Abstract
Peptidoglycan (PGN) is ubiquitous in nearly all bacterial species. The PGN sacculus protects the cells against their own internal turgor making PGN one of the most important targets for antibacterial treatment. Within the last sixty years PGN composition has been intensively studied by various methods. The breakthrough was the application of HPLC technology on the analysis of muropeptides. However, preparation of pure PGN relied on a very time consuming method of about one week. We established a purification protocol for both Gram-positive and Gram-negative bacteria which can be completely performed in plastic reaction tubes yielding pure muropeptides within 24 hours. The muropeptides can be analyzed by UPLC-MS, allowing their immediate determination. This new rapid method provides the feasibility to screen PGN composition even in high throughput, making it a highly useful tool for basic research as well as for the pharmaceutical industry.
Similar content being viewed by others
Introduction
The bacterial cell wall is mainly composed of peptidoglycan (PGN) with attached proteins and modifications like wall teichoic acids (WTAs). PGN is a rigid structure of alternating N-Acetylglucosamine-N-Acetylmuramic acid (GlcNAc-MurNAc) glycan chains cross-linked by peptides. The peptide moiety consists of a stem peptide (L-Alanine – D-iGlutamate/iGlutamine – L-Lysin/meso-Diaminopimelic acid – D-Alanine – D-Alanine). L-Lysin (L-Lys) is mainly a feature of Gram-positives, while meso-Diaminopimelic acid (mDAP) is typical for Gram-negatives as well as for Bacillus subtilis. We will abbreviate the second amino acid of the stem peptide by Glx which is either glutamine (Gln) or glutamate (Glu). Our model organism S. aureus harbors also a five-glycine (Gly5) interpeptide bridge branching from the L-lysine (Lys), which constitutes indirect cross-links between two adjacent stem peptides1. The resulting macromolecule forms a bag shaped sacculus covering the whole bacterial cell. It is unique to bacteria ensuring their shape and protecting them against their own internal turgor. Loss of its integrity results in cell lysis, making PGN one of the most important targets for antibacterial treatment. Indeed, it is the target for well-known therapeutic drugs such as β-lactams or vancomycin, which all inhibit the late steps of glycan chain polymerization and/or cross-linking, both occurring outside the cytoplasmic membrane. With the emergence of new and highly multi-resistant bacterial strains, we are in an urgent need of new anti-microbial drugs. Therefore, it is, amongst others, important to understand the complex interplay between the PGN structure, (changes in) its chemical composition and also subsequent immune responses.
Since its first isolation in 1951 by Salton and Horne2 PGN composition has been intensively studied with various methods. The original determination of the glycan and peptide composition was performed by paper chromatography1. The breakthrough was by Glauner in 19883, who for the first time applied high pressure liquid chromatography (HPLC) technology on the analysis of muropeptides. Muropeptides result from enzymatic digestion of the glycan strands into disaccharides, with part of them still being cross-linked by peptides to various extensions. Glauner extensively studied the effects of pH, buffer concentrations and temperature on HPLC separation of muropeptides of the Gram-negative bacterium E. coli. Later, a protocol for PGN isolation and HPLC analysis of the Gram-positive Staphylococcus aureus was established by deJonge et al.4. Basically, these two protocols have been used ever since. However, both of them are very time consuming taking about a week to obtain pure PGN. Both protocols rely on multiple boiling steps with sodiumdodecylsulfate (SDS), which has to be washed out by extensive ultracentrifugation steps for about an hour each. Last year a protocol for isolation of E. coli PGN for ultra-performance liquid chromatography (UPLC) analysis was reported, that is substantially shorter5. However, it still relies on washing in an ultracentrifuge thereby limiting the amounts of samples that can be prepared in parallel.
PGN research has experienced a revival within the last decade. Even PGN from bacteria like Chlamydiae, which had long been thought to not contain a PGN sacculus, was now successfully isolated and analyzed6. In addition, it had been shown, that growth conditions7,8 as well as various antibiotics as β-lactams and daptomycin affect PGN composition9,10,11. To test PGN composition of different bacteria and under various conditions, a faster analysis method was needed. One which allows to test several conditions in parallel and which can also be applied to bacteria that are not easily grown in high amounts.
