Abstract
Meromictic lakes are useful biogeochemical models because of their stratified chemical gradients and separation of redox reactions down the water column. Perennially ice-covered meromictic lakes are particularly stable, with long term constancy in their density profiles. Here we sampled Lake A, a deep meromictic lake at latitude 83°N in High Arctic Canada. Sampling was before (May) and after (August) an unusual ice-out event during the warm 2008 summer. We determined the bacterial and archaeal community composition by high-throughput 16S rRNA gene tag-pyrosequencing. Both prokaryote communities were stratified by depth and the Bacteria differed between dates, indicating locally driven selection processes. We matched taxa to known taxon-specific biogeochemical functions and found a close correspondence between the depth of functional specialists and chemical gradients. These results indicate a rich microbial diversity despite the extreme location, with pronounced vertical structure in taxonomic and potential functional composition and with community shifts during ice-out.
Similar content being viewed by others
Introduction
Perennially ice-covered meromictic lakes are common in both the Arctic and Antarctic and are formed by snow and glacial meltwater flowing over relict saline water. Because of their physical stability, such lakes have been identified as model systems for inferring biogeochemical processes within water columns1. Biogeochemical intermediates and end products from microbial activity have been observed to accumulate at different depths in these waters. The result is stratified, unusually high concentrations of inorganic phosphorus and ammonium2, dissolved metals3, dimethyl sulfide4 and nitrous oxide5,6; however, the direct association of these chemical features with complex microbial communities has been little explored.
Paleolimnological studies indicate that Lake A, (lat. 83°N, Canadian High Arctic), a deep meromictic lake at the top of Ellesmere Island, has been ice-covered even during summer for much of the last few thousand years7 and a modeling study based on temperature and conductivity profiles of Lake A indicated that the lake has been covered by thick perennial ice most years since the 1930s8. Observations on microbiological diversity in Lake A are sparse. Van Hove et al.9 applied denaturing gradient gel electrophoresis (DGGE) to determine the vertical pattern of Cyanobacteria within the lake and found that Synechococcus ribotypes separated into upper freshwater (mixolimnion) and deeper saline (monimolimnion) clades in both 1999 and 2001. Pouliot et al.10 reported on the Archaea by way of 16S rRNA gene clone libraries using archaeal-specific primers and found that in June 2006, the archaeal communities were different in the upper freshwater and in the deep saline anoxic zone. In addition, they reported a single Marine Group I ribotype within the chemocline, which is between the upper fresh water and the deep anoxic waters. Other studies also showed that biological variables in the lake reflected the physical stability and salinity gradients; for example, pigment profiles clearly separated the upper, chemocline and deep waters11. Metagenomic analysis of saline meromictic Ace Lake in Antarctica has revealed diverse bacterial assemblages and metabolic potential in its surface and anoxic zones12, however there are no reports on heterotrophic bacterial communities in analogous lakes in the North Polar region. Because Lake A and other High Arctic Lakes are difficult to access, all previous studies have been limited to sporadic samples taken in summer and there is no information about seasonal changes that might occur in microbial communities in these far northern lakes.
As part of an International Polar Year initiative in 2008, there was an opportunity to sample Lake A in early May and again in August (Figure 1 and Table 1). Our aim was to investigate the archaeal and bacterial communities to compare the chemocline and deep waters, which were expected to change little over the season and the upper mixed-layer communities, where changes in surface runoff, mixing and irradiance would be predicted to result in environmentally driven differences. A second goal was to relate the bacterial and archaeal taxa found down the water column to gradients in oxygen and other redox-related chemical variables. We applied a high-throughput, tag sequencing approach13 targeting ~400 nucleotides within the V6–V8 variable regions of the 16S rRNA gene of Bacteria and Archaea. Sequences were then classified to the genus level. We then identified heterotrophic and autotrophic taxa that have been previously associated with specific biogeochemical functions. In summer 2008, the Canadian High Arctic experienced unusually warm air temperatures and the perennial ice cover of Lake A completely melted out14. Our two sampling dates in 2008 thus enabled us to identify community changes that may have been influenced by this unusual ice-out event. Such conditions are likely to become increasingly common in the Arctic, given the ongoing climate change and ice loss taking place at high northern latitudes15.
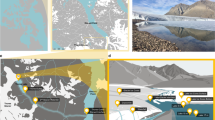
Geography and physicochemical features of Lake A.
(a) General location of the study site on Ellesmere Island in the Canadian High Arctic, with a detailed view of the region showing Lake A and the surrounding geographic features.The represented ice extent from the Canadian Ice Service Archive (http://ice-glaces.ec.gc.ca) corresponds to August 2008. (b) Vertical profiles of Lake A in May (continuous lines) and August (dashed lines) 2008 indicating salinity (thick lines) and temperature (thin lines).
Results
Pyrosequencing overview and community β-diversity (between-sample comparisons)
Bacterial reads averaged 430 bp and archaeal reads slightly longer at 446 bp. At the 97% similarity level, bacterial OTUs ranged from 280–425 and archaeal OTUs from 142–221 per sample (Supplementary Table S1) after equalizing for the same number of sequences per sample. Although there were more initial sequences for the Archaea from deeper waters, the poor yield from surface depths resulted in lower numbers of final reads analyzed after re-sampling. Overall, the Archaea showed significantly higher proportions of abundant OTUs (p < 0.01), defined as OTUs representing >1% of the total sequences16, than the Bacteria (64% vs. 44%). A taxonomy-independent Bray-Curtis similarity tree was constructed based on total OTU compositions of the 12 bacterial and archaeal samples to determine associations among the communities (Figure 2). There was a clear vertical separation between the upper 12 m of the water column (mixolimnion), the halocline at 20 m (metalimnion) and the depths below (monimolimnion). The dendrogram also revealed temporal changes in both Bacteria and Archaea. The August 2 m samples from both domains separated from other mixolimnion samples and the 10 m August samples grouped with the 12 m samples rather than with shallower samples as in May. This assessment was tested by quantitative analysis of the proportion of OTUs shared between the surface samples from the same depths sampled in May and August (Figure 2): the August 2 and 10 m samples shared more OTUs with the depths below them from May, rather than the same depths.
Whole community sample comparisons for Archaea (a) and Bacteria (b).
