Abstract
Adipose tissue functions as an endocrine organ and the development of systemic inflammation in adipose tissue is closely associated with metabolic diseases, such as obesity and insulin resistance. Accordingly, the fine regulation of the inflammatory response caused by obesity has therapeutic potential for the treatment of metabolic syndrome. In this study, we analyzed the role of DJ-1 (PARK7) in adipogenesis and inflammation related to obesity in vitro and in vivo. Many intracellular functions of DJ-1, including oxidative stress regulation, are known. However, the possibility of DJ-1 involvement in metabolic disease is largely unknown. Our results suggest that DJ-1 deficiency results in reduced adipogenesis and the down-regulation of pro-inflammatory cytokines in vitro. Furthermore, DJ-1-deficient mice show a low-level inflammatory response in the high-fat diet-induced obesity model. These results indicate previously unknown functions of DJ-1 in metabolism and therefore suggest that precise regulation of DJ-1 in adipose tissue might have a therapeutic advantage for metabolic disease treatment.
Similar content being viewed by others
Introduction
Adipose tissue is a complex organ that is required for energy homeostasis. This tissue is primarily composed of adipocytes and contains diverse cellular types, including fibroblasts, fibroblastic pre-adipocytes, endothelial cells and immune cells1. Adipocytes contain a single large lipid droplet surrounded by a thin rim of cytoplasm that lies between the droplet and the plasma membrane2. Adipocytes within white adipose tissue (WAT) store excess energy in the form of triglyceride and release free fatty acids in response to energy requirements such as fasting3. Additionally, adipocytes secrete hormones and cytokines (adipokines) that affect whole-body energy metabolism functions such as glucose metabolism, appetite and inflammation. Accordingly, adipose tissue functions as an endocrine organ as well as an energy storage organ4,5. The dysfunction of the adipose tissue is associated with the pathological consequences of metabolic diseases, most notably obesity, insulin resistance and type 2 diabetes.
Many studies have suggested that chronic inflammation is a key feature of obesity-related insulin resistance6,7,8. Obesity alters the cellular composition in WAT. Macrophage infiltration of adipose tissue is observed in obese conditions in both humans and mice and this recruitment is linked to systemic inflammation and insulin resistance. Many pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), are elevated in WAT with obesity and these cytokines have direct effects on cellular metabolism. Additionally, many reports have shown that oxidative stress is increased in WAT as obesity develops9,10. Increased oxidative stress in WAT affects other metabolic organ, including liver and skeletal muscle as well as WAT itself, via the dysregulation of adipokines and leads to insulin resistance and diabetes. These reports proposed that increased oxidative stress is one cause of obesity-associated metabolic syndrome and that factors regulating oxidative stress might be useful target for new therapies.
DJ-1 (PARK7) is a 189-amino-acid protein that is ubiquitously expressed in mammalian tissues11. A loss of function mutation of DJ-1 causes early onset familial Parkinson's disease12. Many reports have proposed that DJ-1 functions as a survival factor and an antioxidant protein and that Cys106 is necessary for antioxidant capacity of DJ-113. DJ-1 prevents cell death from oxidative stress and DJ-1 deficiency increases susceptibility to oxidative stress14,15,16. The antioxidant function of DJ-1 is also observed in tissues other than the brain. Indeed, DJ-1-deficient mice show a reduced β-cell area of pancreatic islets and reduced insulin secretion in response to aging and an high-fat diet (HFD) and it is suggested that DJ-1 deficiency results in the dysregulation of oxidative stress and the dysfunction of pancreatic islets17. However, so far there have been few studies of the effects of DJ-1 on adipogenesis and metabolic regulation.
In the present study, we have investigated the role of DJ-1 in adipogenesis and inflammation associated with obesity-associated metabolism. We observed that DJ-1 deficiency results in reduced adipogenesis and inflammatory cytokine expression in 3T3-L1 cells. Furthermore, to further understand the role of DJ-1 in vivo, we applied the HFD model for 12 weeks and detected reduced inflammation in DJ-1-deficient mice. These results indicate previously unknown functions of DJ-1 in adipogenesis and metabolism and suggest that selective regulation of DJ-1 is a promising new therapeutic target for treating obesity-related metabolic diseases.
