Abstract
Progesterone has been shown to have neuroprotective effects in multiple animal models of brain injury, whereas the efficacy and safety in patients with traumatic brain injury (TBI) remains contentious. Here, a total of seven randomized controlled trials (RCTs) with 2492 participants were included to perform this meta-analysis. Compared with placebo, there was no significant decrease to be found in the rate of death or vegetative state for patients with acute TBI (RR = 0.88, 95%CI = 0.70, 1.09, p = 0.24). Furthermore, progesterone was not associated with good recovery in comparison with placebo (RR = 1.00, 95%CI = 0.88, 1.14, p = 0.95). Together, our study suggested that progesterone did not improve outcomes over placebo in the treatment of acute TBI.
Similar content being viewed by others
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability and without effective treatment in children and young adults1,2,3. Despite improvement in outcome following brain injury in recent years, large numbers of patients remain disabled and dependent2,4. Previous animal studies suggested that progesterone could extenuate neural damage effectively by reducing free radicals, inflammatory cytokines, excitotoxicity, apoptosis and vasogenic edema in the model of neurologic injury5,6,7. However, the relevant clinical trials of progesterone demonstrated different clinical benefits and discrepant conclusions for the treatment of patients with acute TBI8,9. Treatment recommendations may be misleading according to the results of any individual trial. Based on available data, we performed a meta-analysis of randomized clinical trials to compare progesterone with placebo for the treatment of patients with severe or moderate acute TBI. The overall evaluation was performed to accurately detail the efficacy and safety of progesterone.
Results
Study selection and characteristics
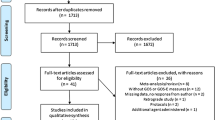
The detailed process of selection is shown in Fig. 1. In total, 295 potential studies were identified with a systematic search of databases. Seventy-five records were excluded as duplicates. We removed 209 apparently unsuitable articles including reviews, case reports and animal experiments after browsing titles and abstracts. The remaining studies were screened and assessed in detail by reviewing full texts. Four articles were excluded due to the following reasons: two excluded studies were not comparative trials, 1 study was an animal experiment and 1 study involved comparison with another drug. Thus, 7 studies meeting our inclusion criteria were selected in this meta-analysis.
These seven studies, with 2492 total participants (Sample size, from 40 to 1179), compared progesterone with placebo in the treatment of acute TBI. Of these, only patients with severe TBI (Glasgow Coma Scale (GCS) ≤8) were included in five studies8,9,10,11,12 and in another 2 studies patients were recruited with moderate-to-severe TBI (GCS ≤12)13,14. All female patients were excluded in one included study12. The primary characteristics and quality assessments of the included RCTs are summarized in Table 1 and 2, respectively.
Meta-analysis outcomes
Death or vegetative state
The meta-analysis of seven RCTs with a random-effects model demonstrated that progesterone did not significantly reduce the rate of death or vegetative state in patients with acute TBI between the two groups (RR = 0.88, 95%CI = 0.70, 1.09, p = 0.24, I2 = 45%) (Fig. 2A). Subgroup meta-analysis was performed (Table 3). Due to the limited number of available studies, meta-regression was not pursued further. A similar result was observed in patients with severe TBI (RR = 0.86, 95%CI = 0.68, 1.09, p = 0.20, I2 = 48%) (Fig. 2B). The sensitivity analysis was performed to examine the influence of different models on the pooled estimates. There were no significant changes to be found with a fixed-effects model (RR = 0.97, 95%CI = 0.84, 1.11, p = 0.65; RR = 0.95, 95%CI = 0.82, 1.10, p = 0.50).
Good recovery
In total, five of the included RCTs had an assessment of good recovery (GOS = 5) at the end of follow-up. No significant heterogeneity was observed in TBI (I2 = 0%). Compared with placebo, the combined data using a fixed-effects model did not show that progesterone significantly increased the rate of good recovery (RR = 1.00, 95%CI = 0.88, 1.14, p = 0.95) (Fig. 3A). There was also no evidence to indicate that progesterone could improve the outcome for a good recovery in severe TBI (RR = 1.04, 95%CI = 0.91, 1.19, p = 0.54, I2 = 0%) (Fig. 3B).
Adverse events
Two studies were included in the meta-analysis of adverse events. A fixed-effects model was used according to heterogeneity. There were no statistically significant differences in pneumonia or sepsis between the two groups (RR = 0.95, 95%CI = 0.85, 1.07, p = 0.42, I2 = 0%; RR = 1.10, 95%CI = 0.76, 1.60, p = 0.61, I2 = 0%) (Fig. 4).
Publication bias
There was no evidence of publication bias (Begg’s test, P = 0.90; Egg’s test, P = 0.059).