Bray-Curtis trees were calculated from total OTU compositions (97% level) for the mixolimnion, metalimnion and monimolimnion samples from May and August. The heatmaps show the proportions of OTUs shared among the mixolimnion depths between May and August. The value for the pairing with the highest match for each column is shown.
Archaeal community structure and distribution
The Bray-Curtis similarity tree indicated that the Archaea communities were stratified by depth and different sequences dominated the different lake strata (Figure 3a and Supplementary Figure S1). The largest difference in community composition was between the monimolimnion (29–60 m) and mixolimnion (2–12 m). The upper waters, as well as the intermediate 20 m sample, were dominated by sequences closely related (99% similarity) to marine clones identified as belonging to the Marine Group I (MG-I, in the phylum Thaumarchaeota). It is, however, important to note that Archaea were difficult to amplify from surface waters (first 10 m) using various archaeal-specific primers (see below) suggesting that there were few Archaea in the upper layers. In the deep layers, our pyrosequencing of the V6–V8 region showed a dominance of Crenarchaeota sequences, specifically the uncultured group C3, which is also referred to as group 1.2 Crenarchaeota17. These deep sequences tended to cluster with clones amplified from marine sediments.
Taxonomic distributions of archaeal sequences and the influence of different primer sets and sequencing methods.
(a) Distributions of archaeal sequences in May (left) and August (right) from our 2008 study using V6–V8 primers and pyrosequencing. CrenA, Crenarchaeota; EuryA, Euryarchaeota; Gp, group; Hydroth., hydrothermal; Unclass., unclassified. (b) Distribution of archaeal sequences previously observed in June 2006 by Pouliot et al.10 using V2–V5 primers and cloning. (c) Comparisons of archaeal patterns obtained using three different primer sets and two sequencing methods (cloning with Sanger sequencing vs. pyrosequencing), using the 2 m and 32 m depths as examples. Data are from this study with the exception of the June 2006 results from Pouliot et al.10. Note that the charts with symbols next to their lower right corners correspond to the same redrawn samples denoted in (a) and (b) by the same symbols. Also note that no amplifications (no amp.) were obtained for two of the primer-sample combinations (see Methods).
As these patterns differed greatly, except for 12 m, from those by Pouliot et al.10 from the same lake in June 2006 (Figure 3b), we investigated further one surface (2 m) and one deep (32 m) sample. First, we tested for biases in sequencing methodology by cloning and Sanger-sequencing the same V6–V8 products used for pyrosequencing. The results showed (Figure 3c) that regardless of method, the surface sample was overwhelmingly MG-I and the deep sample was mostly C3 group. Next, we sent DNA from the same two samples to a commercial pyrosequencing service that used V3–V4 region primers targeting the regions reported in Pouliot et al.10. The commercial laboratory was not able to amplify the 32 m sample, possibly because of metal content at this depth18 and for 2 m they recovered only 15 archaeal reads out of 3384 mostly bacterial sequences, in keeping with few Archaea at the surface. Those 15 archaeal surface reads were all MG-I, which we had also found using the V6–V8 primers. For a final test, we attempted to construct clone libraries using the 2008 samples as template with the same V2–V5 region primers as Pouliot et al.10. We were unable to amplify the 2 m sample, again likely because of low Archaea concentrations in the surface, but we were successful with the 32 m sample. The results were nearly identical to the 2006 results, with a dominance of Euryarchaeota (LDS and Rice Cluster V groups) being recovered. In summary, the primers targeting different variable regions showed distinct biases, but there were no biases detected using different sequencing methods.
Bacterial community structure and distribution
Distinct communities in the mixolimnion, metalimnion and monimolimnion were evident at the level of phylum (Figure 4a and Supplementary Figure S2). For example, although accounting for up to ~45% of the sequences in the mixolimnion, the Actinobacteria and Verrucomicrobia were very rare or absent from the monimolimnion. There were pronounced changes in the photosynthetic bacterial communities down the water column, with Cyanobacteria sequences dominating the oxygenated surface waters, Chlorobi at 20–32 m and Chloroflexi in the deepest depths. The OP clade was present in upper waters, while SAR406 was found in the saline deeper waters. The Proteobacteria represented about one third of the sequences at most depths, but at finer taxonomic resolution there were marked changes (Figure 4b): α-Proteobacteria (Pelagibacter and related sequences) and β-Proteobacteria (Burkholderiales + Methylophilales) were the main sequences in the upper waters, while the monimolimnion contained mostly δ-Proteobacteria (sulfate-reducing bacteria; SRBs). Regardless of taxonomic level, the 29–60 m samples included considerable proportions (14–23%) of unclassified sequences. The dominant bacterial OTUs (Supplementary Figure S2) reflected the sharp vertical physical stratification of the lake. Chlorobi, accounted for ~40% of the sequences at 29 m, followed by a few groups of δ-Proteobacteria (SRBs), OP and SAR406 clade OTUs. Near the surface, Cyanobacteria OTUs constituted up to ~30% of all sequences, but the remaining OTUs were diverse, but primarily within Verrucomicrobia, Bacteroidetes and Actinobacteria (especially group ACK-M1). The α-proteobacterium Pelagibacter accounted for up to ~35% of the sequences and was especially common at 10 and 12 m.
Temporal changes
The Archaea remained relatively constant between sampling dates, with only slight relative increases of MG-I throughout and less Euryarchaeota in the monimolimnion (Figure 3). Bacteria were more dynamic, with major changes within the surface mixolimnion; Bacteroidetes and Cyanobacteria increased in sequence proportions in August compared to May at the expense of Actinobacteria, Verrucomicrobia and other photosynthetic groups (Figure 4). Finally, in the monimolimnion, there was an increase in the proportion of Chlorobi in August.