Results
Expression of DJ-1 is up-regulated during adipogenesis in 3T3-L1 cells
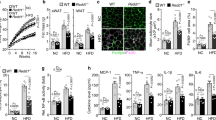
To examine the putative role of DJ-1 in adipogenesis, we analyzed DJ-1 expression in 3T3-L1 cells caused by adipogenic differentiation. The 3T3-L1 cell line is a mouse pre-adipocyte line that is widely used as a cellular model of adipogenesis18. To confirm adipogenic differentiation, the expression of adipogenic markers was also examined. During adipogenesis in 3T3-L1 cells, the mRNA level of DJ-1 is increased (1.6–4.6 folds) and, simultaneously, the expression of adipogenic markers1,18,19, such as peroxisome-proliferator-activated receptor-γ (PPAR-γ) (2.2–57.3 folds), CCAAT/enhancer-binding protein-α (c/EBP-α) (8.0–65.5 folds) and adipocyte protein 2 (aP2) (184.2–3019.5 folds), was up-regulated during adipogenic differentiation (Fig. 1a). Additionally, we confirmed that the protein level of DJ-1 was up-regulated under adipogenic differentiation conditions (Fig. 1b). The protein level of DJ-1 was increased during adipogenesis in 3T3-L1 cells and adipogenic marker expression was also examined. However, the secretion of DJ-1 was not altered during adipogenesis in 3T3-L1 cells (data not shown). These results show that the expression of DJ-1 mRNA and the levels of DJ-1 protein are up-regulated in response to adipogenic conditions from the early stages of differentiation.
Expression of DJ-1 is increased during adipogenesis.
(a) The gene expression level changes of DJ-1 and adipogenic markers, including PPAR-γ, C/EBP-α and aP2 caused by the adipogenic differentiation of 3T3-L1 cells. The mRNA level was determined by quantitative RT-PCR. *, p < 0.05 and **, p < 0.01 versus day 0. (b) The protein levels of DJ-1 and adipogenic markers were examined by immunoblotting during adipogenesis in 3T3-L1 cells. The expression of HSC70 was used as a normalized control. All data are presented as the mean ± s.d.
Endogenous DJ-1 contributes to adipogenesis in 3T3-L1 cells
Because DJ-1 expression was enhanced during adipogenesis in 3T3-L1 cells, we investigated the role of endogenous DJ-1 in adipogenic differentiation by transfecting siRNA targeting DJ-1. The knock-down of DJ-1 by siRNA treatment was confirmed by immunoblotting (Fig. 2a). Cell viability was not affected by DJ-1 deficiency at 0 d or 6 d after adipogenesis induction (Fig. 2b). However, surprisingly, DJ-1 deficiency resulted in reduced adipogenic differentiation and lipid droplet formation compared with the control (Fig. 2c). Lipid droplets formation was detected by oil red O staining at 6 d after adipogenesis induction. Additionally, the expression of adipogenic markers, such as PPAR-γ (Non-targeting, 6.30 ± 0.54; DJ-1, 2.57 ± 0.19 folds), aP2 (Non-targeting, 3.07 ± 0.11; DJ-1, 1.17 ± 0.10 folds) and lipoprotein lipase (LPL) (Non-targeting, 4.78 ± 0.21; DJ-1, 4.05 ± 0.15 folds), was down-regulated in response to DJ-1 deficiency (Fig. 2d). Similarly, the mRNA level of glucose transporter type 4 (GLUT4) was reduced in DJ-1-deficient 3T3-L1 cells (Non-targeting, 5.30 ± 0.26; DJ-1, 4.16 ± 0.29 folds). Adipogenic marker expression was examined by RT-PCR at 6 d after adipogenesis induction. The down-regulation of adipogenic genes in response to DJ-1 knock-down was shown prior to and during the early stages of adipocyte differentiation (Supplementary Fig. S1). Taken together, these results suggest that the endogenous expression of DJ-1 is required for the adipogenic program of 3T3-L1 cells from the early to late stages of differentiation.
DJ-1 deficiency results in impaired adipocyte differentiation.
(a) 3T3-L1 cells were transfected with non-targeting or anti-DJ-1 siRNA (10 nM). After the 3T3-L1 cells were grown to confluence, the cells were cultured under adipogenic induction medium (AIM). The knock-down of DJ-1 was confirmed by immunoblotting at 0 d and 6 d after adipogenesis induction. Quantification of DJ-1/actin protein ratio was shown below each blot. (b) To determine cell viability, the MTT assay was performed. Non-targeting or anti-DJ-1 siRNA-treated 3T3-L1 cells were grown under adipogenic differentiation conditions. Cell viability was examined at 0 d and 6 d after adipogenesis induction. NS, not significant. (c) 3T3-L1 cells were transfected with non-targeting or anti-DJ-1 siRNA and the cells were grown under adipogenic differentiation conditions for 6 d. The knock-down of DJ-1 exhibited a reduced lipid droplet formation, as determined by oil red O staining. Differentiated adipocytes were photographed (x200) at 6 d after adipogenesis induction. Scale bar = 100 μm. (d) The expression of adipocyte-specific marker genes and GLUT4 were lower in anti-DJ-1 siRNA-treated cells at 6 d after adipogenesis. *, p < 0.05 and **, p < 0.01 versus the control. All data are presented as the mean ± s.d.