Discussion
The pathophysiology of acute TBI is a complex, interwoven and multifactorial process, which includes primary and secondary injury15,16. TBI-induced secondary injury has been considered to be a potential target for therapeutic intervention involving reduction and prevention of inflammation, calcium flux, oxidative stress, necrosis and apoptosis17,18. Based on the efficacy and safety in animal models, progesterone has been regarded to be a potent candidate for the treatment of TBI19,20,21,22. However, the relevant clinical trials of progesterone came to inconsistent conclusions10,12,14. The previous review of progesterone for the treatment of TBI included only three small-scale and low-quality studies23. In this current study, we selected 7 relevant RCTs including 2492 patients (progesterone: 1276 cases, placebo: 1216 cases) hospitalized for acute TBI to assess the efficacy of progesterone therapy on the Glasgow Outcome Scale (GOS) score and for adverse events.
Some previous clinical studies demonstrated that progesterone was a neuroprotective agent and improved outcomes for patients with acute severe TBI8,10. However, we found no significant difference between the progesterone-treated group and the placebo group in the rate of death or vegetative state. Moreover, our results showed that progesterone was not associated with good recovery at the end of the follow-up period. To date, various drugs have been investigated in clinical trials, yet none has been proven to reduce mortality significantly at the confirmatory stage24,25,26,27. The trauma of individual patients could not be controlled well in comparison with the animal model. The heterogeneity and variability of TBI may be one of the important reasons14,28. This classification scheme of patients may be relatively insensitive using the Glasgow Coma Scale (GCS) or the Glasgow Outcome Scale-Extended (GOSE)28,29.
Some limitations must be noted in this present study. First, one included study excluded female patients as a result of side effects on the menstrual cycle12. Second, due to the lack of available data, we did not analyze other clinical outcomes except mortality and good recovery. It was unknown whether progesterone promoted the recovery of motor and sensory skills. Finally, the follow-up was short-term and varied across the studies. Thus, an appropriate dosage and a long-term follow-up may be necessary to further investigate the efficacy of progesterone in the treatment of acute TBI.
In conclusion, the pooled data did not support the idea that progesterone was superior to placebo in the treatment of acute TBI. Progesterone may be not effective in lowering the incidence of death or vegetative state in patients with acute TBI.
Methods
Search strategy
Our electronic search was conducted in PubMed, Embase and the Cochrane Library databases until May 10, 2015. The core terms included “progesterone” and “head injury,” “traumatic brain injury,” “TBI,” “random,” and “random*”. There was no language limitation. We also searched Google Scholar and checked the reference lists of the included studies to identify any additional eligible articles.
Inclusion criteria
Studies were included if they met the following criteria: (1) adults (older than 18 years) with a diagnosis of acute TBI, (2) progesterone compared with placebo (or no progesterone) and (3) randomized controlled trials. Duplicate articles, reviews, case reports and studies without extractable data were excluded.
Data extraction and outcome measures
Two authors (CL and HQH) independently extracted the following data from each included study in the standard form: (1) study characteristics (author’s name, date of publication, study design, sample size), (2) characteristics of participants (age and gender), (3) interventions (administration, duration and dosage) and (4) outcomes (GOS and adverse events). Any discrepancies were discussed and resolved by the research team when necessary. The efficacy outcome was assessed with death or vegetative state (GOS = 1 or 2) and good recovery (GOS = 5) at the 6 months after TBI or end of the follow-up period. Adverse events included pneumonia and sepsis.
Quality assessment
The eligible studies were evaluated according to the Cochrane Collaboration’s tool30. The domains were as follow: selection bias (random method and allocation concealment), performance and detection bias (blinding of participants, personnel and outcome assessment), attrition bias (incomplete outcome data) and reporting bias (selective reporting).
Statistical analysis
The data were analyzed with the Cochrane Review Manager 5.3 and STATA 11.0 software according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement31,32. Risk ratios (RR) were calculated and pooled with a 95% confidence interval (CI) for dichotomous variables. The heterogeneity was estimated using the I2 test, which was considered to be low heterogeneity when I2 ≤ 25%. A fixed-effects random effects model was used if the I2 was ≤25%. Otherwise, a random effects model was applied. We used the funnel plot and Eger’s test to assess potential publication bias33.
Additional Information
How to cite this article: Lin, C. et al. Efficacy of progesterone for moderate to severe traumatic brain injury: a meta-analysis of randomized clinical trials. Sci. Rep. 5, 13442; doi: 10.1038/srep13442 (2015).
References
Ghajar, J. Traumatic brain injury. Lancet 356, 923–929 (2000).
Maas, A. I., Stocchetti, N. & Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol 7, 728–741 (2008).
Coronado, V. G. et al. Trends in Traumatic Brain Injury in the U.S. and the public health response: 1995-2009. J Safety Res 43, 299–307 (2012).
Langlois, J. A., Rutland-Brown, W. & Wald, M. M. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 21, 375–378 (2006).