Relationships to biogeochemical cycles
There are limitations using DNA as a template and targeting only 16S rRNA genes; however we found that identified taxon sequence distributions could be mapped onto what is known about biogeochemical cycles that may be present in Lake A18. Cyanobacteria, the only bacterial group capable of oxygenic photosynthesis, represented ~25% of the total sequences in the upper mixed layer (Figure 5). Anoxygenic photosynthesizers (AnoxPS) were mostly just below the chemocline (20 and 29 m) and were dominated by the Chlorobi (mostly Pelodictyon), but other major AnoxPS groups were represented: the Chloroflexi and various α- and β-Proteobacteria belonging to the purple non-sulfur (PNS) bacterial groups (Rhodoferax, Rhodobacterales and Rhodospirillales). Very few of the more recently discovered phylogenetically diverse aerobic anoxygenic phototrophs (AAPs)19 were found. Given that the majority of the AnoxPS sequences belonged to the Chlorobi, the potential AnoxPS, sulfur oxidation and H cycle profiles were similar since Chlorobi oxidize S and H2 and were most strongly represented in the depth range 20–29 m. Dehalogenimonas was found deeper at 60 m, indicating the potential for H cycle activity in the deepest portion of the lake. Other possible S-oxidizing genera that were present in relatively small sequence proportions included Sulfurimonas, Pseudomonas, Rhodobacter and Magnetococcus. SRBs were more prevalent just below the S oxidizer maximum and were less abundant, but much more diverse, with at least 10 genera belonging to 5 orders or families of δ-Proteobacteria. Desulfobacterium was the most common S reducer identified. A few other sequences affiliated with genera that either produce or oxidize H2 were retrieved, but generally at <1% of the total sequences; these included, in decreasing relative abundance, Polaromonas, Acidiphilium, Rhodobacter, Sulfurimonas, Syntrophus, Acidovorax and Pseudomonas.
Profiles of sequence counts of taxa known to be capable of major biogeochemical cycling processes at each depth.
Profiles were created by collating the numbers of sequences belonging to identified taxa known to be involved in the different biogeochemical cycles (note the different x-axis scales). For the Fe and Mn cycle, most bacteria capable of Fe(III)-reduction can also reduce Mn(IV), therefore the counts for the two profiles are treated here as the same. Note that these profiles are based on DNA template that may be derived from both living and dead cells and indicates the original depth of the DNA sequences, not measured activity, but rather potential. Also note that the sums of all sequence counts at each depth (across all five cycles) would not be expected to total 100% since some sequences belong to unidentified taxa which could not be assigned to the subset of biogeochemical cycles here. Finally, some sequences were counted multiple times (across the profiles) as they matched taxa that contributed to multiple cycles, for example anoxygenic photosynthesizers that are also S-reducers (see text for further clarification).
Nearly 20 bacterial genera that could, if active, contribute to N cycling were detected in Lake A. Diazotrophs represented ~2.5% of the sequences near the surface, denitrifiers accounted for ~4.5% at 20 m and nitrifiers had two maxima of ~1% each at the same depths as the diazotroph and denitrifier peaks (Figure 5). Neither Synechococcus nor the Chlorobi were included in these N cycle calculations, as only some species of these genera are reported to fix nitrogen20,21. Only ~1.5% of sequences in the mixolimnion belonged to α- (primarily Methylocapsa) and β-Proteobacteria (primarily Methylotenera), which are methanotrophs and methylotrophs.
Finally, a small maximum (0.9% of the sequences) of possible Fe- or Mn-reducing bacteria was identified (Figure 5). Most bacteria capable of Fe(III)-reduction can also reduce Mn(IV)22, therefore the two were combined. Examples of these bacteria detected in our samples were: Malonomonas, Geopsychrobacter and other Desulfuromonadales (δ-Proteobacteria); Acidiphilium (α-Proteobacteria); and Rhodoferax (β-Proteobacteria). One Chlorobi species, Chlorobium ferrooxidans, is known to oxidize Fe(II) to Fe(III) during photosynthesis23. Since it is not possible to identify our short pyrosequences with 100% accuracy at the species level, we have not included the Chlorobi detected in our samples in the Fe cycle. However, if included, the magnitude of the proportion of the Fe-cycle-capable sequences would increase to ~10% and the maximum would occur at 29 m.
Discussion
The identical clustering for both Bacteria and Archaea communities showing strong differences between the surface (2–12 m) and deeper strata (29–60 m) of Lake A were expected due to the markedly different limnological conditions, corresponding to above and below the pycnocline and oxycline11,14. Similarly disparate communities were detected in the mixolimnion versus monimolimnion of Ace Lake, Antarctica, which, although meromictic, has a period of open water and surface mixing each summer and a salinity-density gradient that is much less than in Lake A12,24.
Quantitative PCR applied to Lake A samples taken in July 2006 indicated that there were few Archaea16S rRNA gene copies in surface waters compared to deeper waters10. From our failed or poor amplification of archaeal 16S rRNA genes using 3 different sets of primers in surface samples, we concluded that Archaea were mostly absent from the oxygenated mixolimnion, as reported in meromictic lakes elsewhere12,25. Low archaeal DNA template concentrations, as well as several primer mismatches (Supplementary Table S3), could account for the community composition differences (Euryarchaeota vs. MG-I) between the different primer sets, as primer biases can be exaggerated under low-template conditions. However, all primer sets detected the dominance of MG-I sequences closely related to Candidatus Nitrosopumilus26 at 12 m. Pouliot et al.10 using qPCR also reported maximum amoA gene copies at this depth, consistent with a high potential for nitrification in the oxycline where oxygen is likely supplied by diffusion from the overlying mixolimnion and NH4+ from the underlying anoxic, ammonium-rich monimolimnion18. In the monimolimnion, the community amplified with the V2-V5 primers was more deeply-branching and belonged to the highly diverse and uncultured RC-V and LDS clusters of Euryarchaeota27, which contrasted with the dominance of Crenarchaeota group C3 sequences detected using primers targeting the V6–V8 region. Metagenomic analysis of Ace Lake showed that Euryarchaeota (mostly methanogens) were the major Archaea in the monimolimnion12, corresponding to the V2–V5 primer results from Lake A. In contrast, direct FISH-counts of Archaea from the coastal Italian meromictic Lake Faro indicated that Crenarchaeota accounted for three quarters of archaeal cells in its monimolimnion25, so either scenario is possible for Lake A. A similar study using fluorescent in situ hybridization of the groups of interest would be needed to resolve the relative importance of the major archaeal groups.
