40× magnification. Tube length data is presented as mean ± standard deviation of three samples for each treatment. (f) H520 or H520-siRNA-STAT3 or H520-siRNA-GIV cells were incubated with or without IL-17 and Western blotting were performed for phosphor-STAT3, total STAT3, GIV and tubulin protein levels.
Others have previously demonstrated that GIV positively autoregulates its own transcription by enhancing STAT3 activation via its GEF motif in invasive cancer cells17. In our study, STAT3 increased GIV expression; depleted STAT3 or GIV in NSCLC cells diminished IL-17-mediated angiogenesis in a microenvironment. However, we did not find that GIV enhances STAT3 activation in NSCLC cells (Fig. 3f).
IL-17 induces VEGF production via STAT3/GIV activation in NSCLC cells
IL-17 is known for eliciting secretion of diverse inflammatory mediators in diverse cell types, including stromal cells and tumor cells. We hypothesized that IL-17 might induce the expression of proangiogenic factors(s) by cancer cells that then act on endothelial cells to promote angiogenesis. We found that IL-17 selectively up-regulates IL-6 and VEGF protein expression in all NSCLC cell lines studied (A549, H520, H1792, H460 cells; Table 1 and Table S1). We observed a similar increase in mRNA levels in A549 cells. The most prominent increase in secretion was for IL-6, with an 8.8-fold increase, followed by VEGF (6.51-fold) in A549 cells (Fig. 4a). By contrast, the production of IL10, IL-8, bFGF, PDGF and Endostain were not significantly affected by exogenous IL-17.
IL-17 stimulates NSCLC cells to produce VEGF via STAT/GIV activation.
(a) A549 cells were treated with PBS or with IL-17 for 24 h. The mRNA levels of proangiogenic factors were analyzed with Realtime-PCR. (b) NSCLC cells were cultured for 24 h with IL-17(100 ng/ml) or PBS. Concentrations of VEGF in culture supernatants were measured by ELISA and mRNA of VEGF in NSCLC cells were measured by Real-time PCR. IL-17 up-regulated the production of VEGF by tumor cells. Both siRNA-STAT3 and siRNA-GIV significantly downregulated the expression of VEGF. (c) A549 cells were cultured for 24 h with IL-17(100 ng/ml) in the presence or absence of VEGF mAb (20 ng/ml), then the CCM harvested. Before CCM added to HUVECs, IL-17 mAb(500 ng/ml) was added to the IL-17 treatment medium. Tubular structures were photographed at 40× magnification. Tube length data is presented as mean ± standard deviation of three samples for each treatment.
VEGF is one of the most important angiogenic factors. We postulated that IL-17 might affect tumor cells’ production of VEGF by activating the STAT3/GIV pathway in tumor cells. Notably, in A549-siRNA-STAT3 cells and H520-siRNA-STAT3 cells, IL-17-induced expression of VEGF was significantly inhibited (Fig. 4b). Meanwhile, GIV-siRNA significantly reversed IL-17-induced VEGF expression at both the protein and mRNA levels in NSCLCs (Fig. 4b), while Il-17-induced IL-6 up-regulation was not affected (Fig. S6). Furthermore, HUVEC cells plated with CCM from A549 cells, neutralizing VEGF by using blocking mAb significantly reduced IL-17-induced tube formation, while using IL-17 mAb treated were not affected (Fig. 4c). These results indicated that VEGF is an important target gene in the STAT3/GIV pathway. Thus, VEGF may be the main downstream target of STAT3/GIV in the context of IL-17-induced tumor angiogenesis (Fig. 5).
IL-17 has recently been reported to activate STAT3 via IL-6 induction28,29. Therefore, we determined whether IL-6 mediated IL-17-driven STAT3 activation in our study. After neutralizing IL-6 with a blocking mAb, IL-17 stimulation still significantly increased p-STAT3 expression in tumor cells even though STAT3 activation was diminished. The results of the tube formation assay also indicated a similar phenomenon in regards to treatment with IL-6 mAb (Fig. S7). Thus, our study showed that IL-6 may be a main upstream activator of pSTAT3 expression, but is not indispensable in the setting of IL-17 stimulation.