DJ-1 deficiency results in reduced expression of pro-inflammatory cytokines
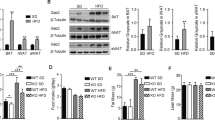
Next, we tested whether the elevated expression of DJ-1 in 3T3-L1 cells is related to the regulation of inflammatory markers. The knock-down of DJ-1 using siRNA treatment in 3T3-L1 cells significantly suppressed the expression level of cytokines, including interleukin-1β (IL-1β) (Non-targeting, 4.17 ± 0.34; DJ-1, 1.81 ± 0.09 folds), IL-6 (Non-targeting, 5.80 ± 0.22; DJ-1, 2.09 ± 0.10 folds) and monocyte chemotactic protein-1 (MCP-1) (Non-targeting, 2.43 ± 0.16; DJ-1, 1.22 ± 0.25 folds) under adipogenic differentiation conditions (Fig. 3a). DJ-1-deficient 3T3-L1 cells showed a reduced expression of these cytokines prior to adipogenic induction (Supplementary Fig. S2). Similarly, the secretion of IL-6 was down-regulated in anti-DJ-1 siRNA-treated cells during adipogenesis (Fig. 3b). IL-6 concentration was enhanced as a result of the adipogenic differentiation of 3T3-L1 cells (8180.84 ± 27.93 pg/ml at 6 d) and DJ-1 deficiency resulted in reduced IL-6 secretion (3652.54 ± 696.15 pg/ml at 6 d). Because IL-1β, IL-6 and MCP-1 are NF-κB target genes20,21 and DJ-1 has been known to enhance NF-κB translocation and transcriptional activity22, we tested the effect of DJ-1 knock-down on NF-κB transcriptional activity by examining reporter-gene activity. Because the transfection efficiency of reporter plasmid was very low in 3T3-L1 cells, we performed this experiment in RAW264.7 cells (a mouse macrophage cell line)23. The basal level of NF-κB transcriptional activity was reduced in DJ-1-deficient cells (Non-targeting, 1.0 ± 0.0; DJ-1, 0.3 ± 0.1 folds). NF-κB luciferase activity was reduced in DJ-1-deficient cells (5.6 ± 0.1 folds) compared with the control (12.0 ± 0.3 folds) in response to lipopolysaccharides (LPS) treatment (Fig. 3c). Additionally, LPS-stimulated nitrate production, which is a marker of NF-κB activation and inflammation24,25, was reduced in DJ-1-deficient RAW264.7 cells (Non-targeting, 22.9 ± 0.3; DJ-1, 8.9 ± 0.7 folds) (Fig. 3d). Similarly, the gene expression levels of IL-1β, IL-6 and MCP-1 were down-regulated in anti-DJ-1 siRNA-treated RAW264.7 cells in the presence of LPS (Supplementary Fig. S3). However, DJ-1 had no effect on the expression of anti-inflammatory cytokines, including IL-10 and Arginase-1 (data not shown). Next, to determine whether DJ-1-mediated NF-κB activity is related to adipogenesis, DJ-1-deficient cells were treated with or without Bay 11-7082 (an inhibitor of NF-κB) for 6 d under adipogenic differentiation conditions. The expression levels of PPAR-γ and LPL were increased by Bay 11-7082 in both non-targeting siRNA and anti-DJ-1 siRNA-treated 3T3-L1 cells, whereas aP2 expression was not changed (Supplementary Fig. S4) and Bay 11-7082 did not increase lipid droplet formation suppressed by DJ-1 knock-down (data not shown). These results suggest that DJ-1 regulates the expression of pro-inflammatory cytokines, possibly through the regulation of NF-κB activity and DJ-1-mediated NF-κB activity might not be sufficient to regulate adipogenesis.
DJ-1 regulates the expression of NF-κB target cytokines.
(a) The expression of pro-inflammatory cytokines, including IL-1β, IL-6 and MCP-1 at 6 d after adipogenesis induction in 3T3-L1 cells. *, p < 0.05 and **, p < 0.01 versus the control. (b) The secretion of IL-6 in anti-DJ-1 siRNA-treated 3T3-L1 cells was determined with a multiplex mouse adipokine assay. The IL-6 concentration in 3T3-L1 cells was examined from 0 d to 6 d after adipogenesis induction. *, p < 0.05 and **, p < 0.01 versus the control. (c) NF-κB activity was examined using the luciferase reporter gene assay in RAW264.7 cells in the absence or presence of LPS (100 ng/ml). Knock-down cells were incubated with LPS for 8 h and luciferase activity was determined. *, p < 0.05 versus the control. #, p < 0.05 versus the vehicle. (d) The concentration of nitrate was detected in non-targeting or anti-DJ-1 siRNA-treated cells. After RAW264.7 cells were transfected with siRNA, the cells were treated with LPS (100 ng/ml) for 8 h. *, p < 0.05 versus the control. #, p < 0.05 versus the vehicle. All data are presented as the mean ± s.d.