The vertical distribution of Bacteria in Lake A was typical of meromictic lakes in terms of depth distribution of photosynthetic and redox conditions12,24,25,28,29,30,31. Aside from the clear separation between surface Cyanobacteria and deep AnoxPS and SRBs, many heterotrophic bacterial groups showed strong vertical structure. The uncultivated SAR406, OP3 and OP9 clades were restricted to the anoxic, saline monimolimnion, while Actinobacteria and Verrucomicrobia were mostly restricted to the freshwater mixolimnion, as reported in other meromictic systems12,32,33,34. Few Pelagibacter OTUs were found in the saline monimolimnion, but those detected were matched to sequences from marine environments, such as those from Antarctic seawater35 or Norwegian fjords (unpublished; accession number FR685069.1). In the surface waters (2–10 m), Pelagibacter OTUs were more diverse and were similar to those previously reported from mainly freshwater environments including from Yellowstone Lake36, Lake Zurich37 and Lake Gatun38. These latter sequences form part of the emerging LD12 clade, a freshwater sister-group to the marine SAR11 Pelagibacter. LD12 termed ultramicrobacteria are abundant microbial species in freshwater lakes39. The group is most often found in surface waters and strains with light-harvesting pigments may contribute significantly to inorganic carbon fixation in some environments. Marine Pelagibacter OTUs were found at the 12 m depth, as were marine Archaea (MG-I), indicating that the 12 m communities were marine in origin. Within the anoxic monimolimnion, the high proportion of taxonomically unassignable OTUs may represent novel sequences or non-curated environmental groups. A manual BLASTn analysis of the top 20 unclassified OTUs in the three deepest depths (Supplementary Table S2) showed that the unknowns were similar to those retrieved from other anoxic environments including the anoxic zones of the marine Saanich Inlet40 and Nitinat Lake, a tidal saltwater fjord41 in British Columbia; South China Sea sediments; one clone from the Cariaco Basin anoxic zone42; and lavas of the Lo'hi seamount43. The majority of these OTUs had similarities >90–95%, to longer, clone-library-based sequences suggesting that they were not chimeras or errors generated by the pyrosequencing. These OTUs most probably represent real taxa and our results highlight the diversity and poor representation of anaerobic bacteria in taxonomic databases.
Unexpectedly, the normally perennial ice covering Lake A completely melted out between May and August 2008 and caused changes in the physical environment of the lake. This provided a unique opportunity to examine community shifts under ice-out conditions, although the microbiological effects directly related to this event remain uncertain given the lack of seasonal succession information under the usual ice-covered condition. The open-water resulted in wind-driven mixing down to the halocline at ~10 m (Figure 1b) and the loss of ice increased the irradiance entering the top of the water column by a factor of 7 and therefore increased photosynthetic potential in the lake14. These influences were consistent with the change in community composition between seasons as August samples showed evidence of mixing effects on OTU compositions among surface samples (Figure 2). The proportion of cyanobacterial sequences increased in August and was more uniform throughout the upper water column, likely as a result of the surface mixing and greatly increased solar irradiance input to the lake. Microscopy counts from the same samples were consistent with our molecular results, with order-of-magnitude increases in the standing stocks of picocyanobacteria between May and August14. Below the mixolimnion, anoxygenic groups such as the Chlorobi (green S bacteria) and Chloroflexi were common and the substantial increase in the former from ~34 to 50% of the total sequences at 29 m in August would also be consistent with the greatly increased available irradiance at depth. Previous work at Lake A based on high pressure liquid chromatography (HPLC) pigment analysis has drawn attention to the responsiveness of these deep-living phototrophs to increased irradiance11. The proportion of Bacteroidetes doubled at the surface in August compared to May, while Actinobacteria and Verrucomicrobia were reduced throughout the surface. This suggests increased availability, or a change in the composition, of organic matter for microbial processes as Bacteroidetes produce various extracellular enzymes which degrade more complex organic molecules44,45. Either allochthonous input or increased exopolymeric substance production by Cyanobacteria46 or other plankton could contribute to Bacteroidetes having a selective advantage.
By collating the numbers of sequences matching taxa reported to be involved in specific biogeochemical pathways, we constructed putative depth profiles of potential activity (Figure 5; Supplementary Table S4). Our sequence results must be interpreted with caution given that: 1) preserved DNA or metabolically inactive cells47 may have contributed to the taxonomic signal; 2) end-point PCR is not quantitative; and 3) rRNA gene copy numbers vary among taxa48. However, the profiles were remarkably coherent with what is known about Lake A vertical gradients in redox chemistry18. The vertical taxonomic signals also followed results of HPLC pigment analysis (see below) and were consistent with patterns from well-studied meromictic lakes elsewhere.
Specifically, we found a high proportion of Chlorobi at 29 m that drove the proposed AnoxPS pattern, consistent with HPLC pigment and spectrophotometric data from previous years where a maximum of bacteriochlorophyll-e and the carotenoid β-isorenieratene, diagnostic pigments of brown-colored Chlorobi, were recorded from around the same depth11,49. Many direct microscopic and molecular methods have shown that Chlorobi occur within a narrow band in, or just under, the chemoclines of the majority of meromictic systems12,25,30,31,32,50,51,52,53. The Chlorobi use sulfur compounds as electron donors and therefore also drive the S-oxidation patterns. A geochemical analysis of Lake A carried out in 1999 reported the predominance of S-redox chemistry within the saline monimolimnion26, as is generally the case when light is available to anoxic regions of stratified lakes54. The more diverse sulfate reducing bacteria had their highest sequence count overlapping the S-oxidizers, as has been observed elsewhere31,32 with the two groups often forming a stable syntrophy21: H2S is oxidized to sulfate and sulfate is reduced to H2S, with various intermediates possible as well.
Sequences matched to bacterial taxa attributed to nitrate and methane cycles represented <5% of the surface community. Gibson et al.18 found little evidence of N redox chemistry or methanogenesis in Lake A; and showed that the dominant form of N below 20 m is ammonium in near-millimolar quantities. This ammonium would be available to the archaeal ammonium oxidizers at 12–20 m where sequences related to the Candidatus Nitrosopumilus (phylum Thaumarchaeota) were found both in May in August. Other meromictic lakes are similar with high ammonium in their anoxic waters55 and little methanogenesis25,28.
Gibson et al.18 and Van Hove et al.49 reported strikingly high concentrations of transition metals at depths from 20–30 m in Lake A, with up to 30 μM total Fe and 176 μM total Mn. These Mn concentrations were more than four times the concentrations in the metal-rich anoxic bottom waters of meromictic Lake Vanda, Antarctica56. This depth region in Lake A corresponded to our small peak of sequences matching Fe-/Mn-reducing bacteria (Figure 5). This proportion could be an underestimate since up to10% of the total sequences belonged to other genera (especially Chlorobium) that could also potentially participate in these pathways. However little information is available on bacteria responsible for metal transformations at such high concentrations, which are rare in natural systems. Bratina et al.56 isolated some Mn-reducers from Lake Vanda that belonged to the genus Carnobacterium, however we did not detect this in our samples even though it was in our reference database. Culturing and targeted molecular analysis of Lake A's metal-transforming groups is warranted.