GIV expression positively correlates with IL-17+ cell and microvessel densities and predicts poor survival of NSCLC patients
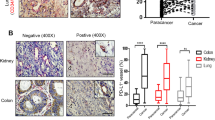
Because high densities of IL-17+ cell tumors have been associated with increased angiogenesis in our studies (Fig. 1b), we further explored whether a similar phenomenon exists regarding GIV in human NSCLC tissue. A correlation analysis on immunostained consecutive tissue sections from 67 NSCLC patients (Table S2) revealed significant positive correlations between GIV expression and MVD (r = 0.512; p < 0.001; Fig. 6a,b), as well as between GIV expression and densities of intratumoral IL-17-producing cells (r = 0.422; p < 0.001; Fig. 6c). Thus, GIV may play an important role in IL-17-induced tumor angiogenesis.
IL-17 and GIV clinical relevance and is associated with poor prognosis.
(a) Serial whole tumor sections from 67 NSCLC patients were used and representative cases of immunostaining of IL-17, GIV and CD34 (MVD) are shown (magnification, 200×). (b,c) Significant positive correlations were found between the GIV expression and MVD (b), GIV expression and IL-17-positive cells intensity (c). (d,e) Kaplan-Meier curves of overall survival analysis between NSCLC patients. GIV (d) or IL-17(e) with high expression patients had significantly poorer OS than low expression patients. (f) NSCLC patients were classified into three groups: group I, high expression both of IL-17+ cells and GIV; group II, high expression of one of the two markers; and group III, low expression of both markers. The 3 year survival rates in group I were significantly lower than those in groups II and III. P values were determined by log-rank test.
We next investigated the prognostic value of IL-17 and its target GIV in NSCLC patients. At the time of data analysis, 42 of the 67 patients had died of the disease during the follow-up period of 36 months. The median cumulative overall survival (OS) was 28 months and the three-year OS rate was 37.3%. Patients with high expression of GIV had significantly poorer OS than those with low expression of GIV (Fig. 6d). Similarly, there was a significant inverse correlation between intratumoral IL-17+ cell density and patient survival (Fig. 6e). These results suggest that increased intratumoral IL-17+ cells and increased GIV expression are associated with NSCLC progression.
Univariate analysis revealed that intratumoral IL-17+ cells and GIV expression were significantly associated with survival (Table 2). Patients were classified into three groups: group I, high expression of both markers; group II, high expression of either marker; group III, low expression of both markers. Significant differences in survival were detected among the three groups (Fig. 6f). Subsequently, in a multivariate COX regression analysis where the presence of IL-17-positive cells, GIV expression and the combination of both markers were simultaneously adopted as covariates (Table 2), only the combination remained statistically significant, whether the comparison was between group I and II or between group I and III. Furthermore, multivariate analyses showed that GIV expression independently correlated with, irrespective of being used alone or in combination (Table S3). These results collectively suggest that intratumoral IL-17+ cells and GIV expression combined serve as a better prognosis marker in NSCLC patients than either marker alone.
Discussion
Tumor angiogenesis, the formation of new capillaries from the existing vascular network, is essential for tumor growth and metastasis. However, the precise molecular events that initiate this complex process of angiogenesis in NSCLCs are poorly understood. In the tumor microenvironment, inflammatory cells and molecules influence almost every aspect of cancer progression. We and others have previously demonstrated that suggests IL-17 is elevated in several types of cancer, but how IL-17 might contribute to tumor angiogenesis is still unclear. Here, we found that IL-17 selectively augments the secretion of various angiogenic factors in tumor cells and promotes endothelial cell tube formation. We suggest that IL-17 mediates these effects by activating STAT3/GIV signaling and by subsequently up-regulating the STAT3/GIV downstream target VEGF. Consistent with these findings, in human NSCLC tissues, IL-17 expression was significantly and positively associated with GIV and increased tumor vascularity. Our findings thus support the notion that IL-17 can promote NSCLC angiogenesis.