DJ-1 knock-out mice show increased level of glucose in the HFD-induced obesity model
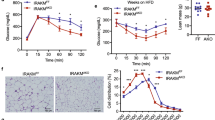
To further investigate the role of DJ-1 in metabolic control, we used the HFD-induced obesity model26,27 in wild-type (WT) and DJ-1 knock-out mice. WT and DJ-1 knock-out (DJ-1 KO) mice were fed an HFD for 12 weeks. Although body weight was increased in both WT and DJ-1 KO mice owing to the HFD, the change in body weight between WT and DJ-1 KO mice was not significant during 12 weeks after the HFD (Supplementary Fig. S6a). To further understand the role of DJ-1 in glucose homeostasis and insulin sensitivity, we performed glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) in WT and DJ-1 KO mice that had been fed an HFD at 6 weeks and 12 weeks (Supplementary Fig. S5,6). Several reports suggest that DJ-1 has a protective role in pancreatic beta-cells and DJ-1 KO mice show the reduced insulin secretion according to the HFD and aging17,28. At 6 weeks, there was no difference in glucose homeostasis and insulin action between WT and DJ-1 KO mice (Supplementary Fig. S5a,b). Interestingly, at 12 weeks, DJ-1 KO mice showed glucose intolerance compared with WT mice in response to glucose injection (Supplementary Fig. S6b). However, in response to insulin administration, there was no difference in DJ-1 KO mice and WT (Supplementary Fig. S6c). In addition, the basal fasting glucose level was increased in DJ-1 KO mice (143.78 mg/dl) compared with WT mice (126.89 mg/dl). These results support that DJ-1 KO mice might have the problem in insulin secretion owing to HFD-induced obesity.
DJ-1 deficiency shows reduced inflammation in WAT
Next, we performed a histological analysis by adopting H&E staining of WAT in WT and DJ-1 KO mice (Fig. 4a). DJ-1-deficient mice showed no remarkable difference in adipocyte size and number. However, mononuclear cells infiltration was increased in WT mice, whereas DJ-1 KO mice exhibited a decrease of mononuclear cell infiltration and a mild inflammatory response. Consistent with the histological analysis, IL-6 expression in the WAT of DJ-1-deficient mice was reduced compared with the WAT of WT mice (WT, 1.00 ± 0.38; DJ-1 KO, 0.21 ± 0.05 folds) (Fig. 4b). Additionally, the secretion level of IL-6 was decreased in the serum of DJ-1 KO mice compared with WT mice (WT, 71.60 ± 26.37; DJ-1 KO, 16.18 ± 4.19 pg/ml) (Fig. 4c). We also examined the expression of adipogenic marker genes and other pro-inflammatory genes. The expression of these genes was also lightly reduced in the WAT of DJ-1 KO mice (Supplementary Fig. S7). However, it did not show a remarkable change. Taken together, these results suggest that DJ-1-deficient mice exhibit a reduced inflammatory response with mild activity in adipocytes under HFD conditions.
Inflammation is down-regulated in DJ-1 KO mice.
(a) Representative H&E-stained images (x200). The white adipose tissue (WAT) samples were obtained from WT and DJ-1 KO mice at 12 week after HFD. Scale bar = 100 μm. Left, image for WAT of WT; Right, image for WAT of DJ-1 KO. Black filled arrows indicate the infiltration of mononuclear cell. (b) The expression levels of IL-6 in the WAT from WT and DJ-1 KO mice fed an HFD for 12 weeks were determined by quantitative RT-PCR. *, p < 0.05 versus the WT mice. n = 9 per group. (c) The IL-6 concentration in the serum from WT and DJ-1 KO mice was detected with a multiplex mouse adipokine assay. *, p < 0.05 versus the WT mice. n = 9 per group. All data are presented as the mean ± s.e.m. (d) Proposed mechanism for functions of DJ-1 in metabolic control.
Discussion
In this study, we investigated the role of DJ-1 in metabolic control using the HFD-induced obesity model. DJ-1 expression was increased during adipogenesis and DJ-1 deficiency resulted in impaired adipogenic differentiation and reduced lipid droplet formation in 3T3-L1 cells. Additionally, our results show that DJ-1 regulates the expression of pro-inflammatory cytokines by regulating NF-κB transcriptional activity. Furthermore, DJ-1-deficient mice showed reduced inflammation in response to HFD. Indeed, IL-6 expression and secretion were down-regulated in DJ-1-deficient mice. Taken together, these results indicate previously unknown functions of DJ-1 in adipogenesis and inflammation in metabolic regulation (Fig. 4d).