Paleolimnological and geophysical studies imply that the distinctive Lake A ecosystem has been mostly ice covered since its formation by isostatic uplift7. However, the lake lost its ice-cover in August 2008 (this study), but remained salinity stratified and meromixis dating several thousand years was maintained. This being said, the stability of the lake may be impacted more in the near future as air temperatures at the northern limit of the terrestrial High Arctic are warming at rates more than three times the global average57. Lake A and similar ecosystems in the region are therefore likely to experience continued perturbation of their light, mixing and nutrient regimes. We found evidence of seasonal change and that the archaeal community differed from 2 years earlier, but multiannual data is required to identify community responses to ongoing climate change.
In conclusion, our high-resolution study revealed diverse, highly stratified communities of Bacteria and Archaea and this taxonomic inventory will serve as a guide for further functional studies. High Arctic lakes are increasingly impacted by climate warming and it is important to establish baseline microbial information about these unique ecosystems. We note that taxa present in very small proportions can play key functional roles in aquatic ecosystems and that high-throughput studies of this and other high-latitude lakes may provide sentinel data to detect the microbiological impacts of planetary change.
Methods
Sample collection and processing
Conductivity, temperature and depth (CTD) profiles of Lake A (82° 59.667′ N 75° 26.602′ W; Figure 1) were taken in May and August 2008 using a XR-420 CTD profiler (RBR Ltd., Ottawa, ON). Water was collected in the field using a closing Kemmerer bottle (Wildlife Supply Company, Yulee, FL) at the depths specified in Table 1. Water was emptied directly into polypropylene containers, which had been cleaned with 5% v/v HCl then sample lake-water-rinsed. The water was kept cool and dark during transport to a field laboratory within four hours. DNA was collected by filtering 3–4 L of water sequentially through a 20 µm pre-filter, a 3 µm pore size 47-mm-diameter polycarbonate (PC) filter (Millipore) and into a 0.2 µm Sterivex unit (Millipore); the unit was filled with buffer (1.8 mL of 50 mM Tris-HCl, 0.75 M sucrose and 40 mM EDTA) and placed in a dry shipper. Samples were subsequently kept at −80°C until DNA was extracted using lysozyme, proteinase K, SDS and a salt-based separation as previously described58.
SSU rRNA gene amplification, 454 pyrosequencing and clone library verification
Extracted DNA from the 0.2–3 µm size-fraction was amplified in three independent 50 µL aliquots per Domain using V6–V8 454 primers as described in Comeau et al.13. Briefly, PCR reactions contained: 1X HF buffer (NEB), 200 µM of each dNTP (Feldan Bio), 0.4 mg/mL BSA (Fermentas), 0.2 µM of each 454 primer (Invitrogen), 1 U of Phusion High-Fidelity DNA polymerase (NEB) and 1–3 µL of template DNA. Three separate DNA concentrations were used for each sample: 1/0.5/0.1X (range of ~0.4–14 ng) for Bacteria and between 3X and 0.1X (~0.4–31 ng) for Archaea which were less abundant than Bacteria. Cycling conditions were: an initial denaturation at 98°C for 30 s, followed by 30 cycles of denaturation at 98°C for 10 s, annealing at 55°C for 30 s, extension at 72°C for 30 s and a final extension at 72°C for 5 min. The triplicate reactions for each Domain were pooled, purified using the QIAquick PCR purification kit (QIAGEN) and quantified spectrophotometrically (Nanodrop ND-1000). The 12 sample-coded amplicons were mixed in equal quantity and 1/8th plate for each Domain was sequenced on a Roche 454 GS-FLX Titanium platform at the IBIS/Université Laval Plate-forme d'Analyses Génomiques (Québec, QC). The raw pyrosequencing reads have been deposited in the NCBI Sequence Read Archive with accession number SRA050236.
Due to the major differences in results from the present study and from earlier archaeal clone libraries for Lake A10, archaeal taxa were further checked by three independent methods using May 2008 samples. First, the 2, 12 and 32 m samples were sent for pyrosequencing by a commercial laboratory (Research and Testing Laboratory, Lubbock, TX) using V3–V4 archaeal primers 340F and 806R which cover the same region as the primers used in Pouliot et al.10. The company failed to amplify the 32 m sample. Second, we were able to amplify the 32 m sample (but the 12 m failed) using the Pouliot et al.10 V2–V5 archaeal primers A109F and A905R which we purified and quantified as above. PCR products were ligated and cloned using the StrataClone PCR Cloning Kit (Agilent). Approximately 20 insert-positive colonies were then sequenced using an Applied Biosystems 3730XL at the CHUL Plateforme de Séquençage (Québec, QC). Finally, the original 12 and 32 m V6–V8 tagged amplicons previously prepared for pyrosequencing were ligated, cloned and sequenced as above.
Pre-processing and quality control of raw sequences
Raw 454 reads were processed to remove low-quality reads defined using the following criteria: (i) presence of uncertain bases (one or more Ns); (ii) short reads (<150 bp after adaptor and bar-code removal); (iii) unusually long reads (greater than expected amplicon size); and (iv) reads with incorrect F primer sequence. Reads were also trimmed of all bases beyond the R primer. These quality-control steps have been shown to reduce 454 sequencing error rates to <0.2%59 without the need for more involved denoising applications60 that can be computationally prohibitive and whose theoretical assumptions are not universally accepted61. The above, high-quality “final reads” were then aligned by Domain using Mothur62,63 (http://www.mothur.org/) against the provided SILVA reference alignments using the ksize = 9 parameter. The resulting alignments were manually refined by removing those reads that were misaligned, generating the high-quality “final aligned reads” used for all downstream analyses (Supplementary Table S1). Input reads for each physical sample associated with its unique identifying bar-code tag were then randomly re-sampled from the total reads available to arrive at the same number of sequences for each, which was equal to the bar-code with the smallest number of sequences: 2223 per sample for Bacteria and 1005 per sample for Archaea.
