Abstract
Anthropogenic atmospheric carbon dioxide (CO2) is being absorbed by seawater resulting in increasingly acidic oceans, a process known as ocean acidification (OA). OA is thought to have largely deleterious effects on marine invertebrates, primarily impacting early life stages and consequently, their recruitment and species’ survival. Most research in this field has been limited to short-term, single-species and single-life stage studies, making it difficult to determine which taxa will be evolutionarily successful under OA conditions. We circumvent these limitations by relating the dominance and distribution of the known polychaete worm species living in a naturally acidic seawater vent system to their life history strategies. These data are coupled with breeding experiments, showing all dominant species in this natural system exhibit parental care. Our results provide evidence supporting the idea that long-term survival of marine species in acidic conditions is related to life history strategies where eggs are kept in protected maternal environments (brooders) or where larvae have no free swimming phases (direct developers). Our findings are the first to formally validate the hypothesis that species with life history strategies linked to parental care are more protected in an acidifying ocean compared to their relatives employing broadcast spawning and pelagic larval development.
Similar content being viewed by others
Introduction
We focused on the unique coastal vent ecosystem of Ischia island (Italy), where underwater CO2 volcanic emissions interact with a seagrass and rocky reef habitat1. CO2 bubbling from the seafloor drives the seawater pH down to equal to or lower than business-as-usual IPCC projections for 2100 (pH 6.5–7.81,2), effectively creating a “chemical island” approximately 2,000 years old3. Our biological focus is on polychaete worms, as they are an abundant taxonomic group in the vents1. Their consistent vent-dominance and the trends seen in their seasonal abundances indicate the possibility of either multi- and/or transgenerational exposure4,5,6,7. Furthermore, the group exhibits highly diverse reproductive and developmental modes8.
We related the type of early life history strategies employed by species living in the vents with their known distribution and abundances1,5,6. We found twelve of the total thirteen species with known reproductive characteristics colonizing high CO2 vent areas to be brooding or direct developers (eggs kept in protected maternal environment/no free-swimming larval phases). Ten had higher abundances in the venting areas than in nearby ambient CO2 areas (Table 1). The exception was one species, morphologically appearing to be Platynereis dumerilii (Audouin & Milne-Edwards, 1834), the only broadcast spawning pelagic developer with higher abundances in the vents5,7,9.
The observation that brooding polychaete species dominate the CO2 vent areas, along with evidence for physiological and genetic adaptation in vent-inhabiting Platynereis dumerilii6, prompted further examination of this particular species. To determine whether these adaptations have led to reproductive isolation, we attempted to crossbreed Platynereis individuals collected from within the vent sites with those collected from control sites outside the vent sites, in the laboratory.
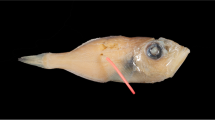
A male from the control population in the initial stages of transforming into a pelagic, swimming reproductive P. dumerilii was introduced into a container with an immature adult Platynereis sp. from the vent population. Within two hours, the male prompted this vent-originating worm to develop large yellow eggs, likely a pheromone-induced response between the two sexes10. These eggs filled the female body cavity and were five times larger than the average P. dumerilii eggs. The female proceeded to build a complex tube structure consisting of inner microtubes where she deposited large, fertilized eggs that immediately stopped developing (Fig. 1).
a. Initial cross-breeding activity with (top) Platynereis dumerilii male transforming into a pelagic, swimming epitoke full of sperm and (below) the Platynereis massiliensis female developing large yellow yolky eggs, (250 μm in diameter); b. Female inside tube laying and moving 74 eggs into inner brood tubes after 12 h of pairing with the male; c. Close-up of inner-parental mucus tubes holding large yellow eggs. Scale: 0.5 mm.