To date, the role of DJ-1 in cell differentiation and adipogenesis has been largely unknown29. Because DJ-1 has important roles in oxidative stress regulation and cell survival, the role of DJ-1 has been suggested to be related to insulin secretion. In DJ-1-deficient mice, the defense mechanism against oxidative stress is absent. Thus, the dysregulation of the oxidative stress caused by an HFD and aging leads to the disruption of pancreatic islets, resulting in reduced insulin secretion17,30. In our experiments, DJ-1-deficient mice showed a significant increase in fasting glucose level at 12 weeks after HFD (Supplementary Fig. S6b,c). These results suggest that DJ-1 deficiency induces the reduction in insulin secretion as previously reported17. On the other hand, several previous reports showed that DJ-1 expression was higher in obesity-resistant rats and suggested a possible role of DJ-1 in adipogenesis associated with androgen receptor-mediated signaling pathway31,32,33. It is supposed that androgen inhibits adipogenesis of mouse pluripotent cells. However, it is unclear that DJ-1 deficiency causes obesity and DJ-1 regulates androgen receptor in 3T3-L1 cells. The effect of DJ-1 on adipogenesis might be dependent on differentiation stage and cellular context. Still, the putative activity of DJ-1 in adipogenesis and metabolic function has been poorly understood.
Our findings show that DJ-1 mRNA and protein expression was up-regulated owing to the adipocyte differentiation of 3T3-L1 cells. However, DJ-1 secretion was not altered during adipogenesis. Accordingly, endogenous intracellular DJ-1 levels might be required for adipogenic differentiation. DJ-1 deficiency resulted in reduced lipid droplet formation and the down-regulation of adipocyte-specific marker genes in 3T3-L1 cells. Indeed, the mRNA levels of adipogenic genes, including PPAR-γ, were reduced in DJ-1-deficient cells without adipogenic induction (Supplementary Fig. S1) and these genes were down-regulated up to terminal differentiation (Fig. 2d). These results show that reduced expression of PPAR-γ due to the loss of DJ-1 might be sufficient to impair adipogenic differentiation. These results suggest that DJ-1 might control adipogenesis through the regulation of adipogenic gene expression. However, the detailed molecular mechanism underlying DJ-1-mediated adipogenesis needs further investigation.
Several reports have suggested that DJ-1 has anti-inflammatory functions in astrocytes and microglia associated with Parkinson's disease pathogenesis34,35. To date, there is no direct evidence to show the role of DJ-1 in pro-inflammatory response. However, our results show that DJ-1 increases the transcriptional activity of NF-κB and the expression of its target genes. These results support that DJ-1 enhances NF-κB nuclear translocation and transcriptional activity22. The expression of NF-κB target genes, such as IL-1β, IL-6 and MCP-1, was down-regulated in DJ-1-deficient 3T3-L1 cells and RAW264.7 cells (Fig. 3a, Supplementary Fig. S2,3). Accordingly, the role of DJ-1 in inflammation might be dependent on cellular type and DJ-1 seems to have different intracellular signaling pathways. The investigation of DJ-1-mediated signaling pathways in various cellular types would be needed to define the function of DJ-1 in inflammatory response. On the other hand, the inhibition of NF-κB activity did not show a significant effect on adipogenesis in DJ-1-deficient 3T3-L1 cells, although adipogenic marker gene expression was slightly increased (Supplementary Fig. S4). Numerous reports have suggested that the NF-κB signaling pathway and PPAR-γ activation have an inverse relationship and that PPAR-γ has anti-inflammatory effects36,37,38,39. However, in our results, DJ-1 deficiency resulted in the down-regulation of both PPAR-γ expression and NF-κB transcriptional activity, suggesting that DJ-1 might control adipogenesis and inflammation through distinct mechanisms. DJ-1-mediated NF-κB activity might not be involved in adipogenic differentiation.
Furthermore, we examined the role of DJ-1 in metabolic control by using the HFD-induced obesity model. DJ-1-deficient mice fed an HFD showed a significant reduction in inflammation, whereas there was no remarkable difference in adipocyte size and number at 12 weeks after HFD (Fig. 4a). Additionally, the expression and secretion of IL-6 was decreased in DJ-1-deficient mice (Fig. 4b,c). IL-6 is a key pro-inflammatory cytokine that is closely associated with insulin resistance in obesity40,41. Accordingly, DJ-1 might function as a strong regulator in pro-inflammation rather than adipogenesis in vivo. However, adipose tissue-specific or macrophage-specific DJ-1-deficient mice might have different phenotypes and developing conditional knock-out mice might be a useful tool for defining the function of DJ-1 clearly in metabolic regulation.
