Abstract
Study design:
Neurotrimin (Ntm) is a member of the family of neural cell adhesion molecules. Its expression pattern suggests that Ntm promotes axonal fasciculation, guides nerve fibers to specific targets and stabilizes synapses as it accumulates coincident with synaptogenesis. Strong labeling of Ntm was observed in motor and sensory areas of the postnatal rat cortex. It is not known whether Ntm is present in adult human spinal cord (SC). In the present study, a monoclonal antibody specific for Ntm (1B1), is applied to the first study of the expression of Ntm in normal and injured adult human SC.
Objective:
(1) To investigate the expression pattern of Ntm in adult normal human SC, and (2) to observe the changes of Ntm expression after SC injury and compare the differences between normal and injured adult human SC.
Methods:
Human SC tissue was obtained from necropsies of patients with (n=5) and without (n=4) SC injury. The 1B1 Ntm monoclonal antibody was used for immunohistochemical staining on paraffin embedded sections with an ABC kit.
Results:
(1) In total, 12 slides were analyzed for each group from both cervical and thoracic levels. Motor neurons and Clarke's neurons and glial-like cells were mild to moderately positive in all uninjured SC specimens. (2) In injured SC, no staining was observed in the injury epicenter between two and three levels proximally and distally, but was detected five levels away. (3) In patients older than 67 years of age, Ntm-positive inclusions were present in the white matter of the SC with or without injury. (4) Some meningeal cells were strongly Ntm-positive, especially in the uninjured human SC.
Conclusion:
Ntm is expressed by motor and Clarke's neurons and glial cells in uninjured human SC. The downregulation of Ntm in the injured SC suggests that its expression is regulated by afferent input.
Similar content being viewed by others
Introduction
During development, neuronal networks are formed by a wide variety of membrane-associated and soluble proteins that direct growing axons toward their targets via growth-promoting and inhibiting effects.1 These processes require two kinds of proteins, one promotes neurite outgrowth such as the immunoglobulin of cell adhesion molecules (IgCAMs), cadherin, and integrin superfamilies;2, 3, 4 and the other protein inhibits neurite outgrowth during axonal path finding such as ephrins, semaphorins, and netrins, which inhibit outgrowth in a cell type-specific manner.5, 6, 7
IgCAMs are the key proteins that regulate neurite outgrowth and the development of specific projections. Among the IgCAMs, limbic system-associated membrane protein (LAMP), opioid-binding cell adhesion molecule (OBCAM), and neurotrimin (Ntm) are founding members of the IgLON family,8, 9, 10 which also includes Kilon/neurotractin,11 they represent the earliest and most abundant glycosylphosphatidylinositol (GPI)-anchored proteins expressed by neurons.12
Ntm is expressed at high levels in several developing projection systems of the rat: neurons of the thalamus, subplate lower cortical laminae in the forebrain and in the pontine nucleus, cerebellar granule cells, and Purkinje cells in the hindbrain. Ntm is also expressed at high levels in the olfactory bulb, neural retina, dorsal root ganglia, spinal cord (SC), and in a graded distribution in the basal ganglia and hippocampus.10 In the cerebral cortex, laminae V and VI express the highest levels of Ntm between P0 and P4, although all layers except lamina I show some expression. In later ages and into the adult, strong labeling was observed in motor and sensory areas of the cortex.11 The highest levels at laminae V and VI, as well as in the subplate10 suggest that subplate neurons are required for cortical neurons from layer VI to grow into the thalamus, and neurons from layer V to select their targets in the colliculus, pons, and SC.13 A wide variety of CAMs direct growing axons toward their targets via growth-promoting and inhibiting effects during development, and maintain the precise patterns of neuronal connectivity in the mature nervous system.14
Neural cell adhesion molecule (N-CAM), to which Ntm belongs, is present in adult rat CNS: the molecular layer of the cerebellum, ependymal cells surrounding the central canal, axons of the white matter, and in Lamina X of the gray matter of the SC. It is not known whether Ntm is present in adult human SC. The present study, using a new monoclonal antibody that specifically recognizes Ntm, is designed to study the expression of Ntm in the normal and injured adult human SC.