We matched the reproductive description of the female’s brooding behaviour to the parent’s genetic identities using a COI barcoding approach (Supplementary Methods). While the COI sequence of the pelagic form was only 0.7% different from the published sequence of P. dumerilii, the brooding form’s sequence was 26% different, indicating that it represents a separate species. Observational results confirm that the female found in the vents is actually Platynereis massiliensis (Moquin-Tandon, 1869), a sibling species of P. dumerilii11. These two sibling species are morphologically indistinguishable as immature adults but are easily discernible upon maturation, having evolved opposing reproduction modes with morphologically different gametes11,12. Platynereis massiliensis are protandric sequential hermaphrodites that first mature as males and fertilize a female partner’s eggs laid inside a brood tube. The female then dies and the male continues ventilating and protecting the developing embryos inside the tube as they develop into young worms11, after which the father changes sex and the process is repeated in the next reproductive event. Platynereis dumerilii have separate sexes and maturation invokes morphological changes allowing the benthic forms to leave their tubes and swarm in a single spawning event in the surface water. Adults swim to the surface, in synchronization with the full moon, in a pheromone-induced search for the opposite sex11,13. They then release their gametes and die. Fertilization occurs in the sea water and the larvae go through a subsequent six-week pelagic phase10.
Our COI analysis provides the first genetic record for P. massiliensis, as well as a genetic template to match previously sequenced individuals from both inside and outside the venting areas to their correct species identity. We did this using published sequence data from Calosi et al. (2013) for P. dumerilii. Results suggest that the vent site is dominated by brooding P. massiliensis (10:1 with P. dumerilii) and the control site is dominated by broadcasting P. dumerilii (15:1 with P. massiliensis), these differences being significant (Χ2: 9.808, p < 0.005). Additionally, we observed several mating pairs successfully producing juveniles inside their maternal tubes from P. massiliensis parents collected exclusively from the vent site.
It is not known what prompted speciation in these two species11. Existing ecological knowledge suggests that they have comparable sizes, habitats and functions and as such are overcoming similar mechanical, chemical and physical constraints11. Additionally, the known species ranges appear to overlap on a large spatial scale: ripe females and adult males of P. massiliensis have been found in the Gulf of Naples (Italy)12, Banyuls-Sur-Mer (France)11, on the Isle of Man coast (British Sea)14, in a Denmark fjord15 and in Norfolk (UK)16. Platynereis dumerilii is also found in these localities, however we are cautious to compare the species’ global distributions from current records, as observations are limited and not confirmed on a molecular basis17. Speciation may have been sympatric in the past (occurring in the same habitat), but the distribution of the brooding P. massiliensis in the localized venting area of this study clearly shows how this species favours this high CO2 habitat, whereas the sibling broadcasting P. dumerilii species does not. This pattern can be interpreted as a solid example of pH-driven brooding preference18.
Using the local distribution information of these congeners, we revisit the synthesis of life history strategies for the complete vent polychaete community and affirm that each dominant species exhibits parental care by a form of brooding or direct development (Table 1). The most parsimonious mechanism driving this trend appears to be that of the direct physical protection of early life stages from the water conditions19,20,21. Alternatively, or in part, this trend may be attributed to (1) an evolutionarily based selection for phenotypes tolerant to low pH among brooding species, (2) selection of traits associated with brooding; or (3) selection through some other vent characteristics besides low pH conditions. The possibility that these CO2-dominating brooding species have selected phenotypes tolerant to low pH is supported by the general ability of polychaetes to rapidly adapt to chronically disturbed habitats8,22. Furthermore, the traits commonly associated with brooders, such as short larval dispersal, continuous reproduction, in part through hermaphroditism and small adult sizes having smaller broods per reproductive event, support respective population’s survival by continuously selecting for fitness to a specific habitat8,23,24. Low pH habitat-based changes may be indirect factors influencing brooding preference as well4,9,25. For instance, habitat complexity and increased algal growth may cause a loss of brooder predators or competitors not as phenotypically plastic to CO2 stress, such as microbial shifts deterring pelagic larval recruitment26 Alternatively, a greater availability of sheltered habitat-based types of refugia and/or better food resources for brooding interstitial species living in the algae may occur27,28,29,30. The thirteen polycheate species in this study live in the low pH vent habitat and have many of these traits (Table 1), but further investigation of OA-mediated biological and ecological effects on species’ long-term OA tolerance is needed to distinguish the exact mechanisms responsible for low pH brooding dominance31,32.
These possibilities show that brooding and/or direct development may not be solely contingent on water chemistry, however the dominant species in this open ‘chemical island’ CO2 vent habitat do appear to be adapted to OA conditions in their reproductive and developmental modes. To broaden and further corroborate our evidence on a relationship between species life history strategy and tolerance to an important global change driver such as OA, we found examples in the literature from other polychaete worms, starfish, cowries and oysters, all following parallel adaptive pathways under climate and environmental-related stressors (Table 2). These species have been found inhabiting areas undergoing rapid environmental alterations and appear to have evolved direct development from broadcasting ancestors to enable them to counteract the detrimental effects of continuous disturbances. Many of these examples show species complexes in which broadcast spawning ancestors retain sensitivity to high CO2/low pH and other environmental extremes marked by their absence in disturbed sites, while species showing forms of parental care persist in the disturbed area33,34.