In conclusion, our study identifies previously undefined functions of DJ-1 in adipogenesis and inflammation in metabolic regulation. The loss of DJ-1 resulted in impaired adipogenesis and reduced inflammation in vitro and a mild inflammatory response in vivo. Taken together, these results suggest that precise regulation of DJ-1 in metabolic organ might bring a therapeutic advantage for obesity and obesity-related metabolic disease treatment.
Methods
Cell culture
Mouse pre-adipocyte 3T3-L1 cells were purchased from ATCC. 3T3-L1 cells were grown in Dulbecco's Modified Eagle Medium (DMEM) (Lonza, Walkersville, MD) supplemented with 10% fetal bovine serum (FBS, Gibco BRL, Grand Island, NY), 100 units/ml penicillin and 100 μg/ml streptomycin (Gibco BRL). For adipogenesis induction, 3T3-L1 cells were plated at 105 cells/well in a six-well plate. After the 3T3-L1 cells were grown to confluence, the cells were cultured under adipogenic induction medium (AIM, 10% FBS, 1 μM dexamethasone, 500 μM 3-isobutyl-1-methylxanthine and 1 μM insulin in DMEM). The cells were treated with AIM every 2 d for 6 or 8 d. To verify the effect of DJ-1 knock-down on adipogenesis of 3T3-L1 cells, only insulin was included in AIM. All components of AIM were purchased from Sigma-Aldrich. RAW264.7 cells were maintained in DMEM supplemented with 10% FBS, 100 units/ml penicillin and 100 μg/ml streptomycin.
Oil red O staining
To detect lipid droplet formation in differentiated 3T3-L1 cells, oil red O staining was performed as previously described with some modifications42. Images were visualized using an Olympus IX 71 microscope equipped with an Olympus DP72 camera.
Knock-down of DJ-1
Non-targeting siRNA duplexes (5′-CGUUAAUCGCGUAUAAUACGCGUA-3′) and anti-DJ-1 siRNA duplexes (5′ACCUUGCUAGUAGAAUAAACAGU-3′) were purchased from Integrated DNA Technologies (Coralville, IA). 3T3-L1 cells or RAW264.7 cells were transfected with against non-targeting and anti-DJ-1 siRNA (10 nM) using Lipofectamin RNAiMAX transfection reagent (Invitrogen, Carlsbad, CA). Cells were used for the experiment after 48–72 h of culture.
MTT assay
Cell viability was determined using the MTT assay. Briefly, 3T3-L1 cells were plated at 8 × 104 cells/well in six-well plates. After the transfection of non-targeting or DJ-1-targeting siRNA, 3T3-L1 cells were grown to confluence. The MTT assay was performed at 0 d and 6 d after adipogenesis induction. The cells were incubated with the MTT solution (0.5 mg/ml MTT in PBS) for 3 h at 37°C. The newly formed crystals were dissolved in DMSO and absorbance was measured at 540 nm.
cDNA synthesis and quantitative RT-PCR
Total RNA was extracted from cells and adipose tissues using TRIzol (Invitrogen, Grand Island, NY). cDNA synthesis and quantitative RT-PCR were performed as previously reported with some modifications29. SYBR Green I Master mix (Roche, Indianapolis, IN) and a Roche LightCycler 480 RT-PCR system were used. The primers used for PCR are described in Supplementary Table S1. The expression levels of the mRNAs were normalized to the expression level of 36B4 and then compared.
Detection of IL-6 concentration
IL-6 secretion in cell culture supernatant and serum was analyzed by using the multiplex mouse adipokine kit (Millipore) according to the manufacturer's instructions.
NF-κB luciferase assay
siRNA-transfected cells were transfected with 0.5 μg of reporter plasmid (pGL4.32[luc2P/NF-KB-RE/Hygro], Promega) using Lipofectamine 2000 reagent (Invitrogen). After 24 h, the cells were treated with LPS (R&D Systems, Minneapolis, MN) for 8 h. The cell lysates were prepared with a passive lysis buffer and used to measure the luciferase activity according to the manufacturer's instructions for the luciferase reporter assay system (Promega). The luciferase assays were carried out using a GloMax 96 Microplate Luminometer (Promega). Reporter activity was normalized to the protein content in the cell extracts. The values shown are averages and standard errors from at least three independent experiments.
Nitrate assay
The production of nitrite, a stable end product of NO oxidation, was used as a measure of iNOS activity. The nitrite present in the conditioned media was determined by a method based on the Griess reaction43. An aliquot of each supernatant (50 μl) was mixed with the same volume of Griess reagent (0.1% (w/v)N-(1-naphathyl)-ethylenediamine and 1% (w/v) sulfanilamide in 5% (v/v) phosphoric acid) for 10 min at room temperature. The absorbance of the final product was measured at 520 nm and the nitrite concentration in the samples was determined from a standard curve of sodium nitrite prepared in culture medium.