Methods
Generation of anti-Ntm monoclonal antibody
Anti-Ntm monoclonal antibody was generated at the Department of Cell Biology, New York University School of Medicine as described previously.11 In brief, a total of 160 μg of soluble recombinent Ntm-Fc protein was injected into a BALB/c mouse footpad four times. After fusion of the mouse nodal lymphocytes with the myeloma cell line NS-1, cells were distributed into 96-well plates containing HAT (hypozanthin, aminopterin, and thymidine) selection medium as described previously.15 A screening for antibodies was performed approximately 2 weeks later by ELISA with Ntm-Fc in order to select clones reactive with the Ntm portion of the chimeric protein. A second stage of screening was performed by immunofluorescence with the CHO cells transfected with myc-tagged Ntm.16 Hybridomas that elicited a positive ELISA reaction or immunostaining were expanded and cloned by the limiting dilution method.15 One line out of 16, termed clone 1B1, produced an antibody that reacted positively with Ntm-Fc by ELISA. Clone 1B1 produced an antibody of the immunoglobulin G1 (IgG1) subclass as determined by clonotyping system/HRP (Southern Biotechnology Associates, Birmingham, AL, USA). Previous research determined that 1B1 is an IgG1 subclass monoclonal antibody specific to Ntm detection by immunocytochemical staining, but does not recognize Ntm-Fc or lysates of Ntm-transfected cells on Western blots.11
Human SC specimens
Five injured human SCs including the injured epicenter were obtained from the Spinal Cord Tissue Bank of the Miami Project to Cure Paralysis, University of Miami School of Medicine (Miami FL, USA). The four uninjured SCs were obtained from the Pathology Department of Specialties Hospital, Centro Medico Nacional Siglo XXI, Instituto Mexicano del Seguro Social (IMSS), Mexico. The demographic information about the patients from both groups is presented in Table 1. The human SC tissues collected from necropsy of patients were fixed in 10% formaldehyde for 7 days, then washed and embedded in paraffin. Serial coronal sections in 10 μm thickness were cut using a microtome.
Immunocytochemical staining standardization
For testing the optimal dilution of the 1B1 Ntm antibody and its immunocytochemical staining profile, two rat pups (P7) were subjected to transcardiac perfusion with 10% formaldehyde. The intact thoracic SC was removed and put in paraformaldehyde overnight. Then the specimens were dehydrated and embedded in paraffin. The SCs were cut serially and coronally into sections with a thickness of 10 μm using a microtome, and mounted on poly-L-lysine-coated slides (Sigma Diagnostics, St Louis, MO, USA). Sections were selected and treated with 0.3% hydrogen peroxide for 30 min to quench endogenous peroxidase reactivity, and then thoroughly washed in changes of TBS (Tris-buffered saline, 0.9% sodium chloride plus 0.05 M Tris base and add 1 N HCL to pH 7.4) and TBST (TBS+0.25% Triton). After a preblocking with a solution of 3% normal goat serum (NGS), these sections were incubated overnight at 4°C in a variety of concentrations of 1B1 Ntm monoclonal antibody (from 1:500 to 1:3000 dilution). Control sections were incubated with mouse IgG (Vector Lab., Burlingame, CA, USA) instead of 1B1 antibody. After repeated washings with TBS and TBST plus 1% NGS, sections were incubated for 1 h with anti-mouse IgG1a (diluted 1:200, Vector Lab., Burlingame, CA, USA), then 1 h with Avidin-Biotin Complex (ABC Elite kit, Vector Laboratory). 3,3′-Diaminobenzidine tetrahydrochloride dihyadrate (DAB, Sigma Chemical Co., St Louis, MO, USA) was used as the chromogen. After staining, the sections were dehydrated in alcohol and coverslipped with Permouth.