Previous studies have shown that IL-17 has diverse effects on inflammatory cells and stromal cells, with most effects relating to angiogenesis stimulation and inflammation13,14,30. In our study, HUVECs treated with IL-17 showed increased formation of vessel-like tubes and IL-17 expression significantly correlated with MVD in human NSCLC tissues. Recently, IL-17 has also been reported to mediate the release of proinflammatory factors and chemokines from tumor cells, including renal, hepatocellular and colorectal cancers20,29,31. Here, IL-17R was highly expressed in all NSCLC cell lines. We thus hypothesize that IL-17-mediated promotion of tumor angiogenesis involves an effect on NSCLC cells, which up-regulate their production of proangiogenic factors. In support of this hypothesis, we found that exogenous IL-17 stimulation increases IL-6 and VEGF levels in all NSCLC cell lines studied, increased IL-8 expression in H460 and H520 cells and decreased expression of Endostain in A549 cells.
In numerous inflammatory cells and tumor cells, IL-17 mediates signaling through distinct pathways, such as the MAPK, NF-κB and STAT3 pathways32. Previously, IL-17 has been reported to stimulate production of IL-6 and STAT3 activation in inflammatory cells and fibroblasts in an autoimmune disease33 as well as in cancer cells24,34. Furthermore, the STAT3 signaling pathway controls a number of important biological responses, including immune functions, cellular growth, cellular differentiation and hematopoiesis24. In this study, we found that IL-17 could stimulate the production of IL-6 and STAT3 activation in NSCLC cells. Moreover, we observed that in HUVECs incubated with CCM from A549-siRNA-STAT3 cells, the ability of IL-17 to promote tube formation was diminished, as evidenced by reduced tube length. This result suggests an important contribution from STAT3 in IL-17-mediated tumor angiogenesis. In a wide range of cancers, immunohistochemical studies have indicated that VEGF expression is correlated with elevated STAT3 activity15,35,36. Indeed, STAT3 has been shown to be a direct transcriptional activator of the VEGF gene37. Of the various factors involved in angiogenesis, members of the VEGF family have predominant roles6,38. In our present study, HUVEC tube formation assays show that CCM from A549 cells treated with IL-17 significantly enhanced tube formation, while VEGF mAbs significantly reversed the IL-17-stimulated tube formation. However, different cell types appear to respond differently to IL-17 in terms of target gene expression. Although IL-8 has been reported to be affected by IL-17 in other cell types29,39, cytokines in all NSCLC cells were not significantly altered in the presence of IL-17.
STAT3 is a downstream target of several cell surface receptors that can be activated by a plethora of soluble mediators, including interleukins (IL-3, IL-6, IL-10) as well as other cytokines(G-CSF, EGF, FGF, PDGF) and hormones (growth hormone, leptin)28,29,40,41. We found that IL-17 up-regulates IL-6 expression in NSCLC cell lines. When we used neutralizing IL-6 treated with IL-17 induced cells, we found that IL-17-induced STAT3 activation and increased the tube length were diminished. By contrast, the production of IL10, bFGF and PDGF were not significantly affected by exogenous IL-17. Our study showed that IL-6 may be a main upstream activator of pSTAT3 expression, but is not indispensable in the setting of IL-17 stimulation. However, it is true that pSTAT3 expression still increases in response to IL-17 after neutralizing IL-6 (figure S7). As an explanation for this result, this increase could be due to other known STAT3 activators that could be produced by IL-17 treatment of tumor cells. More recently, Hu et al. demonstrated that TGF-β increased the levels of STAT3 in prostate cancer cells42 and another reports suggested that STAT3 was activated by G-CSF in myeloma and ovarian cancer cells43,44. Importantly, although GSM-CSF, G-CSF, TNF-α, TGF-β have been reported to be affected by IL-17 on other cell types45, production of these cytokines in NSCLC cells remain elusive and should be investigate in future studies.
Recently, Dunkel and colleagues have shown that GIV is a direct target of STAT3 and that GIV positively autoregulates its own transcription by enhancing STAT3 activation via its GEF motif17. Our results show that IL-17 induces angiogenesis in NSCLC cells by activating the STAT3/GIV signaling pathway and that knockdown of STAT3 significantly reduces GIV expression. Furthermore, others also found GIV to be an important metastasis-related protein in the progression of diverse cancers26,46,47. In our study, GIV expression in A549 cells is important for IL-17-induced endothelial cell tube formation and GIV expression is positively correlated with IL-17 and MVD in human NSCLC tissue. These results provide a mechanistic explanation for the prognostic studies that have directly linked IL-17, STAT3 signaling and GIV with tumor recurrence, tumor metastasis and poor survival in cancer patients. To the best of our knowledge, this study is the first to demonstrate that IL-17-induced STAT3/GIV activation occurs during tumor cell angiogenesis.