DJ-1 knock-out mice and high-fat diet condition
DJ-1 knock-out mice were obtained from Dr. Charles R. Gerfen44. The mice were fed a high-fat diet (60% kcal fat, D12492, Research Diets Inc.) for 12 weeks. The serum samples were obtained by tail vein bleeds at the indicated intervals. All animal experiments were performed according to procedures approved by the Ulsan National Institute of Science and Technology's Institutional Animal Care and Use Committee.
H&E staining for adipose tissue
Epididymal white adipose tissue was collected under deep anesthesia after a 16 h fast. The tissue was fixed in 4% paraformaldehyde and embedded in Tissue Path. Tissue sections (4 μm) were prepared, stained in hematoxylin and eosin (H&E) and examined under a microscope.
Immunoblotting
Immunoblotting was performed as previously described29. Signals were visualized by chemiluminescence (ECL system, Amersham Biosciences, Piscataway, NJ) and detected with an LAS4000 biomolecular imager (GE healthcare). The primary antibody against DJ-1 was obtained from Abcam (UK) and other primary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The horseradish peroxidase-conjugated secondary antibodies were purchased from KPL (Gaithersburg, MA). The blots were quantified using NIH ImageJ software and normalized to actin.
Statistical analysis
The data were analyzed using Student's t- test; p < 0.05 and p < 0.01 were considered to be significant.
References
Cristancho, A. G. & Lazar, M. A. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol 12, 722–734 (2011).
Tang, Q. Q. & Lane, M. D. Adipogenesis: From Stem Cell to Adipocyte. Annu Rev Biochem 81, 715–736 (2012).
Camp, H. S., Ren, D. & Leff, T. Adipogenesis and fat-cell function in obesity and diabetes. Trends Mol Med 8, 442–447 (2002).
Galic, S., Oakhill, J. S. & Steinberg, G. R. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 316, 129–139 (2010).
Wolfgang, M. J. & Lane, M. D. Control of energy homeostasis: Role of enzymes and intermediates of fatty acid metabolism in the central nervous system. Annu Rev Nutr 26, 23–44 (2006).
Ouchi, N., Parker, J. L., Lugus, J. J. & Walsh, K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11, 85–97 (2011).
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Shoelson, S. E., Lee, J. & Goldfine, A. B. Inflammation and insulin resistance. J Clin Invest 116, 1793–1801 (2006).
Furukawa, S. et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114, 1752–1761 (2004).
Ahima, R. S. Connecting obesity, aging and diabetes. Nat Med 15, 996–997 (2009).
Thomas, B. & Beal, M. F. Parkinson's disease. Hum Mol Genet 16, R183–R194 (2007).
Bonifati, V. et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299, 256–259 (2003).
Canet-Aviles, R. M. et al. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. P Natl Acad Sci USA 101, 9103–9108 (2004).
Inden, M. et al. PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson's disease rat model. Neurobiol Dis 24, 144–158 (2006).
Martinat, C. et al. Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism. PLoS Biol 2, e327 (2004).
Kim, R. H. et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A 102, 5215–5220 (2005).
Jain, D. et al. Age- and diet-dependent requirement of DJ-1 for glucose homeostasis in mice with implications for human type 2 diabetes. J Mol Cell Biol 4, 221–230 (2012).
Rosen, E. D. & MacDougald, O. A. Adipocyte differentiation from the inside out. Nature reviews. Mol Cell Biol 7, 885–896 (2006).
Maeda, K. et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab 1, 107–119 (2005).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, Inflammation and Cancer. Cell 140, 883–899 (2010).
Gouranton, E. et al. Lycopene inhibits proinflammatory cytokine and chemokine expression in adipose tissue. J Nutr Biochem 22, 642–648 (2011).
McNally, R. S. et al. DJ-1 enhances cell survival through the binding of Cezanne, a negative regulator of NF-kappaB. J Biol Chem 286, 4098–4106 (2011).
Frazier-Jessen, M. R. et al. NF-kappaB elements contribute to junB inducibility by lipopolysaccharide in the murine macrophage cell line RAW264.7. FEBS Lett 513, 203–207 (2002).
Jones, E., Adcock, I. M., Ahmed, B. Y. & Punchard, N. A. Modulation of LPS stimulated NF-kappaB mediated Nitric Oxide production by PKCepsilon and JAK2 in RAW macrophages. J Inflamm 4, 23 (2007).
Joseph, S. B., Castrillo, A., Laffitte, B. A., Mangelsdorf, D. J. & Tontonoz, P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med 9, 213–219 (2003).
Hariri, N. & Thibault, L. High-fat diet-induced obesity in animal models. Nutr Res Rev 23, 270–299 (2010).