Immunocytochemical staining of human SC tissue
Sections from injured epicenter and a variety of levels of injured or uninjured SC from the two groups of patients were selected for immunocytochemical staining. The procedure was performed as described previously with the SC tissue from rat pups, except that a 3% normal human serum was used in the procedure instead of 3% NGS. To test the optimal reaction concentration once more, uninjured SC sections were incubated overnight at 4°C with a variety of diluted 1B1 Ntm monoclonal antibody solution (1:500–1:3000). Eventually, the most specific staining was observed with 1:1000 dilution of 1B1 antibody, which was consistent with the staining of rat pups SCs. This dilution was consequently used in all subsequent stainings. Control sections were always incubated without antibody, carried out in parallel. Sections selected from the injured SC included the injury epicenter, and two levels from the epicenter both proximally and distally, or five levels away from injury epicenter (as show in Table 1). The intensity of reaction product in the cells was visualized with a Zeiss Axiophot microscopy and scored as negative (−), lightly positive (+), moderately positive (++) and strongly positive (+++).
Results
The best immunostaining reaction for rat pup SC tissue was obtained by using 1:1000 diluted 1B1 solution (data not shown). Although the staining was not strong, many moderately stained motoneurons were clearly observed. Coincidentally, the optimal concentration of 1B1 solution for adult human SC tissue was found to be the same as 1:1000 dilution.
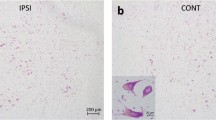
All four uninjured SC specimens showed light or moderate positive inmmunostaining within motor neurons of the ventral horn and Clarke's nucleus (Figure 1a and b). Both cervical and thoracic motor neurons were moderately stained with Ntm. By contrast, among the injured SCs at the injury epicenter, the neurons were always found to be negative for Ntm immunostaining (Figure 2a). Neurons located two levels away from the injury epicenter both proximally and distally were also negative, except on one occasion: lightly positive neurons were showing in a section from C7, which was two levels below the injury epicenter (Figure 2b). In one case, the negative neurons were three levels below the C6 epicenter. However, among the injured SCs, the sections taken five levels away from the injury epicenter showed positive staining. Interestingly, glial-like cells were also observed to be Ntm-positive in uninjured SCs (Figures 1a and 3), but negative in injured SCs around the epicenter (Figure 2a). Among the patients who were older than 67 years of age, inclusions could be seen within the white matter that also showed moderate positive staining for Ntm, which was often observed both in the injured and uninjured SCs (Figure 4a and b). Surprisingly, some leptomeningeal cells in the spinal meninges were stained strongly positive for Ntm, especially in the uninjured SC. Relatively fewer of such positive cells presented around the injury epicenter in the injured SC (Figure 5a and b).
An uninjured human thoracic spinal cord (SC) – 10 μm-thick paraffin section was immunostained with 1:1000 diluted 1B1 Ntm antibody by ABC method. (a) Neurons (arrows) and glial-like cells (arrowheads) with moderately positive staining (++) for Ntm are seen in the anterior horn of the SC. (b) A higher-power view of Ntm-positive motoneurons in the thoracic SC. Neurons within Clarke's nucleus are well delineated and have a distinct nucleus
An injured human cervical SC in 10-μm-thick section was immunostained with 1:1000 diluted 1B1 Ntm antibody by ABC method. (a) At C5 of the injured SC epicenter, it is completely negative for Ntm staining. (b) At C7, which is two levels below the injured epicenter, neurons with lightly positive (+) staining begin to appear (arrows)
(a) In an 86-year-old patient with SC injury, inclusions are showing in the white matter on the paraffin section of the cervical SC (arrows, H and E staining). (b) These inclusions are moderately positive (++) for Ntm when immunostained with 1:1000 diluted 1B1 Ntm antibody by ABC method (arrows). Similar staining appeared in both injured and uninjured human SCs patients over 67 years old
The injured and uninjured human SCs were immunostained with 1:1000 diluted 1B1 Ntm antibody by ABC method on their 10-μm-thick paraffin section. (a) Leptomeningeal cells within the leptomeninx are strongly positive (+++) for Ntm (arrows) in an uninjured SC. (b) The strongly positive (+++) meningeal cells for Ntm are also showing within an injured SC section that is nine levels away from the injury epicenter
Discussion
Interactions between CAMs of the growth cone membrane and molecules in the extracellular matrix or on the membranes of other cells in contact with the growth cone, can give cues that direct axonal outgrowth along appropriate projectional pathways.14 Among the IgCAMs, the IgLON family comprises LAMP, OBCAM, Ntm, and Kilon.9, 10, 17, 18 In rats Ntm is detectable from E15 prenatally, rises gradually, and peaks during the first and second postnatal week. Thereafter, Ntm message levels gradually decline to about half maximal levels in the adult.10, 19
In the current study, Ntm was detected in motoneurons of the rat at day 7, although staining was not strong. Furthermore, we have characterized the expression of Ntm in human tissue and demonstrated that motor neurons were lightly or moderately positive in all of uninjured SC specimens. These results are consistent with the description of adult rat tissue by Struyk et al10 as well as the positive staining of rat motor neurons in vitro (P Shrager, J Salzer, unpublished observations).