We established a purification protocol which can be completely performed in 2 ml reaction tubes, allowing the isolation of up to 48 samples in parallel or even in 96 well plates, yielding pure muropeptides in 24 hours. The muropeptides can be subsequently analyzed by UPLC. This reduces the amount of sample needed and the analysis time from about three hours (HPLC) to 70 min per sample. In addition, the LC conditions were adapted to mass spectrometry (MS) with suitable solvents so that the whole PGN can be directly analyzed by UPLC-MS. This allows for muropeptide determination without first collecting and desalting LC peaks and results in a complete PGN mapping. Taken together, the whole procedure is reduced from about two weeks to 24 hours. Furthermore, this fast isolation method also makes it much easier to obtain larger amounts of PGN which can be used to collect special muropeptides that can then be tested for their ability to stimulate the immune system12.
Results and Discussion
Peptidoglycan isolation
All steps are performed in 2 ml reaction tubes with a U-shaped bottom. A detailed step-to-step protocol is given in the online method. The method is based on the isolation of PGN by boiling in 1 M NaCl. In the case of some Gram-positive strains we experienced that NaCl treatment is not sufficient. A 0.25% sodium dodecylsulfate (SDS) solution in 0.1 M Tris/HCl (pH 6.8) was then used instead. The SDS has to be washed out again thoroughly. A test for residual SDS is available and should be performed13. The resulting cell walls are washed with water and broken into smaller fragments by sonication. DNAse, RNAse and trypsin are used to digest residual nucleic acids as well as cell wall bound proteins. The enzymes are inactivated by boiling and the cell walls are washed again with water. Treatment with 1 N HCl releases bound wall teichoic acids (WTA) or other glycophosphates. Higher concentrations of HCl must be avoided, as they result in clumping of the sample. In former protocols4, 48% HF was used to release WTA, but comparison of PGN treated with HCl or with HF showed no significant differences (see Fig. S1). Washing the pellet until the pH is neutral results in pure PGN that can be digested by cell wall hydrolases. In our case the PGN was digested with mutanolysin for 16 hours at 37°C shaking.
Peptidoglycan analysis by UPLC
Prior to UPLC analysis the MurNAc residues of the muropeptides are reduced into NAc-muraminitol by sodium borohydrate (NaBH4). Otherwise, mutarotation of the non-reduced glycan end would result in double peaks. Analysis is performed by UPLC (or HPLC) using trifluoracetic acid (TFA) and a methanol gradient from 5 to 30% (for Gram-positives) or from 0 to 30% (for Gram-negatives) in 70 min. TFA is an ion pairing agent that forms an ion bond with the muropeptides resulting in a good separation of mixed analyts. As TFA is volatile it can also be used for MS analysis. However, it reduces the sensitivity of the mass spectrometer which might require extra washing after use. After testing several different columns, the reversed phase UPLC column CSH C18, 130Å, 1.7 µm, 2.1 mm × 100 mm of Waters was best suited for our needs.
Peptidoglycan mapping by UPLC-MS
The samples can also be directly analyzed by UPLC-MS. We used a Synapt G2 mass spectrometer coupled to a Waters Acquity H-class and operated in positive ESI mode with a scan range from 50–2,000. The data was analyzed by MassLynx. As an example for PGN isolation by this new method, UPLC analysis and MS-mapping are presented in Fig. 1 and Fig. 2. For Gram-positives we used Staphylococcus aureus (S. aureus) SA11314 and for Gram-negatives Escherichia coli (E. coli) Nissle 191715.
Muropeptide profile of S. aureus SA113 obtained by UPLC and UPLC/MS.
(a) Muropeptide profile of S. aureus SA113 obtained by reversed phase UPLC. (b) TIC of UPLC/MS analysis of S. aureus SA113 obtained by reversed-phase UPLC coupled to MS. Masses of indicated peaks are shown in table 1 and table S1 including molecule composition and proposed sum formula.
Muropeptide profile of E. coli Nissle 1917 obtained by UPLC and UPLC/MS.
(a)Muropeptide profile of E. coli Nissle 1917 obtained by reversed phase UPLC. (b) TIC of UPLC/MS analysis of E. coli Nissle 1917 obtained by reversed-phase UPLC coupled to MS. Masses of indicated peaks are shown in table 2 and table S2 including molecule composition and proposed sum formula.