This multispecies comparative method substantiates the idea that today’s organisms exhibiting brooding or direct development may be more successful in responding to future OA than their pelagic broadcast spawning counterparts. One important consideration in this proposed response hinges on dispersal capacity and extinction of brooders in the future ocean. Brooding dispersal capacity is theoretically limited by low mobility of the early developmental phases, but existing evidence counter-intuitively indicate high dispersal ability in many brooder species35,36. The “Rockall paradox” reviews examples of such situations, where isolated islands are void of any pelagic broadcast spawning invertebrates. In these cases, it is noted that pelagic spawning parents assume a risk that their offspring will find suitable habitats for survival and reproduction. This strategy potentially presents difficulties, as pelagic larvae may not be able to find, settle and reproduce in distant places35. The possible link of these isolated islands to the “chemical island” of Ischia’s vents may be that pelagic larval settlement and recruitment success in acidified oceans is highly reduced4,5,7,26, supporting the hypothesis of direct developer pH tolerance. On the global scale of OA, pelagic larvae may be searching in vain for a ‘less acidified’ habitat that can retain a viable population base.
Current research on evolution and adaptation to OA is primarily focused on quantifying genetic variability of OA tolerant traits as an indicator of adaptive capacity into the expected future oceanic conditions37,38,39,40. Within this context, brooders may reach extinction far before their pelagic counterparts, as they typically hold lower genetic variability24. However, our evidence points to the opposite pattern. It would be worthwhile to investigate extinction risks of brooding and pelagic-developing species in the context of global OA at different spatial and temporal scales, in an attempt to constrain the effects of both exposure to ongoing global OA and local extreme events. In fact, while brooding-associated traits may be less advantageous under local extreme events, due to dispersal limitation on a short time scale – within a generation, they may actually prove to be more adaptive in a globally disturbed ocean (on a longer time scale: across multiple generations). Our polychaete-based analysis, supported by a selection of other invertebrate taxa, provides compelling comparative evolutionary-relevant evidence that direct developers/brooders may do better in the globally acidifying ocean than their relatives employing broadcast spawning and pelagic larval development. The general principle we present here will be useful to inform our capacity to identify which marine taxa will likely be more tolerant to ocean acidification, largely advancing our predictive ability on the fate of marine biodiversity simply based on an aspect of species’ life history strategies.
Methods for the sequencing procedure
DNA was extracted from two partial specimens of confirmed reproductive modes using the DNEasy Blood and Tissue Kit (Qiagen), following the manufacturer’s protocol. A ~600 base pair segment of the mitochondrial cytochrome c oxidase subunit I was amplified using universal primers41 for Platynereis massiliensis and polychaete-specific PolyLCO/Poly-HCO primers for P. dumerilii42. PCR products were cleaned with Exo-SapIT (Affymetrix). Cycle sequencing was performed using BigDye Terminator v 3.1 (Life Technologies). Sequences were cleaned using Zymo Research DNA Sequencing Clean-up Kit™. Sequences were analyzed in an ABI3130 Genetic Analyzer (Life Technologies) and edited in Sequencher v. 4.8 (Genecodes). Sequence alignment and calculation of Kimura 2-parameter genetic distances were conducted in MEGA 643. The sequences have been deposited in GenBank under accession numbers KP127953 (P. massiliensis) and KP127954 (P. dumerilii).
Additional Information
How to cite this article: Lucey, N. M. et al. To brood or not to brood: Are marine invertebrates that protect their offspring more resilient to ocean acidification?. Sci. Rep. 5, 12009; doi: 10.1038/srep12009 (2015).
References
Kroeker, K. J., Micheli, F., Gambi, M. C. & Martz, T. R. Divergent ecosystem responses within a benthic marine community to ocean acidification. Proc. Natl. Acad. Sci. USA 108, 14515–20 (2011).
IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. [ Stocker, T. F., Qin, D., Plattner, G. K., Tignor, M., Allen, S. K., Boschung, J., Nauels, A., Xia, Y., Bex, V. & Midgley, P. M. (eds.)] Cambridge University Press, Cambridge, United Kingdom and New York, N.Y, USA, 1535 pp. (2013).
Lombardi, C., Gambi, M. C., Vasapollo, C., Taylor, P. & Cocito, S. Skeletal alterations and polymorphism in a Mediterranean bryozoan at natural CO2 vents. Zoomorphology 135–145 (2011) 10.1007/s00435-011-0127-y.
Kroeker, K. J., Micheli, F. & Gambi, M. C. Ocean acidification causes ecosystem shifts via altered competitive interactions. Nat. Clim. Chang. 3, 156–159 (2013).
Cigliano, M., Gambi, M. C., Rodolfo-Metalpa, R., Patti, F. P. & Hall-Spencer, J. M. Effects of ocean acidification on invertebrate settlement at volcanic CO2 vents. Mar. Biol. 157, 2489–2502 (2010).
Calosi, P. et al. Adaptation and acclimatization to ocean acidification in marine ectotherms: an in situ transplant with polychaetes at a shallow CO2 vent system. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 368, (2013) org/10.1098/rstb.2012.0444.
Ricevuto, E., Kroeker, K. J., Ferrigno, F., Micheli, F. & Gambi, M. C. Spatio-temporal variability of polychaete colonization at volcanic CO2 vents indicates high tolerance to ocean acidification. Mar. Biol. (2014) 10.1007/s00227-014-2555-y.
Giangrande, A. Polychaete Reproductive Patterns, Life Cycles and Life Histories: An Overview. Oceanogr. Mar. Biol. An Annu. Rev. 35, 323–386 (1997).
Giangrande, A., Gambi, M. C., Micheli, F. & Kroeker, K. J. Fabriciidae (Annelida, Sabellida) from a naturally acidified coastal system (Italy) with description of two new species. J. Mar. Biol. Assoc. United Kingdom 1–11 (2014) 10.1017/S0025315414000678.
Fischer, A. & Dorresteijn, A. The polychaete Platynereis dumerilii (Annelida): a laboratory animal with spiralian cleavage, lifelong segment proliferation and a mixed benthic/pelagic life cycle. Bioessays 26, 314–25 (2004).
Schneider, S., Fischer, A. & Dorresteijn, A. A morphometric comparison of dissimilar early development in sibling species of Platynereis (Annelida, Polychaeta). Roux’s Arch. Dev. Biol. 201, 243–256 (1992).
Hauenschild, C. Nachweis der sogenannten atoken Geschlechtsform des Polychaeten Platynereis dumerilii Aud. et M. Edw. als eigene Art auf Grund von Zuchtversuchen. Nachdruck verboten. Ubersetzungsr. Vor. 107–128 (1951).
Fischer, A. H., Henrich, T. & Arendt, D. The normal development of Platynereis dumerilii (Nereididae, Annelida). Front. Zool. 7, 31 (2010).
Southward, E. C. On some polychaeta of the Isle of Man. Supplied by Br. Libr. ‘World’s Knowledge’ XXIX, 263 (1923).
Rasmussen, E. Systematics and ecology of the Isefjord marine fauna (Denmark). Ophelia 11, 78–80 (1973).
Hamond, R. The polychaeta of the coast of Norfolk. Cah. Biol. Mar. 1966, 383–436 (1960).
Fischer, G., Stuttgart, J. & Ulm, L. Annelida, Borstenwürmer, Polychaeta. 211–214 (1996).
Calosi, P. et al. Distribution of sea urchins living near shallow water CO2 vents is dependent upon species acid-base and ion-regulatory abilities. Mar. Pollut. Bull. 73, 470–84 (2013).
Dupont, S., Lundve, B. & Thorndyke, M. Near Future Ocean Acidification Increases Growth Rate of the Lecithotrophic Larvae and Juveniles of the Sea Star Crossaster papposus. J. Exp. Zool. (Mol. Dev. Evol.) 314B, 382–389 (2010).
Melzner, F. et al. Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6, 2313–2331 (2009).
Kurihara, H. Effects of CO2 -driven ocean acidification on the early developmental stages of invertebrates. Mar. Ecol. Prog. Ser. 373, 275–284 (2008).
Wilson, H. W. Sexual Reproductive Modes in Polychaetes: Classification and Diversity. Bull. Mar. Sci. 48, 500–516 (1991).