Clarke's neurons are the cells of origin of the spinocerebellar tract; their axons ascend in the lateral SC to reach the cerebellum on the dorsolateral aspect of medulla oblongata and pons. Both cerebellar afferent neurons and their fiber tract projections to granule cells and Purkinje cells having high levels of Ntm expression in early postnatal stages, but low levels of immunoreactivity in adult rats.20 The finding that Ntm is present in neurons of Clarke's nucleus in adult human SC may indicate the importance of Ntm on development and stabilization of cerebellar afferent pathways, together with its proposed role in the establishment of sensory-motor circuits.
In adult human injured SC, no Ntm immunoreactivity was detected at the injury epicenter and two levels proximally and distally, whereas positive staining persisted five levels away from the injury epicenter. The loss of Ntm expression within and around the injury epicenter suggests it is downreglated secondary to loss of afferent input. A similar loss of LAMP expression following deafferentation in the cat SC has been reported,20 suggesting that IgLON expression may generally be regulated by activity-dependent mechanisms. Alternatively, Ntm expression may decline in response to direct injury.
In patients older than 67 years of age, moderate Ntm-positive-stained inclusions could be seen around white matter. These inclusions are most likely associated with lipofusion accumulations and consist mainly of amyloid. Staining may reflect false positivity.
In addition to Ntm expression by neurons, we present the first evidence that some non-neuronal cells also express Ntm, including glial-like cells in the human SC, as well as leptomeningeal cells. Previous reports indicate that Ntm is not expressed at significant levels by cultured astrocytes or Schwann cells based on biochemistry and PCR analysis.10, 12 Meningeal cells have been shown to produce neuronal factors,21, 22, 23 which may inhibit axonal regrowth. However, Lakatos et al24 recently reported that adding meningeal cells to transplanted olfactory ensheathing cells (OECs) after SC injury, significantly improved the extent of remyelination compared with the purified OECs. Therefore, it is possible that meningeal cells may be involved in axonal re-growth. The precise cells in the human SC that are Ntm-positive and the significance of this expression will require further study.
References
Yu TW, Bargmann CI . Dynamic regulation of axon guidance. Nat Neurosci 2001; 4 (Suppl 1): 169–1176.
Salzer JL, Colman DR . Mechanisms of cell adhesion in the nervous system: role of the immunoglobulin gene superfamily. Dev Neurosci 1989; 11: 377–390.
Matsunaga M, Hatta K, Nagafuchi A, Takeichi M . Guidance of optic nerve fibers by N-cadherin adhesion molecules. Nature 1988; 334: 62–64.
Reichardt LF, Tomaselli KJ . Extracellular matrix molecules and their receptors: functions in neural development. Annu Rev Neurosci 1991; 14: 531–570.
Drescher U, Bonhoeffer F, Müller BK . The Eph family in retinal axon guidance. Curr Opin Neurobiol 1997; 7: 75–80.
Kolodkin AL, Matthes DJ, Goodman CS . The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 1993; 75: 1389–1399.
Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessel TM, Tessier-Lavigne M . The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 1994; 78: 409–424.
Schofield PR et al. Molecular characterization of a new immunoglobulin superfamily protein with potential roles in opioid binding and cell contact. EMBO J 1989; 8: 489–495.
Pimenta AF et al. The limbic system-associated membrane protein is an Ig superfamily member that mediates selective neuronal growth and axon targeting. Neuron 1995; 15: 287–297.
Struyk AF, Canoll PD, Wolfgang MJ, Rosen CL, D'ustachio P, Salzer JL . Cloning of neurotrimin defines a new subfamily of differentially expressed neural cells adhesion molecules. J Neurosci 1995; 15: 2141–2156.
Salzer JL, Rosen CL, Struyk AF . GPI anchored proteins in neural cell adhesion. In: Colman DR (ed) Cell Adhesion, Vol. 16, Advances in Molecular and Cellular Biology. CT Jai: Greenwich 1996, pp 193–222.
Gil OD et al. Complementary expression and heterophilic interactions between IgLON family members Neurotrimin and LAMP. J Neurobiol 2002; 51: 190–204.
McConnell SK, Ghosh A, Shatz CJ . Subplate pioneers and the formation of descending connections from cerebral cortex. J Neurosci 1994; 14: 1892–1907.
Doherty P, Walsh FS . Cell adhesion molecules, second messengers and axonal growth. Curr Opin Neurobiol 1992; 2: 595–601.
Hockfield S, Carlson S, Evans C, Levitt P, Pintar J, Silberstein L (eds). Selected Methods for Antibody and Nucleic Acid Probes. Cold Spring Harbor Laboratory Press: Plainview, NY 1993, pp 59–109.
Gil OD, Zannazi G, Struyk AF, Salzer JL . Neurotrimin mediates bifunctional effects on neurite outgrowth via homophilic and heterophilic interactions. J Neurosci 1998; 18: 9321–9325.
Schofield PR et al. Molecular characterization of a new immunoglobulin superfamily protein with potential roles in opioid binding and cell contact. EMBO J 1989; 8: 489–495.
Miyata S et al. Biochemical and ultrastructural analyses of IGLON cell adhesion molecules, Kilon and OBCAM in the rat brain. Neuroscience 2003; 117: 645–658.
Chen S, Gil O, Ren YQ, Zanazzi G, Salzer JL, Hillman DE . Neurotrimin expression during cerebellar development suggest roles in axon fasciculation and synaptogenesis. J Neurocytol 2001; 30: 927–937.
Goldberger ME, Paige E, Croul S, Levitt P . Partial deafferentation of cat spinal neurons results in permanent changes in cell surface molecular expression and metabolic activity. Exp Neurol 1993; 123: 74–80.
Niclou SP, Franssen EHP, Ehlert EME, Taniguchi M, Verhaagen J . Meningeal cell-derived semaphorin 3A inhibits neurite outgrowth. Mol Cell Neurosc 2003; 24: 902–912.
Shearer MC et al. The astrocyte/meningeal cell interface is a barrier to neurite outgrowth which can be overcome by manipulation of inhibitory molecules or axonal signaling pathways. Mol Cell Neurosci 2003; 24: 913–925.
Hirsch S, Bahr M . Immunocytochemical charaterization of reactive optic nerve astrocytes and meningeal cells. GLIA 1999; 26: 36–46.
Lakatos A, Smith PM, Barnett SC, Franklin RJM . Meningeal cells enhance limited CNS remyelination by transplanted olfactory enheathing cells. Brain 2003; 126: 598–609.
Acknowledgements
This study was supported by The Health Foundation of South Florida and The Miami Project to Cure Paralysis. We gratefully acknowledge Dr Lourdes Cabrera for providing uninjured adult human spinal cord samples.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grijalva, I., Li, X., Marcillo, A. et al. Expression of neurotrimin in the normal and injured adult human spinal cord. Spinal Cord 44, 280–286 (2006). https://doi.org/10.1038/sj.sc.3101842
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101842