Exemplary analysis of S. aureus SA113 by UPLC and UPLC-MS
The methicillin sensitive S. aureus strain SA113 was grown over night in BM medium. We isolated its PGN and analyzed it by UPLC as well as by UPLC-MS. This enabled us to directly determine the masses of all muropeptide peaks we got by UPLC separation. The total ion current (TIC) chromatogram obtained by UPLC-MS was almost identical to the UV pattern of UPLC alone with two exceptions: 1) Retention time of the peaks in the quaternary UPLC was about 1 min longer than in the binary UPLC-MS system. Therefore, in tables 1 and S1 only the TIC retention times are given. 2) The signal of some muropeptides, that turned out to be O-acetylated, were stronger in the TIC than in the UV (e.g. peaks 9 in Fig. 1a and b).
The UV chromatogram of the UPLC as well as the TIC chromatogram of the MS show the expected pattern as it had been published before for the methicillin resistant S. aureus COL by de Jonge et al. in 19924,16. By coupling UPLC to MS we could directly determine the masses and subsequently the potential structures of almost all peaks. The obtained masses verified that the main peaks contained the expected muropeptides starting with the monomeric peak 5 (Penta(Gln)Gly5) which is the basic structure of all muropeptides. Peaks 11, 15, 16, 17 and 18 were composed of this basic structure cross-linked by the Gly5 interpeptide bridge into dimers, trimers, tetramers, pentamers and hexamers, respectively (for general structures see Fig. 3). This proved that our substantially shortened purification method as well as the adaption of UPLC-MS is working properly. Tab. 1 gives an overview on the muropeptides of the main peaks that we detected in our analysis which had already been determined before4,17. The complete results of our PGN mapping of S. aureus SA113 is given in Tab. S1. All structures were drawn by ChemDrawUltra 13.0 (PerkinElmer), which automatically calculated the mass of the molecule and the proposed sum formula. The latter is given. As can be judged by the peak forms in the UPLC chromatogram, most peaks are composed of several muropeptides. While all of them have the same basic structure (MurNac-GlcNac with stem peptide and Gly5 interpeptide bridge), several modifications occur, which will be discussed in detail:
Schematic structure of muropeptides of S. aureus and E. coli.
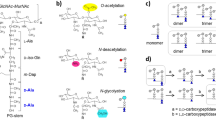
The basic structure consists of the disaccharide (GlcNAc-MurNAc) with an adjacent stem peptide (L-Ala – D-iGlx – L-Lys – D-Ala – D-Ala) with Glx being either Gln or Glu. Our model organism S. aureus, harbors also a five-glycine interpeptide bridge branching from the L-Lys, which constitutes indirect cross-links between two adjacent stem peptides, as it is depicted in the multimeric muropeptide. The C at position 6 of the MurNAc can be O-acetylated.
The lengths of the stem peptides are variable. Monomeric stem peptides of S. aureus are mostly composed of the following five amino acids (L-Ala – D-iGln – L-Lys– D-Ala – D-Ala) with the D-iGln resulting from the amidation of the original D-iGlu18,19. These structures are called “Pentas”. Cross-linking of several muropeptides by their interpeptide bridges forms multimeric muropeptides with normally one Penta and several Tetras, as the last D-Ala is cleaved off during the transpeptidation reaction. The resulting muropeptides are called “Penta-Tetran”. However, several of the higher cross-linked muropeptides had lost the last D-Ala on the Penta stem peptide shortening it to a Tetra, forming “Tetran” muropeptides. No further degradation into tripeptides was observed suggesting that S. aureus does not possess an L,D-carboxypeptidase activity.
In S. aureus, the D-iGlu at position two of the stem peptide is almost quantitatively amidated resulting in D-iGln18,19. However, some muropeptides still harbor the original D-iGlu. While these non-amidated forms have been published to have a shorter retention time than their amidated counterparts18, in our system they had longer retention times. This might be due to the different column used for analysis.