Strathmann, R. R. & Strathmann, M. F. The Relationship Between Adult Size and Brooding in Marine Invertebrates. Am. Nat. 119, 91–101 (1982).
Strathmann, R. R. The Spread of Sibling Larvae of Sedentary Marine Invertebrates. Am. Nat. 108, 29–44 (1974).
Fabricius, K. E., De’ath, G., Noonan, S. & Uthicke, S. Ecological effects of ocean acidification and habitat complexity on reef-associated macroinvertebrate communities. Proc. R. Soc. B Biol. Sci. 281, (2014) org/10.1098/rspb.2013.2479.
Connell, S. D., Kroeker, K. J., Fabricius, K. E., Kline, D. I. & Russell, B. D. The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 368, (2013) org/10.1098/rstb.2012.0442.
Porzio, L., Buia, M. C., & Hall-Spencer, J. M. Effects of ocean acidification on macroalgal communities. J. Exp. Mar. Bio. Ecol. 400, 278–287 (2011).
Rouse, G. & Fitzhugh, K. Broadcasting fables: Is external fertilization really primitive? Sex, size and larvae in sabellid polychaetes. Zool. Scr. 23, 271–312 (1994).
Worsaae, K. & Kristensen, R. M. Evolution of interstitial Polychaeta (Annelida). Hydrobiologia 535, 319–340 (2005).
Westheide, W. Polychaetes: Interstitial families. Synopsis of the British Fauna (New Series). 1–152. Universal Book Services/Dr W. Backhuys, Oegstgeest (1990).
Dupont, S. & Thorndyke, M. C. Impact of CO2 -driven ocean acidification on invertebrates early life-history – What we know, what we need to know and what we can do. Biogeosciences Discuss. 6, 3109–3131 (2009) 10.1098/rspb.2008.1935.
Byrne, M. Impact of ocean warming and ocean acidification on marine invertebrate life history stages: Vulnerabilities and potential for persistence in a changing ocean. Ocean. Mar. Biol. Annu. Rev. 49, 1–42 (2011).
Gray, J. S. Pollution-induced changes in populations. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 286, 545–561 (1979).
Åkesson, B. Reproduction and Larval Morphology of Five Ophryotrocha Species (Polychaeta, Dorvilleidae). Zool. Scr. 2, 145–155 (1973).
Johannesson, K. The paradox of Rockall: why is a brooding gastropod (Littorina saxatilis) more widespread than one having a planktonic larval dispersal stage (L. littorea)? Mar. Biol. 99, 507–513 (1988).
Levin, L. A. Life History and Dispersal Patterns in a Dense Infaunal Polychaete Assemblage: Community Structure and Response to Disturbance. Ecology 65, 1185–1200 (1984).
Kelly, M. W., Padilla-Gamiño, J. L. & Hofmann, G. E. Natural variation and the capacity to adapt to ocean acidification in the keystone sea urchin Strongylocentrotus purpuratus. Glob. Chang. Biol. (2013) 10.1111/gcb.12251.
Kelly, M. W. & Hofmann, G. E. Adaptation and the physiology of ocean acidification. Funct. Ecol. (2012) 10.1111/j.1365-2435.2012.02061.x.
Sunday, J. M., Crim, R. N., Harley, C. D. G. & Hart, M. W. Quantifying rates of evolutionary adaptation in response to ocean acidification. PLoS One 6, e22881 (2011).
Sunday, J. M. et al. Evolution in an acidifying ocean. Trends Ecol. Evol. 1–9 (2013) 10.1016/j.tree.2013.11.001.
Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299 (1994).
Carr, C. M., Hardy, S. M., Brown, T. M., Macdonald, T. A. & Hebert, P. D. N. A tri-oceanic perspective: DNA barcoding reveals geographic structure and cryptic diversity in Canadian polychaetes. PLoS One 6, (2011).
Kumar, S., Tamura, K. & Nei, M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief. Bioinform. 5, 150–63 (2004).
Rouse, G. W. & Gambi, M. C. Evolution of reproductive features and larval development in the genus Amphiglena (Polychaeta: Sabellidae). Mar. Biol. 131, 743–754 (1998).
Giangrande, A., Quarta, S. & Caroppo, C. Observations of Spio decoratus Polychaeta Spionidae life history under laboratory conditions, with taxonomic considerations. Oebalia 18, 83–93 (1992).