OTU and taxonomic analyses
The final aligned reads were clustered into Operational Taxonomic Units (OTUs) at the ≥97% similarity level using furthest-neighbor clustering in Mothur63 which approximates genus or species, depending on the group64, for Bacteria and Archaea. Singletons, OTUs comprised of single sequences occurring only once in the dataset, were removed at this step. Measures of diversity, rarefaction and community similarity analyses were carried out in Mothur. Bacteria and Archaea OTUs were taxonomically identified within Mothur with 50% bootstrap cut-off, using user-designed taxonomy outlines and reference sequence databases which were trimmed to the V6–V8 region, as recommended by Werner et al.65. Multiple studies on real and simulated pyrosequencing reads, often on previous-generation reads of smaller size than ours, have shown classification accuracies around 95% at the genus level59,66,67 and have found that they accurately recreate community phylogeny68 and diversity69,70. Bacterial classification was based upon the 33 315 sequence “GreenGenes97” reference files for pyrosequencing65 (greengenes.lbl.gov/Download/) modified to remove a small amount of sequences with little taxonomic information (unclassified at the phylum level) and modified to include missing genus information based upon a consensus between the original GreenGenes classification and the results of the Classifier tool of the RDP database71 using the 95% bootstrap cut-off value. Archaeal classification was based on the SILVA taxonomy outline and reference sequence set provided with Mothur (2288 sequences; www.mothur.org/wiki/Silva_reference_files) which we modified to include recent proposals of Thaumarchaeota classification72. The modified reference sequence databases and taxonomy outlines are available upon request. Common “unclassified OTUs” generated from the above techniques were further identified using BLASTn at the NCBI. Following a literature search, approximately 31% of the total bacterial sequences (all depths/samples combined) could be matched to genera or species (Supplementary Table S4) with known physiology and were classified as photosynthetic or involved in sulfur, nitrogen, hydrogen, methane and Fe/Mn biogeochemical cycles (Figure 5). Statistical analyses (Mann-Whitney test of non-normally distributed sample means) were carried out with PAST (http://folk.uio.no/ohammer/past/).
References
Coolen, M. J. L. et al. Evolution of the methane cycle in Ace Lake (Antarctica) during the Holocene: Response of methanogens and methanotrophs to environmental change. Org. Geochem. 35, 1151–1167 (2004).
Canfield, D. E. & Green, W. J. The cycling of nutrients in a closed-basin Antarctic Lake: Lake Vanda. Biogeochem. 1, 233–256 (1984).
Green, W. J., Canfield, D. E., Lee, G. F. & Jones, R. A. Mn, Cu and Cd distributions and residence times in closed basin Lake Vanda (Wright Valley, Antarctica). Hydrobiologia 134, 237–248 (1986).
Lee, P. A. et al. Elevated levels of dimethylated-sulfur compounds in Lake Bonney, a poorly ventilated Antarctic lake. Limnol. Oceanogr. 49, 1044–1055 (2004).
Priscu, J. C. The biogeochemistry of nitrous oxide in permanently ice-covered lakes of the McMurdo Dry Valleys, Antarctica. Glob. Change Biol. 3, 301–315 (1997).
Vincent, W. F., Downes, M. T. & Vincent, C. L. Nitrous oxide cycling in Lake Vanda, Antarctica. Nature 292, 618–620 (1981).
Tomkins, J. D., Lamoureux, S. F., Antoniades, D. & Vincent, W. F. Sedimentology of perennial ice-covered, meromictic Lake A, Ellesmere Island, at the northern extreme of Canada. Can. J. Earth Sci. 46, 83–100 (2009).
Vincent, A. C., Mueller, D. R. & Vincent, W. F. Simulated heat storage in a perennially ice-covered high Arctic lake: Sensitivity to climate change. J. Geophy. Res. 113, C04036 (2008).
Van Hove, P., Vincent, W. F., Galand, P. E. & Wilmotte, A. Abundance and diversity of picocyanobacteria in High Arctic lakes and fjords. Algo. Stud. 126, 209–227 (2008).
Pouliot, J., Galand, P. E., Lovejoy, C. & Vincent, W. F. Vertical structure of archaeal communities and the distribution of ammonia monooxygenase A gene variants in two high Arctic lakes. Environ. Microbiol. 11, 687–699 (2009).
Antoniades, D. et al. Bacterial dominance of phototrophic communities in a High Arctic lake and its implications for paleoclimate analysis. Polar Sci. 3, 147–161 (2009).
Lauro, F. M. et al. An integrative study of a meromictic lake ecosystem in Antarctica. ISME J. 5, 879–895 (2011).
Comeau, A. M., Li, W. K. W., Tremblay, J.-E., Carmack, E. C. & Lovejoy, C. Changes in Arctic Ocean microbial community structure following the 2007 record sea ice minimum. PLoS ONE 6, e27492 (2011).
Veillette, J., Martineau, M.-J., Antoniades, D., Sarrazin, D. & Vincent, W. F. Effects of loss of perennial lake ice on mixing and phytoplankton dynamics: Insights from High Arctic Canada. Ann. Glaciol. 51, 56–70 (2010).
Prowse, T. et al. Past and future changes in lake and river ice. Ambio 40, 53–62 (2011).
Galand, P. E., Casamayor, E. O., Kirchman, D. L., Potvin, M. & Lovejoy, C. Unique archaeal assemblages in the Arctic Ocean unveiled by massively parallel tag sequencing. ISME J. 3, 860–869 (2009).
DeLong, E. F. & Pace, N. R. Environmental diversity of Bacteria and Archaea. Syst. Biol. 50, 470–478 (2001).
Gibson, J. A. E. et al. Geochemistry of ice-covered meromictic Lake A in the Canadian High Arctic. Aquat. Geochem. 8, 1–23 (2002).
Yurkov, V. V. in The Prokaryotes: A Handbook on the Biology of Bacteria. Volume 5: Proteobacteria: Alpha and Beta Subclasses (eds M. M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer & E. Stackebrandt) (Springer, 2006).
Martinez-Romero, E. in The Prokaryotes: A Handbook on the Biology of Bacteria. Volume 2: Ecophysiology and Biochemistry (eds M. M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleife & E. Stackebrandt) (Springer, 2006).