The length and the composition of the interpeptide bridge varies. Gly numbers that are not a multiple of 5 indicate that this very muropeptide was originally part of a bigger molecule but had been reorganized within the PGN sacculus. For example we found monomeric muropeptides with 6, 7, 8 or 9 Gly residues instead of 5 and dimeric muropeptides with 6, 7, 8 or 9 Gly residues instead of 10. In Tab. 1 and Tab. S1 the sum of all Gly residues of each muropeptide is given. In addition, two muropeptides contained Ala in exchange for one Gly in the interpeptide bridge. Muropeptides harboring various numbers of Gly residues or Ala in their interpeptide bridge have been published before4.
There also occur changes in the saccharide moiety. Several muropeptides had lost one GlcNAc residue. In rare cases we also found an additional GlcNAc residue, which resulted in a muropeptide with a trisaccharide, but we did not observe the addition of an extra MurNac. In table S1 the addition or loss of a GlcNAc residue is given as +1 or −1 respectively.
Even though O-acetylation had been thought to be lost during acidic treatment, we detected that most of the small peak groups, as well as some of the peak shoulders, both of which had not been analyzed so far, were composed of acetylated muropeptides. Even treatment with HF did not result in a loss of the O-acetylated muropeptides (see Fig. S1). As an example, peak 9 contained only the O-acetylated monomer Penta-Gly5. If HF caused de-O-acetylation, this peak should be missing in Fig. S1B, but it is not. The PGN of pathogenic staphylococcal strains is highly O-acetylated on the 6th C-atom of the MurNAc, which renders the bacterium resistant against lysozyme20. Lysozyme is an enzyme produced by humans and animals as a defense mechanism. It hydrolyzes PGN and therefore kills bacteria. Even though we cannot exclude that part of the O-acetylation is lost during isolation, a change of the respective peaks would be an indication for changes in the O-acetylation grade and could be useful to screen for possible mutants or for drugs affecting O-acetylation.
A very small amount of MurNac residues seemed not to be completely reduced to NAc-muraminitol. This also resulted in longer retention times compared to the reduced counterparts.
Exemplary analysis of E. coli Nissle 1917 by UPLC and UPLC-MS
As an example for Gram-negative bacteria we grew E. coli Nissle 1917 overnight in LB medium and isolated its PGN. The PGN was digested by mutanolysin and analyzed by UPLC and UPLC-MS (Fig. 2). Again, the UV pattern was very similar to the TIC chromatogram and we could determine the masses of most muropeptide peaks. The masses we found resulted in proposed muropeptide structures that are in accordance with the ones previously published by Glauner3 (Tab. 2). The complete analysis is given in Tab. S2.
We found the expected monomeric muropeptides with the Tri and the Tetra stem-peptide (peak 1 and 4, respectively), the dimeric muropeptides Tetra-Tri and Tetra-Tetra (peaks 7 and 8, respectively) as well as in small amounts the trimeric muropeptide Tetra-Tetra-Tetra (peak 10). (For a schematic drawing of the Gram-negative muropeptides see Fig. 3b). Interestingly, for the Tetra, the Tetra-Tri and the Tetra-Tetra, we always got two peaks in the TIC chromatogram but only one in the UV chromatogram. We fragmented the respective peaks and they seem to be stereo-isomers as in all three cases closely related fragmentation spectra with different relative fragment peak intensities were obtained. Fig. S2 gives the exemplary fragmentation of peaks 8a and 8b, both being the Tetra-Tetra muropeptide. We did not get any hints for mDap-mDap-crosslinking, as it had been seen in strain E. coli W73.
The monomeric peak 3 in the TIC chromatogram is not in correlation with any peak of the UV chromatogram. Vice versa, the UV chromatogram has two unlabeled peaks, for which we cannot assign a muropeptide. Peak 3 of the TIC chromatogram contains 4 muropeptides (Tab. S2) with two of them being already known from the Glauner analysis3, the Di and the Penta-Gly(5) monomer. While these two peaks in the Glauner analysis have a higher retention time than the Tetra, they have a shorter retention time when analyzed with our conditions. The same is seen for Tetra-Penta-Gly(5) (peak 6) which in our system elutes before the Tetra-Tri and not after it.