Stein, D. G., Wright, D. W. & Kellermann, A. L. Does progesterone have neuroprotective properties. Ann Emerg Med 51, 164–172 (2008).
Pascual, J. L. et al. Neuroprotective effects of progesterone in traumatic brain injury: blunted in vivo neutrophil activation at the blood-brain barrier. Am J Surg 206, 840–845 (2013).
Si, D. et al. Progesterone treatment improves cognitive outcome following experimental traumatic brain injury in rats. Neurosci Lett 553, 18–23 (2013).
Xiao, G., Wei, J., Yan, W., Wang, W. & Lu, Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care 12, R61 (2008).
Skolnick, B. E. et al. A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med 371, 2467–2476 (2014).
Xiao, G. M. et al. [Clinical study on the therapeutic effects and mechanism of progesterone in the treatment for acute severe head injury]. Zhonghua Wai Ke Za Zhi 45, 106–108 (2007).
Aminmansour, B. et al. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: A randomized clinical trial with placebo group. Adv Biomed Res 1, 58 (2012).
Shakeri, M. et al. Effect of progesterone administration on prognosis of patients with diffuse axonal injury due to severe head trauma. Clin Neurol Neurosurg 115, 2019–2022 (2013).
Wright, D. W. et al. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med 49, 391–402 (2007).
Wright, D. W. et al. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med 371, 2457–2466 (2014).
Maas, A. I., Stocchetti, N. & Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol 7, 728–741 (2008).
Mustafa, A. G. & Alshboul, O. A. Pathophysiology of traumatic brain injury. Neurosciences (Riyadh) 18, 222–234 (2013).
Park, E., Bell, J. D. & Baker A. J. Traumatic brain injury: can the consequences be stopped? CMAJ 178, 1163–1170 (2008).
Margulies, S. & Hicks, R. Combination therapies for traumatic brain injury: prospective considerations. J Neurotrauma 26, 925–939 (2009).
Drew, P. D. & Chavis, J. A. Female sex steroids: effects upon microglial cell activation. J Neuroimmunol 111, 77–85 (2000).
He, J., Hoffman, S. W. & Stein, D. G. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci 22, 19–31 (2004).
Cutler, S. M. et al. Progesterone improves acute recovery after traumatic brain injury in the aged rat. J Neurotrauma 24, 1475–1486 (2007).
Li, Z. et al. Progesterone increases circulating endothelial progenitor cells and induces neural regeneration after traumatic brain injury in aged rats. J Neurotrauma 29, 343–353 (2012).
Ma, J., Huang, S., Qin, S. & You, C. Progesterone for acute traumatic brain injury. Cochrane Database Syst Rev 10, CD008409 (2012).
Narayan, R. K. et al. Clinical trials in head injury. J Neurotrauma 19, 503–557 (2002).
Schouten, J. W. Neuroprotection in traumatic brain injury: a complex struggle against the biology of nature. Curr Opin Crit Care 13, 134–142 (2007).
Loane, D. J. & Faden, A. I. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 31, 596–604 (2010).
Maas, A. I., Roozenbeek, B. & Manley, G. T. Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics 7, 115–126 (2010).
Manley, G. T. & Maas, A. I. Traumatic brain injury: an international knowledge-based approach. JAMA 310, 473–474 (2013).
Wilson, J. T., Pettigrew, L. E. & Teasdale, G. M. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma 15, 573–585 (1998).
Higgins, J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928 (2011).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151, W65–94 (2009).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6, e1000097 (2009).
Egger, M., Davey, S. G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 81471269 (BA14)).
Author information
Authors and Affiliations
Contributions
C.L. and H.-H.Q. designed the experiments. C.L. and Z.L. performed the experiments. C.-H.L. analyzed the data. C.L. and J.J. wrote the manuscript. Critical revision, final drafting and text approval were performed by L.-Y.L., C.L. and N.L.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lin, C., He, H., Li, Z. et al. Efficacy of progesterone for moderate to severe traumatic brain injury: a meta-analysis of randomized clinical trials. Sci Rep 5, 13442 (2015). https://doi.org/10.1038/srep13442
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep13442
This article is cited by
-
Understanding microglial responses in large animal models of traumatic brain injury: an underutilized resource for preclinical and translational research
Journal of Neuroinflammation (2023)
-
Neurosteroid Receptor Modulators for Treating Traumatic Brain Injury
Neurotherapeutics (2023)
-
Effects of Progesterone on Preclinical Animal Models of Traumatic Brain Injury: Systematic Review and Meta-analysis
Molecular Neurobiology (2022)
-
The effect of age and sex on outcomes following isolated moderate to severe traumatic brain injury
European Journal of Trauma and Emergency Surgery (2022)
-
Drugs with anti-inflammatory effects to improve outcome of traumatic brain injury: a meta-analysis
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.