Previous results have shown a protumor effect of IL-17 in hepatocellular and colorectal carcinoma; IL-17 was shown to be an independent prognostic factor for OS and DFS21,29. Consistent with these previous results, we found that IL-17 is associated with poor prognosis and that intratumoral IL-17-producing cells are positively and significantly correlated with MVD in human NSCLC tissues. Although consistent with several recent publications regarding the role of IL-17 in promoting tumor progress, these findings contradict other reports suggesting that IL-17 can provide an antitumor effect against certain tumors8,30. Furthermore, GIV expression positively correlated with the presence of IL-17+ cells and both were associated with the presence of microvascular invasion and poor survival in NSCLC patients. The combination of intratumoral IL-17+ cells and GIV expression served as a better predictor of poor survival than either alone in NSCLC patients.
In conclusion, our results suggest that IL-17-mediated tumor angiogenesis involves activation of the STAT3/GIV signaling pathway and subsequent up-regulation of VEGF production in NSCLC cells. These might be the mechanisms that underlie the correlation between IL-17 and poor prognosis and angiogenesis. Therapies that target IL-17 and GIV may be developed as potential therapeutic approaches to inhibit NSCLC.
Materials and Methods
Patients
A total of 67 patients who underwent surgery for histologically verified NSCLC at the Department of Pathology, The Harbin Medical University Cancer Hospital between 2006 and 2010 were enrolled in this study. None of the patients received any anticancer therapy prior to sample collection. The tumor stage was determined according to the 2010 American Joint Committee on Cancer and International Union Against Cancer tumor-node-metastasis (TNM) classification system. Tumor differentiation was graded according to the Edmondson and Steiner grading system. All experiments were performed in accordance with the relevant guidelines and regulations of Harbin Medical University. And this study was approved by the Ethics Committee of Harbin Medical University and written informed consent was obtained from each patient.
Cell culture
The human lung adenocarcinoma cell lines A549, H1792 and H522, lung squamous carcinoma cell line H520 and H226, large cell lung cancer cell line H460 and HUVECs were purchased from American Type Cell Collection (ATCC, Manassas, VA, USA). Lung cancer cells were cultured in RPMI 1640 (Gibco, USA) supplemented with 10% fetal calf serum. HUVECs were maintained using the EGM-2 bullet kit (Lonza, Basel, Switzerland) in a humidified atmosphere of 5% CO2 at 37 °C.
The NSCLC cells were treated with medium alone or with various concentrations (10 ng/ml, 50 ng/ml and 100 ng/ml) of recombinant human interleukin-17 (R&D Systems, Minneapolis, MN, USA) in RPMI-1640 medium for 24 h or at 100 ng/ml for the indicated time. To prevent the effects of cytokines, IL-17 mAb, IL-6 mAb and VEGF mAb (R&D Systems, Minneapolis, MN, USA), were added to the IL-17 treatment medium.
Transient transfection with siRNA
STAT3- and GIV- siRNAs, as well as negative control RNA molecules with mismatched sequences, were synthesized by Oligofectamine (Invitrogen,San Diego, CA) using the following sense and anti-sense strands: STAT3 sense, 5′ GGGACCUGGUGUGAAUUAUTT 3′ and antisense, 5′ AUAAUUCACACCAGGUCCCTT 3′; GIV sense, 5′ GAGGCAGACAGUGUCAUUATT 3′; antisense, 5′ UAAUGACACUGUCUGCCUCTT 3′. Transfection into NSCLC cells was performed using Lipofectamine™ 2000 (Invitrogen) according to the manufacturer’s instructions.
Western blot analysis
The cells were lysed with lysis buffer (Cell Signaling Technology, Danvers, MA, USA) containing a protease inhibitor (Sigma Chemical company, St. Louis, MO, USA). Protein concentration was quantified using the BCA protein assay kit (Santa Cruz, USA). Western blotting was performed as previously described48. Specific primary antibodies against p-STAT3 (Y705), STAT3 (Cell Signaling Technology, Beverly, MA) and GIV (Millipore, MA, USA) were used in our study. Tubulin (Sigma, USA) was used as a loading control.