With these few exceptions the elution profile of the E. coli muropeptides under our conditions is the same as already known. We also found one anhydro Tetra-Tetra muropeptide (peak 11), but we did not find any structures still containing lysine and arginine, which would be remnants from Braun's Lipoprotein21. Instead we found masses corresponding to muropeptides which had lost the GlcNAc moiety (Tab. S2). These are probably features of the strain we used. As expected, in all muropeptides with a Penta stem-peptide, the amino acid in position five is a Gly, as the last D-Ala is cleaved off by the D,D-carboxypeptidase penicillin-binding protein (PBP) 522. We did obtain masses for the peaks between minutes 14 and 21, but they did not fit any muropeptide structures and are therefore not given.
Conclusion
We have presented here a very quick method for PGN isolation with additional analysis on UPLC-MS all within 24 hours. This method is suitable for high throughput screening of various bacterial strains or growth conditions, as it is performed in either 2 ml plastic reaction tubes or even in microtiter plates. With this new procedure we mapped the whole PGN of S. aureus SA113 up to the hexameric cross-linked muropeptide. In addition to the already known muropeptide structures of the prevalent peaks we have also presented masses and structures for the so far unidentified smaller peak groups in between. As an example for Gram-negatives we analyzed the PGN of E. coli Nissle 1917 and corroborated former publications.
Methods
The protocols will also be provided at www.nature.com/protocolexchange.
Bacterial strains
S. aureus SA11314 and E. coli Nissle 191715.
Cell growth
The cells were grown in their respective medium to the needed OD. Basic medium (BM) for S. aureus consisted of Soy Peptone (10 g; Plato), Yeast Extract (5 g; Deutsche Hefewerke), NaCl (5 g; Carl-Roth), Glucose (1 g; Carl Roth) and K2HPO4 (1 g; Applichem). Deionized water was added to a final volume of 1 liter and pH was adjust to 7.2. LB medium for E. coli consisted of Peptone (10 g; Plato), Yeast Extract (5 g; Deutsche Hefewerke) and NaCl (5 g; Carl Roth). Deionized water was added to a final volume of 1 liter and pH was adjust to 7.2.
Reagents
Unless otherwise stated, all reagents were bought from Sigma-Aldrich.
Midipreparation for UPLC/MS or HPLC/MS analysis
This protocol results in sample amounts suitable for several analyses. From 2 ml of culture of OD578 ≈ 10 about 300 µl purified PGN are gained. Spin down 2 ml of an overnight culture in a 2 ml microcentrifuge tube (Eppendorf) for 5 min at 10,000 rpm. Alternatively: Spin down 2 × 2 ml of a culture with a lower OD. Resuspend the pellet in 1 ml solution A (1 M sodium chloride) and boil the suspension for 20 minutes at 100°C in a heating block. [Δ CRITICAL STEP 1: Sometimes, NaCl treatment is not sufficient for peptidoglycan isolation from the cells. Use 0.25% SDS solution in 0.1 M Tris/HCl (pH 6.8) instead. SDS has to be washed out thoroughly after boiling. Make sure the samples are boiling at 100°C. Bad isolation results are mostly caused by too low heat.] Spin down the suspension (5 min at 10,000 rpm), wash it at least twice with 1.5 ml ddH2O and resuspend the pellet in 1 ml ddH2O. Put the sample to a sonifier waterbath for 30 minutes. Add 500 µl of solution B (15 μg/ml DNase and 60 μg/ml RNase in 0.1 M TRIS/HCl, pH 6.8) and incubate for 60 minutes at 37°C in a shaker. Add 500 µl of solution C (50 μg/ml trypsin in ddH2O) and incubate for additional 60 minutes at the same conditions. To inactivate the enzymes boil the suspension for 3 minutes at 100°C in a heating block, then spin the sample down (5 min at 10,000 rpm) and wash it once with 1 ml ddH2O. To release WTA resuspend the pellet in 500 µl of 1 M HCl (ready-to-use solution from Applichem) and incubate for 4 h at 37°C in a shaker. Spin down the suspension (5 min at 10,000 rpm) and wash with ddH2O until the pH is 5–6. Afterwards, resuspend the pellet in 100–250 µl digestion buffer (12.5 mM sodium dihydrogen-phosphate, pH 5.5) to an OD578 of 3.0 and add 1/10 volume of mutanolysin solution (5.000 U/ml of mutanolysin in ddH2O). [Δ CRITICAL STEP 2: If OD578 is too high the sample is too concentrated. Therefore, the digestion with mutanolysin might be disturbed. Measurement of OD is tricky, because peptidoglycan sinks to the bottom. Mix the suspension carefully with a pipette and measure OD rapidly.] Then incubate the sample for 16 h at 37°C (150 rpm shaking). Inactivate mutanolysin by boiling (100°C) for 3 min. Spin the sample down (5 min at 10,000 rpm) and use the supernatant. Before applying the sample to the UPLC system, MurNAc has to be reduced to NAc-muraminitol. Therefore, add 50 µl of reduction solution (10 mg/ml sodium borohydrate in 0.5 M borax in ddH2O at pH 9.0; both reagents were purchased from Merck) and incubate the sample for 20 minutes at room temperature. Stop the reaction with 10 µl phosphoric acid (98%). The resulting pH must be between 2 and 3. Then analyze the sample by UPLC/MS or HPLC/MS.