Kupriyanova, E. K., Nishi, E., Harry A. Ten Hove & Rzhavsky, A. V. Life-History Patterns in Serpulimorph Polychaetes: Ecological and Evolutionary Perspectives. Oceanogr. Mar. Biol. an Annu. Rev. 39, 1–100 (2001).
Mastrodonato, M. et al. External gestation of Exogone naidina Öersted, 1845 (Polychaeta, Syllidae): ventral attachment of eggs and embryos. Tissue Cell 35, 297–305 (2003).
Franke, H. Reproduction of the Syllidae (Annelida: Polychaeta). Hydrobiologia 402, 39–55 (1999).
Meyer, C. P. Molecular systematics of cowries (Gastropoda: Cypraeidae) and diversification patterns in the tropics. Biol. J. Linn. Soc. 79, 401–459 (2003).
Chaparro, O. R., Montory, J. A., Segura, C. J. & Pechenik, J. A. Effect of reduced pH on shells of brooded veligers in the estuarine bivalve Ostrea chilensis Philippi 1845. J. Exp. Mar. Bio. Ecol. 377, 107–112 (2009).
Chaparro, O. R., Charpentier, J. L. & Collin, R. Embryonic Velar Structure and Function of Two Sibling Species of Crepidula With Different Modes of Development. 203, 80–86 (2002).
Hettinger, A. et al. The influence of food supply on the response of Olympia oyster larvae to ocean acidification. Biogeosciences 10, 6629–6638 (2013).
Puritz, J. B. et al. Extraordinarily rapid life-history divergence between Cryptasterina sea star species. Proc. Biol. Sci. 279, 3914–22 (2012).
Byrne, M. Viviparity in the sea star Cryptasterina hystera (Asterinidae) -conserved and modified features in reproduction and development. Biol. Bull. 208, 81–91 (2005).
Noisette, F. et al. Does encapsulation protect embryos from the effects of ocean acidification? The example of Crepidula fornicata. PLoS One 9, e93021 (2014).
Grassle, J. P. & Grassle, F. Sibling Species in the Marine Pollution Indicator Capitella (Polychaeta). Science 192, 567–569 (1976).
Grassle, J. F. & Grassle, J. P. Temporal Adaptations in Sibling Species of Capetella, in Ecology of Marine Benthos, Coull, B. C. Ed., Columbia University of South Carolina Press, Columbia, SC 177–189 (1977).
Garaffo, G. V. et al. Sewage-induced polychaete reefs in a SW Atlantic shore: rapid response to small-scale disturbance. Mar. Ecol. 33, 272–279 (2012).
Acknowledgements
NML was supported by a MARES PhD scholarship (FPA 2011-0016). The UKOA Research Programme Benthic Consortium # (NE/H017127/1) and the NSERC Discovery Programme supported PC. We thank Massa–Gallucci A. and Valvassori G. for observations of brooding P. massiliensis specimens from the venting site and Suaria G. for helpful discussions and revisions.
Author information
Authors and Affiliations
Contributions
1) N.M.L. conceived the idea; 2) All co-authors planned the project/methods; 3) N.M.L., L.D. and A.S. carried out the experiment/sequencing; 4) N.M.L. interpreted the results with support from M.C.G., A.S. and P.C.; 5) N.M.L. wrote the first draft of this MS and all co-authors contributed to the writing of the finalised version.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lucey, N., Lombardi, C., DeMarchi, L. et al. To brood or not to brood: Are marine invertebrates that protect their offspring more resilient to ocean acidification?. Sci Rep 5, 12009 (2015). https://doi.org/10.1038/srep12009
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep12009
This article is cited by
-
Microbial communities in developmental stages of lucinid bivalves
ISME Communications (2022)
-
Systematic review of Neanthes Kinberg, 1865 (Annelida: Errantia: Nereididae) from southern Africa, including a preliminary molecular phylogeny of the genus
Marine Biodiversity (2022)
-
The Nereid on the rise: Platynereis as a model system
EvoDevo (2021)
-
Temperature and salinity, not acidification, predict near-future larval growth and larval habitat suitability of Olympia oysters in the Salish Sea
Scientific Reports (2020)
-
Spermcast mating with release of zygotes in the small dioecious bivalve Digitaria digitaria
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.