Real-time RT-PCR analysis
Total RNA from the cultured lung cancer cells was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Complementary DNA was synthesized from 1 ng of total RNA using the PrimeScriptTM RT reagent kit with gDNA Eraser (Takara Bio, Inc, Dalian, China) in a final reaction volume of 20 μl. Semiquantitative real-time PCR was performed using the SYBR Green Master Mix (Roche Applied Science, Mannheim, Germany) on an ABI7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control to correct for variations in the cDNA content among the samples. The primers were synthesized by Oligofectamine (Invitrogen, San Diego, CA). No amplification of nonspecific products was observed in any of the reactions, as determined from an analysis of the dissociation curves. The data were normalized to the GAPDH expression levels and are presented as the averages from three repeated experiments. The relative gene expression levels were calculated using the comparative Ct (△△Ct) method; the relative expression is calculated as 2−△△Ct, where Ct represents the threshold cycle.
Conditioned medium and ELISA
Supernatants from NSCLC cells treated with or without IL-17 (100 ng/ml) for 24 h were collected. The medium was centrifuged to remove the cellular debris and frozen at −80 °C until subjected to ELISA to assay levels of angiogenic factor. Angiogenic factor concentrations were determined using a commercial ELISA kit (Uscn Life Science, Houston, USA) according to the manufacturer’s instructions. The level of angiogenic factor was expressed in picograms per milliliter.
Immunohistochemistry and assessment
Immunohistochemistry to detect IL-17, CD34 and GIV expression was performed as previously described49. The primary antibodies and dilutions were as follows: rabbit polyclonal anti-IL-17 antibody (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti-GIV antibody (1:100, Millipore, MA, USA) and rabbit monoclonal anti-CD34 antibody (1:200, Zhongshan Company, Beijing, China). The negative control sections were treated with PBS instead of the primary antibodies.
Stained tissue sections were evaluated using light microscopy at 200× or 400× magnification by two pathologists. Five representative fields of each case were captured. To calculate the percentages of IL-17- and GIV-positive cells, the number of positive-staining cells and total number of cells in five count areas of each photograph were measured using Image-Pro Plus v6.0 software (Media Cybernetics, Inc.). The mean percentage of positive cells was calculated as the quotient of the number of positive cells divided by the total number of cells. The MVD was assessed via immunohistochemistry using a CD34 marker. The stained sections were screened at 200× magnification for hot spots and the average number of microvessels was recorded. Any red-staining cells that were morphologically compatible with endothelial cells and that were in a cluster containing or lacking a lumen (rudimentary or well-formed) were considered microvessel cells and were counted as a vessel. Two observers were responsible for the microvessel number counting and the mean value was used for analysis.
Immunofluorescence staining
A549 cells and H520 cells were cultured in slides within 6-well plates. Tumor cells and siRNA-treated tumor cells were treated with recombinant IL-17 (100 ng/mL) for 24 h. At the end of the incubation, cells were fixed with 4% paraformaldehyde for 15 minutes at RT and permeabilized by treating with 100% methanol for 10 minutes at 20 °C. Next, slides were washed with PBS, blocked with normal goat IgG for 1 hour at room temperature and incubated overnight at 4 °C with rabbit anti-p-STAT3 or rabbit anti-GIV antibodies. After washing with PBS, chamber slides were incubated with secondary antibodies (goat anti-rabbit-IgG-Alexa Fluor 594). Slides were then mounted with Prolong Gold Antifade Reagent with 40,6-diamidino-2-phenylindole (DAPI; Invitrogen). The fluorescent images were analyzed by fluorescence microscopy.
Tube formation assay
The tube formation assay was performed as described previously50. Briefly, a 96-well plate was coated with 60 μl of matrigel (BD Biosciences, USA), which was allowed to polymerize and solidify at 37 °C for 30 min. The HUVECs were seeded onto the matrigel layer in the presence or absence of various concentrations of IL-17 (10, 50 and 100 ng/ml) or conditioned cell media (CCM). After 8–16 h, blood-vessel-like tubules from three randomly chosen fields were counted and photographed under a microscope (Nikon, Japan). The tube length was quantified using the Image-Pro Plus v6.0 software (Media Cybernetics, Inc.). Results are represented as total tube length (μm) for three photographic fields per experimental condition. Each treatment was performed in duplicate and the experiment was independently repeated three times.