Minipreparation in 96 well plate for UPLC/MS analysis
This protocol gives just enough material for one sample for UPLC/MS analysis. For different growth parameters, use a divisible 96 well plate. Use a multichannel pipette for all resuspension steps. Always cover the samples properly with a foil (Greiner) or lid to avoid evaporation or mixing of samples!
Always use a 96 well-plate with U-shaped bottom (Greiner). Spin down 200 µl of the culture for 10 min at 4,700 rpm in a plate centrifuge. Resuspend each pellet in 200 µl solution A (1 M sodium chloride) and boil the suspension for 30 minutes at 100°C in a heating block. [Δ CRITICAL STEP 1: Sometimes, NaCl treatment is not sufficient for peptidoglycan isolation from the cells. Use 0.25% SDS solution in 0.1 M Tris/HCl (pH 6.8) instead. SDS has to be washed out thoroughly after boiling.] The plate must be covered with foil. Make sure the samples are boiling at 100°C. Bad isolation results are mostly caused by too low heat. Spin down the suspension (10 min at 4,700 rpm with foil coverage or lid) and wash at least twice with 200 µl ddH2O. Afterwards, resuspend each pellet in 150 µl ddH2O. Close the wells carefully with a foil coverage (NOT lid), so that no water can ingress to the sample. Place the plate to a sonifier waterbath for 30 minutes (plate floats). Spin down the suspension (10 min at 4,700 rpm) and resuspend each pellet in 100 µl of solution B (15 μg/ml DNase and 60 μg/ml RNase in 0.1 M TRIS/HCl, pH 6.8). Properly close plate with foil coverage and incubate for 60 minutes at 37°C in a shaker at 150 rpm. It is better to place samples at the edge of the shaker than in the middle. Add 100 µl of solution C (50 μg/ml trypsin in ddH2O), properly close plate with foil coverage and incubate for another 60 minutes at 37°C in a shaker at 150 rpm. To inactivate the enzymes boil the suspension for 5 minutes at 100°C. Spin down the suspension (10 min at 4,700 rpm) and wash each pellet once with 200 µl ddH2O. To release WTA resuspend each pellet in 200 µl of 1 M HCl (ready-to-use solution from Applichem) and incubate for 4 h at 37°C in a shaker (150 rpm, with foil coverage). Spin down the suspension (10 min at 4,700 rpm) and wash pellets with ddH2O until the pH is 5–6. Resuspend each pellet in 50 µl digestion buffer (12.5 mM sodium dihydrogen-phosphate, pH 5.5) and add 5 µl mutanolysin solution (5.000 U/ml of mutanolysin in ddH2O). Incubate the well plate for 16 h at 37°C (at 150 rpm, with foil coverage). Boil the samples on the heating block (100°C) for 5 minutes to inactivate mutanolysin. Afterwards spin the plate down (10 min at 4,700 rpm) and use the supernatant. Before applying the samples to the UPLC system, MurNAc has to be reduced to NAc-muraminitol. Add 10 µl of the reduction solution (10 mg/ml sodium borohydrate in 0.5 M borax in ddH2O at pH 9.0; both reagents were purchased from Merck) to each sample and incubate for 20 min at room temperature. [Δ CRITICAL STEP 2: The reduction solution contains a lot of bubbles. Transfer of the exact volume of 10 µl reduction solution is not possible. Set your pipette to a volume of 100 µl and add 1 drop to each sample. This accords to the needed volume.] Stop the reaction with 5 µl phosphoric acid (50%). The pH must be between 2 and 3 (add phosphoric acid very slow and carefully!). Then analyze the samples by UPLC or UPLC/MS.