Boucher, J. et al. Impaired thermogenesis and adipose tissue development in mice with fat-specific disruption of insulin and IGF-1 signalling. Nat Commun 3, 902 (2012).
Inberg, A. & Linial, M. Protection of pancreatic beta-cells from various stress conditions is mediated by DJ-1. J Biol Chem 285, 25686–25698 (2010).
Kim, J. M. et al. DJ-1 promotes angiogenesis and osteogenesis by activating FGF receptor-1 signaling. Nat Commun 3 (2012).
Inberg, A. & Linial, M. Protection of Pancreatic beta-Cells from Various Stress Conditions Is Mediated by DJ-1. J Biol Chem 285, 25686–25698 (2010).
Joo, J. I. et al. Differential expression of adipose tissue proteins between obesity-susceptible and -resistant rats fed a high-fat diet. Proteomics 11, 1429–1448 (2011).
Taira, T., Iguchi-Ariga, S. M. & Ariga, H. Co-localization with DJ-1 is essential for the androgen receptor to exert its transcription activity that has been impaired by androgen antagonists. Biol Pharm Bull 27, 574–577 (2004).
Singh, R., Artaza, J. N., Taylor, W. E., Gonzalez-Cadavid, N. F. & Bhasin, S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 144, 5081–5088 (2003).
Waak, J. et al. Regulation of astrocyte inflammatory responses by the Parkinson's disease-associated gene DJ-1. Faseb J 23, 2478–2489 (2009).
Trudler, D., Weinreb, O., Mandel, S. A., Youdim, M. B. & Frenkel, D. DJ-1 deficiency triggers microglia sensitivity to dopamine toward a pro-inflammatory phenotype that is attenuated by rasagiline. J Neurochem (2013).
Remels, A. H. et al. PPARgamma inhibits NF-kappaB-dependent transcriptional activation in skeletal muscle. Am J Physiol Endocrinol Metab 297, E174–183 (2009).
Kersten, S., Desvergne, B. & Wahli, W. Roles of PPARs in health and disease. Nature 405, 421–424 (2000).
Tham, D. M. et al. Angiotensin II is associated with activation of NF-kappa B-mediated genes and downregulation of PPARs. Physiol Genomics 11, 21–30 (2002).
Gustafson, B., Hammarstedt, A., Andersson, C. X. & Smith, U. Inflamed adipose tissue - A culprit underlying the metabolic syndrome and atherosclerosis. Arterioscl Throm Vas 27, 2276–2283 (2007).
Klover, P. J., Zimmers, T. A., Koniaris, L. G. & Mooney, R. A. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes 52, 2784–2789 (2003).
Hoene, M. & Weigert, C. The role of interleukin-6 in insulin resistance, body fat distribution and energy balance. Obes Rev 9, 20–29 (2008).
Kim, J. M. et al. An activator of the cAMP/PKA/CREB pathway promotes osteogenesis from human mesenchymal stem cells. J Cell Physiol 228, 617–626 (2013).
Green, L. C. et al. Analysis of nitrate, nitrite and [15N]nitrate in biological fluids. Anal Biochem 126, 131–138 (1982).
Manning-Bog, A. B. et al. Increased vulnerability of nigrostriatal terminals in DJ-1-deficient mice is mediated by the dopamine transporter. Neurobiol Dis 27, 141–150 (2007).
Acknowledgements
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (2012-000891). The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Contributions
J.-M.K. wrote the manuscript and performed most of the experiments. H.-J.J. and S.Y.C. performed and analyzed the experiments and contributed to the writing of the manuscript. S.-A.P. and I.S.K. assisted with the experiments. Y.Y.R. and Y.W.L. assisted with the in vivo study. S.H.R. contributed to the experimental design and the discussion. P.-G.S. supervised the study and advised during the designing of the figures and the writing of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information_JM Kim et al.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Kim, JM., Jang, HJ., Choi, S. et al. DJ-1 contributes to adipogenesis and obesity-induced inflammation. Sci Rep 4, 4805 (2014). https://doi.org/10.1038/srep04805
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04805
This article is cited by
-
Dj1 deficiency protects against atherosclerosis with anti-inflammatory response in macrophages
Scientific Reports (2021)
-
DJ-1 maintains energy and glucose homeostasis by regulating the function of brown adipose tissue
Cell Discovery (2017)
-
DJ-1 deficiency attenuates expansion of liver progenitor cells through modulating the inflammatory and fibrogenic niches
Cell Death & Disease (2016)
-
Differential effect of DJ-1/PARK7 on development of natural and induced regulatory T cells
Scientific Reports (2015)
-
DJ-1 deficiency alleviates steatosis in cultured hepatocytes
Biotechnology and Bioprocess Engineering (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.