Overmann, J. in The Prokaryotes: A Handbook on the Biology of Bacteria. Volume 7: Proteobacteria: Delta and Epsilon Subclasses, Deeply Rooting Bacteria (eds M. M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer & E. Stackebrandt) (Springer, 2006).
Lovley, D. in The Prokaryotes: A Handbook on the Biology of Bacteria. Volume 2: Ecophysiology and Biochemistry (eds M. M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleife & E. Stackebrandt) (Springer, 2006).
Heising, S., Richter, L., Ludwig, W. & Schink, B. Chlorobium ferrooxidans sp. nov., a phototrophic green sulfur bacterium that oxidizes ferrous iron in coculture with a “Geospirillum” sp. strain. .Arch. Microbiol. 172, 116–124 (1999).
Rankin, L. M., Gibson, J. A. E., Franzmann, P. D. & Burton, H. R. The chemical stratification and microbial communities of Ace Lake, Antarctica: A review of the characteristics of a marine-derived meromictic lake. Polarforschung 66, 33–52 (1999).
Lentini, V., Gugliandolo, C. & Maugeri, T. L. Vertical distribution of Archaea and Bacteria in a meromictic lake as determined by fluorescent in situ hybridization. Curr. Microbiol. 64, 66–74 (2012).
Mincer, T. J. et al. Quantitative distribution of presumptive archaeal and bacterial nitrifiers in Monterey Bay and the North Pacific Subtropical Gyre. Environ. Microbiol. 9, 1162–1175 (2007).
Barberán, A., Fernández-Guerra, A., Auguet, J.-C., Galand, P. E. & Casamayor, E. O. Phylogenetic ecology of widespread uncultured clades of the Kingdom Euryarchaeota. Mol. Ecol. 20, 1988–1996 (2011).
Dimitriu, P. A., Pinkart, H. C., Peyton, B. M. & Mormile, M. R. Spatial and temporal patterns in the microbial diversity of a meromictic soda lake in Washington State. Appl. Environ. Microbiol. 74, 4877–4888 (2008).
Gibson, J. A. E. The meromictic lakes and stratified marine basins of the Vestfold Hills, East Antarctica. Antarct. Sci. 11, 175–192 (1999).
Gregersen, L. H. et al. Dominance of a clonal green sulfur bacterial population in a stratified lake. FEMS Microbiol. Ecol. 70, 30–41 (2009).
Koizumi, Y., Kojima, H. & Fukui, M. Dominant microbial composition and its vertical distribution in saline meromictic Lake Kaiike (Japan) as revealed by quantitative oligonucleotide probe membrane hybridization. Appl. Environ. Microbiol. 70, 4930–4940 (2004).
Galand, P. E. et al. Phylogenetic and functional diversity of Bacteria and Archaea in a unique stratified lagoon, the Clipperton atoll (N Pacific). FEMS Microbiol. Ecol. 79, 203–217 (2012).
Humayoun, S. B., Bano, N. & Hollibaugh, J. T. Depth distribution of microbial diversity in Mono Lake, a meromictic soda lake in California. Appl. Environ. Microbiol. 69, 1030–1042 (2003).
Lehours, A.-C., Evans, P., Bardot, C., Joblin, K. & Gérard, F. Phylogenetic diversity of Archaea and Bacteria in the anoxic zone of a meromictic lake (Lake Pavin, France). Appl. Environ. Microbiol. 73, 2016–2019 (2007).
Murray, A. E., Peng, V., Tyler, C. & Wagh, P. Marine bacterioplankton biomass, activity and community structure in the vicinity of Antarctic icebergs. Deep Sea Res. II 58, 1407–1421 (2011).
Clingenpeel, S. et al. Yellowstone Lake: High-energy geochemistry and rich bacterial diversity. Environ. Microbiol. 13, 2172–2185 (2011).
van den Wyngaert, S., Salcher, M. M., Pernthaler, J., Zeder, M. & Posch, T. Quantitative dominance of seasonally persistent filamentous cyanobacteria (Planktothrix rubescens) in the microbial assemblages of a temperate lake. Limnol. Oceanogr. 56, 97–109 (2011).
Shaw, A. K. et al. It's all relative: Ranking the diversity of aquatic bacterial communities. Environ. Microbiol. 10, 2200–2210 (2008).
Salcher, M. M, Pernthaler, J. & Posch, T. Seasonal bloom dynamics and ecophysiology of the freshwater sister clade of SAR11 bacteria ‘that rule the waves’ (LD12). ISME J. 5, 1242–1252 (2011).
Walsh, D. A. et al. Metagenome of a versatile chemolithoautotroph from expanding oceanic dead zones. Science 326, 578–582 (2009).
Schmidtova, J., Hallam, S. J. & Baldwin, S. A. Phylogenetic diversity of transition and anoxic zone bacterial communities within a near-shore anoxic basin: Nitinat Lake. Environ. Microbiol. 11, 3233–3251 (2009).
Madrid, V. M., Taylor, G. T., Scranton, M. I. & Chistoserdov, A. Y. Phylogenetic diversity of bacterial and archaeal communities in the anoxic zone of the Cariaco Basin. Appl. Environ. Microbiol. 67, 1663–1674 (2001).
Santelli, C. M. et al. Abundance and diversity of microbial life in ocean crust. Nature 453, 653–656 (2008).
Bauer, M. et al. Whole genome analysis of the marine Bacteroidetes ‘Gramella forsetii’ reveals adaptations to degradation of polymeric organic matter. Environ. Microbiol. 8, 2201–2213 (2006).
Grossart, H.-P., Levold, F., Allgaier, M., Simon, M. & Brinkhoff, T. Marine diatom species harbour distinct bacterial communities. Environ. Microbiol. 7, 860–873 (2005).
Pereira, S. et al. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 33, 917–941 (2009).
Borin, S. et al. DNA is preserved and maintains transforming potential after contact with brines of the deep anoxic hypersaline lakes of the Eastern Mediterranean Sea. Saline Systems. 4, 10 (2008).
Pei, A. Y. et al. Diversity of 16S rRNA genes within individual prokaryotic genomes. Appl. Environ. Microbiol. 76, 3886–3897 (2010).
Van Hove, P., Belzile, C., Gibson, J. A. E. & Vincent, W. F. Coupled landscape-lake evolution in High Arctic Canada. Can. J. Earth Sci. 43, 533–546 (2006).