UPLC/MS ANALYSIS
An Acquity UPLC was coupled to a SynaptG2 mass spectrometer (both Waters). We used a C18 CSH 130Å, 1.7 µm, 2.1 mm × 100 mm column with the respective guard column (C18 CSH 130Å, 1.7 µm, 2.1 mm × 5 mm) available at Waters. As MS standard L-Enk was used.
The column temperature was 52°C and the injection volume was 10 µl with no loop overfill. Muropeptides were detected at 210 nm (DAD) or by MS. The MS was set to positive ESI mode with a scan range from 50–2,000. The capillary voltage was 3 kV. The sampling cone was set to 30 and the extraction cone to 3.0. Source temperature was set to 120°C and desolvation temperature to 450°C. The flow of the cone gas was 10 l/h and of the desolvation gas 800 l/h.
For muropeptide separation by UPLC/MS we used solvent A (0.1% TFA in 5% methanol; for Gram-negatives omit methanol) and solvent B (0.1% TFA in 30% methanol). TFA (trifluoroacetic acid) was bought from Carl Roth, methanol (UV grade) from Sigma-Aldrich. We applied a flow rate of 0.176 ml/min starting with 100% solvent A for 1 min. Afterwards, a linear gradient was run in 59 min to 100% solvent B. The post run was 5 min with 100% solvent B and additional 10 min with 100% solvent A for re-equilibration. [Δ CRITICAL STEP: A long and intensive equilibration of the column and an exact column temperature is very important. Wash the column 30 min with methanol, 30 min with ddH2O water, 30 min with solvent B and 30 min with solvent A to a steady baseline. Degassed solvents should be self-evident.]
References
Schleifer, K. H. & Kandler, O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev 36, 407–477 (1972).
Salton, M. R. J. & Horne, R. W. Studies of the bacterial cell wall. 2. Methods of preparation and some properties of cell walls. Biochimica et Biophysica Acta 7, 177–197 (1951).
Glauner, B. Separation and quantification of muropeptides with high-performance liquid chromatography. Anal Biochem 172, 451–464 (1988).
de Jonge, B. L., Chang, Y. S., Gage, D. & Tomasz, A. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2A. J Biol Chem 267, 11248–11254 (1992).
Desmarais, S. M., Cava, F., de Pedro, M. A. & Huang, K. C. Isolation and Preparation of Bacterial Cell Walls for Compositional Analysis by Ultra Performance Liquid Chromatography. JoVE e51183, 10.3791/51183 (2014).
Pilhofer, M. et al. Discovery of chlamydial peptidoglycan reveals bacteria with murein sacculi but without FtsZ. Nat Commun 4, 2856, 10.1038/ncomms3856 (2013).
Takacs, C. N. et al. Growth medium-dependent glycine incorporation into the peptidoglycan of Caulobacter crescentus. PLoS One 8, e57579, 10.1371/journal.pone.0057579 (2013).
Zhou, X. & Cegelski, L. Nutrient-dependent structural changes in S. aureus peptidoglycan revealed by solid-state NMR spectroscopy. Biochemistry 51, 8143–8153, 10.1021/bi3012115 (2012).
Bertsche, U. et al. Increased cell wall teichoic acid production and D-alanylation are common phenotypes among daptomycin-resistant methicillin-resistant Staphylococcus aureus (MRSA) clinical isolates. PLoS One 8, e67398, 10.1371/journal.pone.0067398 (2013).
Bertsche, U. et al. Correlation of daptomycin resistance in a clinical Staphylococcus aureus strain with increased cell wall teichoic acid production and D-alanylation. Antimicrob Agents Chemother 55, 3922–3928, 10.1128/AAC.01226-10 (2011).
Baek, K. T. et al. beta-Lactam Resistance in Methicillin-Resistant Staphylococcus aureus USA300 Is Increased by Inactivation of the ClpXP Protease. Antimicrob Agents Chemother 58, 4593–4603, 10.1128/AAC.02802-14 (2014).