Statistics
Statistical analysis was performed with SPSS 17.0 software (SPSS, Chicago, IL). Each treatment was performed in duplicate and the experiment was independently repeated three times. Measurement values were expressed as the means ± standard deviations. Student’s t test and Spearman’s r correlation were used as appropriate. The survival rates were performed by the Kaplan-Meier method (log-rank test). Cox multivariate analysis with a stepwise method (forward, likelihood ratio) was used to determine the independent prognostic factors. A p value < 0.05 was judged to indicate significant results. The median values of the percentage of IL-17+ cells and of GIV+ cells were used as cutoffs to dichotomize the immunostaining.
Additional Information
How to cite this article: Pan, B. et al. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci. Rep. 5, 16053; doi: 10.1038/srep16053 (2015).
Change history
27 May 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Smith, R. A., Cokkinides, V. & Brawley, O. W. Cancer screening in the United States, 2009: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 59, 27–41 (2009).
Sun, S., Schiller, J. H., Spinola, M. & Minna, J. D. New molecularly targeted therapies for lung cancer. J Clin Invest 117, 2740–50 (2007).
Mantovani, A., Romero, P., Palucka, A. K. & Marincola, F. M. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet 371, 771–83 (2008).
Solan, M. J. & Werner-Wasik, M. Prognostic factors in non-small cell lung cancer. Semin Surg Oncol 21, 64–73 (2003).
Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1, 27–31 (1995).
Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–57 (2000).
Miossec, P., Korn, T. & Kuchroo, V. K. Interleukin-17 and type 17 helper T cells. N Engl J Med 361, 888–98 (2009).
Kryczek, I. et al. Phenotype, distribution, generation and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 114, 1141–9 (2009).
Steiner, G. E. et al. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic and malignant prostate. Prostate 56, 171–82 (2003).
Zhang, B. et al. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun 374, 533–7 (2008).
Chang, S. H. et al. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci USA 111, 5664–9 (2014).
Pan, B. et al. Interleukin-17 levels correlate with poor prognosis and vascular endothelial growth factor concentration in the serum of patients with non-small cell lung cancer. Biomarkers 20, 232–9 (2015).
Numasaki, M. et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood 101, 2620–7 (2003).
Tartour, E. et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res 59, 3698–704 (1999).
Niu, G. et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21, 2000–8 (2002).
Kortylewski, M. & Yu, H. Role of Stat3 in suppressing anti-tumor immunity. Curr Opin Immunol 20, 228–33 (2008).
Dunkel, Y. et al. STAT3 protein up-regulates Galpha-interacting vesicle-associated protein (GIV)/Girdin expression and GIV enhances STAT3 activation in a positive feedback loop during wound healing and tumor invasion/metastasis. J Biol Chem 287, 41667–83 (2012).
Ghosh, P., Garcia-Marcos, M., Bornheimer, S. J. & Farquhar, M. G. Activation of Galphai3 triggers cell migration via regulation of GIV. J Cell Biol 182, 381–93 (2008).
Iida, T. et al. Tumor-infiltrating CD4+Th17 cells produce IL-17 in tumor microenvironment and promote tumor progression in human gastric cancer. Oncol Rep 25, 1271–7 (2011).
Liu, J. et al. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun 407, 348–54 (2011).
Zhang, J. P. et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol 50, 980–9 (2009).
He, S. et al. Distribution and clinical significance of Th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. Int J Mol Sci 12, 7424–37 (2011).
Numasaki, M. et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol 175, 6177–89 (2005).
Yu, H., Kortylewski, M. & Pardoll, D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 7, 41–51 (2007).
Cailleau, R., Olive, M. & Cruciger, Q. V. Long-term human breast carcinoma cell lines of metastatic origin: preliminary characterization. In Vitro 14, 911–5 (1978).
Garcia-Marcos, M. et al. Expression of GIV/Girdin, a metastasis-related protein, predicts patient survival in colon cancer. FASEB J 25, 590–9 (2011).
Weng, L., Enomoto, A., Ishida-Takagishi, M., Asai, N. & Takahashi, M. Girding for migratory cues: roles of the Akt substrate Girdin in cancer progression and angiogenesis. Cancer Sci 101, 836–42 (2010).
Wang, L. et al. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med 206, 1457–64 (2009).