Meyer, K. M. et al. Carotenoid biomarkers as an imperfect reflection of the anoxygenic phototrophic community in meromictic Fayetteville Green Lake. Geobiol. 9, 321–329 (2011).
Ng, C. et al. Metaproteogenomic analysis of a dominant green sulfur bacterium from Ace Lake, Antarctica. ISME J. 4, 1002–1019 (2010).
Halm, H. et al. Co-occurrence of denitrification and nitrogen fixation in a meromictic lake, Lake Cadagno (Switzerland). Environ. Microbiol. 11, 1945–1958 (2009).
Øvreås, L., Forney, L., Daae, F. L. & Torsvik, V. Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl. Environ. Microbiol. 63, 3367–3373 (1997).
van Gemerden, H. & Mas, J. in Anoxygenic Photosynthetic Bacteria (eds RE Blankenship, MT Madigan & CE Bauer) (Springer, 1995).
Hamersley, M. R. et al. Water column anammox and denitrification in a temperate permanently stratified lake (Lake Rassnitzer, Germany). Syst. Appl. Microbiol. 32, 571–582 (2009).
Bratina, B., Stevenson, B., Green, W. & Schmidt, T. Manganese reduction by microbes from oxic regions of the Lake Vanda (Antarctica) water column. Appl. Environ. Microbiol. 64, 3791–3797 (1998).
Vincent, W. F. et al. Extreme ecosystems and geosystems in the Canadian High Arctic: Ward Hunt Island and vicinity. Ecoscience 18, 236–261 (2011).
Harding, T., Jungblut, A., Lovejoy, C. & Vincent, W. F. Microbes in High Arctic snow and implications for the cold biosphere. Appl. Environ. Microbiol. 77, 3234–3243 (2011).
Huse, S. M., Huber, J. A., Morrison, H. G., Sogin, M. L. & Welch, D. M. Accuracy and quality of massively parallel DNA pyrosequencing. Genome Biol. 8, R143 (2007).
Quince, C. et al. Accurate determination of microbial diversity from 454 pyrosequencing data. Nature Meth. 6, 639–641 (2009).
Behnke, A. et al. Depicting more accurate pictures of protistan community complexity using pyrosequencing of hypervariable SSU rRNA gene regions. Environ. Microbiol. 13, 340–349 (2010).
Schloss, P. D. et al. Introducing mothur: Open source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
Schloss, P. D. A high-throughput DNA sequence aligner for microbial ecology studies. PLoS ONE 4, e8230 (2009).
Fraser, C., Alm, E. J., Polz, M. F., Spratt, B. G. & Hanage, W. P. The bacterial species challenge: Making sense of genetic and ecological diversity. Science. 323, 741–746 (2009).
Werner, J. J. et al. Impact of training sets on classification of high-throughput bacterial 16S rRNA gene surveys. ISME J. 6, 94–103 (2012).
Claesson, M. J. et al. Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS ONE 4, e6669 (2009).
Sundquist, A. et al. Bacterial flora-typing with targeted, chip-based Pyrosequencing. BMC Microbiol 7, 108 (2007).
Jeraldo, P., Chia, N. & Goldenfeld, N. On the suitability of short reads of 16S rRNA for phylogeny-based analyses in environmental surveys. Environ. Microbiol. 13, 3000–3009 (2011).
Liu, Z., Lozupone, C., Hamady, M., Bushman, F. D. & Knight, R. Short pyrosequencing reads suffice for accurate microbial community analysis. Nucl. Acids Res. 35, e120 (2007).
Youssef, N., Sheik, C. S., Krumholz, L. R., Najar, F. Z., Roe, B. A. & Elshahed, M. S. Comparison of species richness estimates obtained using nearly complete fragments and simulated pyrosequencing-generated fragments in 16S rRNA gene-based environmental surveys. Appl. Environ. Microbiol. 75, 5227–5236 (2009).
Cole, J. R. et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucl. Acids Res. 37, D141–145 (2009).
Brochier-Armanet, C., Boussau, B., Gribaldo, S. & Forterre, P. Mesophilic Crenarchaeota: Proposal for a third archaeal phylum, the Thaumarchaeota. Nat. Rev. Microbiol. 6, 245–252 (2008).
Acknowledgements
We thank our colleagues at the IBIS/Université Laval Plate-Forme d'Analyses Génomiques for the DNA pyrosequencing; the IBIS Centre de Bio-informatique et de Biologie Computationnelle, Compute-Canada and the CLUMEQ Supercomputing Facility for bioinformatics support. The majority of funding was from Natural Sciences and Engineering Research Council of Canada (NSERC) grants to CL and WFV; other support was from the Canada Research Chair program to WFV, the Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT), the Network of Centres of Excellence (NCE) program ArcticNet and Polar Continental Shelf Program. PG is financially supported by the CNRS. TH was supported by a post graduate fellowship from NSERC. Additional logistics and travel support from Parks Canada and the Northern Scientific Training Program from Aboriginal Affairs and Northern Development Canada is acknowledged. We are indebted to Julie Veillette, Dermot Antoniades, Sophie Charvet and Denis Sarrazin for field and laboratory assistance.
Author information
Authors and Affiliations
Contributions
This work was conceived by WFV and CL Laboratory work was done by AMC and TH. Analysis was carried out by all authors. The manuscript was written and approved by all authors.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Figures and Tables
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Comeau, A., Harding, T., Galand, P. et al. Vertical distribution of microbial communities in a perennially stratified Arctic lake with saline, anoxic bottom waters. Sci Rep 2, 604 (2012). https://doi.org/10.1038/srep00604
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00604
This article is cited by
-
Genomic insights into cryptic cycles of microbial hydrocarbon production and degradation in contiguous freshwater and marine microbiomes
Microbiome (2023)
-
Anthropogenic activities mediate stratification and stability of microbial communities in freshwater sediments
Microbiome (2023)
-
Discovery of a novel bacterial class with the capacity to drive sulfur cycling and microbiome structure in a paleo-ocean analog
ISME Communications (2023)
-
Archaeal and Bacterial Diversity and Distribution Patterns in Mediterranean-Climate Vernal Pools of Mexico and the Western USA
Microbial Ecology (2023)
-
Genomic evidence of functional diversity in DPANN archaea, from oxic species to anoxic vampiristic consortia
ISME Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.