Müller-Anstett, M. A. et al. Staphylococcal peptidoglycan co-localizes with Nod2 and TLR2 and activates innate immune response via both receptors in primary murine keratinocytes. PLoS One 5, e13153, 10.1371/journal.pone.0013153 (2010).
Hayashi, K. A rapid determination of sodium dodecyl sulfate with methylene blue. Anal Biochem 67, 503–506 (1975).
Iordanescu, S. Host controlled restriction mutants of Staphylococcus aureus. Archives roumaines de pathologie experimentales et de microbiologie 34, 55–58 (1975).
Jacobi, C. A. & Malfertheiner, P. Escherichia coli Nissle 1917 (Mutaflor): new insights into an old probiotic bacterium. Digestive diseases (Basel, Switzerland) 29, 600–607, 10.1159/000333307 (2011).
de Jonge, B. L., Chang, Y. S., Gage, D. & Tomasz, A. Peptidoglycan composition in heterogeneous Tn551 mutants of a methicillin-resistant Staphylococcus aureus strain. J Biol Chem 267, 11255–11259 (1992).
Boneca, I. G., Xu, N., Gage, D. A., de Jonge, B. L. & Tomasz, A. Structural characterization of an abnormally cross-linked muropeptide dimer that is accumulated in the peptidoglycan of methicillin- and cefotaxime-resistant mutants of Staphylococcus aureus. J Biol Chem 272, 29053–29059 (1997).
Figueiredo, T. A. et al. Identification of genetic determinants and enzymes involved with the amidation of glutamic acid residues in the peptidoglycan of Staphylococcus aureus. PLoS Pathog 8, e1002508, 10.1371/journal.ppat.1002508 (2012).
Münch, D. et al. Identification and in vitro analysis of the GatD/MurT enzyme-complex catalyzing lipid II amidation in Staphylococcus aureus. PLoS Pathog 8, e1002509, 10.1371/journal.ppat.1002509 (2012).
Bera, A., Biswas, R., Herbert, S. & Götz, F. The presence of peptidoglycan O-acetyltransferase in various staphylococcal species correlates with lysozyme resistance and pathogenicity. Infect. Immun. 74, 4598–4604 (2006).
Braun, V. & Wolff, H. The murein-lipoprotein linkage in the cell wall of Escherichia coli. Eur J Biochem 14, 387–391 (1970).
Potluri, L. et al. Septal and lateral wall localization of PBP5, the major D,D-carboxypeptidase of Escherichia coli, requires substrate recognition and membrane attachment. Molecular Microbiology 77, 300–323, 10.1111/j.1365-2958.2010.07205.x (2010).
Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft (SFB766 to UB) and Baden-Württemberg Stiftung (P-BWS-Glyko/21-Götz) to DDD. We thank F. Götz for helpful discussions and F. Götz and P. Schwartz for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
D.K., M.S., D.D.D., Conception and design, Acquisition of data, Analysis and interpretation of data; U.B., Conception and design, Interpretation of data, Writing the article.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Kühner, D., Stahl, M., Demircioglu, D. et al. From cells to muropeptide structures in 24 h: Peptidoglycan mapping by UPLC-MS. Sci Rep 4, 7494 (2014). https://doi.org/10.1038/srep07494
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07494
This article is cited by
-
Tumescenamide C, a cyclic lipodepsipeptide from Streptomyces sp. KUSC_F05, exerts antimicrobial activity against the scab-forming actinomycete Streptomyces scabiei
The Journal of Antibiotics (2024)
-
Synergistic Inhibitory Effect of Lactobacillus Cell Lysates and Butyrate on Poly I:C-Induced IL-8 Production in Human Intestinal Epithelial Cells
Probiotics and Antimicrobial Proteins (2024)
-
Staphylococcus aureus sacculus mediates activities of M23 hydrolases
Nature Communications (2023)
-
SepN is a septal junction component required for gated cell–cell communication in the filamentous cyanobacterium Nostoc
Nature Communications (2022)
-
Nesterenkonia sedimenti sp. nov., isolated from marine sediment
Archives of Microbiology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.