Gu, F. M. et al. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer 10, 150 (2011).
Benchetrit, F. et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood 99, 2114–21 (2002).
Inozume, T., Hanada, K., Wang, Q. J. & Yang, J. C. IL-17 secreted by tumor reactive T cells induces IL-8 release by human renal cancer cells. J Immunother 32, 109–17 (2009).
Gaffen, S. L. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 9, 556–67 (2009).
Ogura, H. et al. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 29, 628–36 (2008).
Gao, Q. et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 15, 971–9 (2009).
Xie, T. X. et al. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res 66, 3188–96 (2006).
Gong, W. et al. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res 11, 1386–93 (2005).
Wei, D. et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene 22, 319–29 (2003).
Coultas, L., Chawengsaksophak, K. & Rossant, J. Endothelial cells and VEGF in vascular development. Nature 438, 937–45 (2005).
Pappu, R., Ramirez-Carrozzi, V. & Sambandam, A. The interleukin-17 cytokine family: critical players in host defence and inflammatory diseases. Immunology 134, 8–16 (2011).
Rane, S. G. & Reddy, E. P. JAKs, STATs and Src kinases in hematopoiesis. Oncogene 21, 3334–58 (2002).
Levy, D. E. & Darnell, J. E. Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 3, 651–62 (2002).
Hu, Q. et al. Periostin Mediates TGF-beta-Induced Epithelial Mesenchymal Transition in Prostate Cancer Cells. Cell Physiol Biochem 36, 799–809 (2015).
Kumar, J. et al. Granulocyte colony-stimulating factor receptor signalling via Janus kinase 2/signal transducer and activator of transcription 3 in ovarian cancer. Br J Cancer 110, 133–45 (2014).
Chakraborty, A. et al. Granulocyte colony-stimulating factor activation of Stat3 alpha and Stat3 beta in immature normal and leukemic human myeloid cells. Blood 88, 2442–9 (1996).
Onishi, R. M. & Gaffen, S. L. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 129, 311–21 (2010).
Jiang, P. et al. An actin-binding protein Girdin regulates the motility of breast cancer cells. Cancer Res 68, 1310–8 (2008).
Liu, C. et al. Girdin protein: a new potential distant metastasis predictor of breast cancer. Med Oncol 29, 1554–60 (2012).
Yang, X. R. et al. Cytokeratin 10 and cytokeratin 19: predictive markers for poor prognosis in hepatocellular carcinoma patients after curative resection. Clin Cancer Res 14, 3850–9 (2008).
Chen, X. et al. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer 69, 348–54 (2010).
Huh, J. E. et al. Bee venom inhibits tumor angiogenesis and metastasis by inhibiting tyrosine phosphorylation of VEGFR-2 in LLC-tumor-bearing mice. Cancer Lett 292, 98–110 (2010).
Acknowledgements
This work was funded by The fund of Harbin city science and Technology Bureau (2014RFXGJ039), Funded by Wu jieping project (320.6750.12311 and 320.6750.12204), the Special Chinese National Postdoctoral Science Foundation (No. 2013T60389) and Young People Foundation of Heilongjiang Provincial of China (No. QC2012C013).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: B.P., Y.Y. Performed the experiments: J.S., D.C., J.C., S.J. and S.C. Performed the statistical analyses: F.L. and Y.Z. Wrote the paper: B.P. and L.S. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Pan, B., Shen, J., Cao, J. et al. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci Rep 5, 16053 (2015). https://doi.org/10.1038/srep16053
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16053
This article is cited by
-
Nailfold capillaroscopy in Egyptian systemic lupus erythematosus (SLE) patients: correlation with demographic features and serum levels of IL 17A and IFNs I
Egyptian Rheumatology and Rehabilitation (2023)
-
DM-MOGA: a multi-objective optimization genetic algorithm for identifying disease modules of non-small cell lung cancer
BMC Bioinformatics (2023)
-
Increased circulating Th17 cell populations in patients with pancreatic ductal adenocarcinoma
Immunogenetics (2023)
-
Neutrophils and polymorphonuclear myeloid-derived suppressor cells: an emerging battleground in cancer therapy
Oncogenesis (2022)
-
Recent advances in the role of Th17/Treg cells in tumor immunity and tumor therapy
Immunologic Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.