Abstract
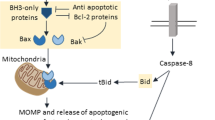
Apoptosis is accompanied by the activation of a number of apoptotic proteases (caspases) which selectively cleave specific cellular substrates. Caspases themselves are zymogens which are activated by proteolysis. It is widely believed that `initiator' caspases are recruited to and activated within apoptotic signalling complexes, and then cleave and activate downstream `effector' caspases. While activation of the effector caspase, caspase-3, has indeed been observed as distal to activation of several different initiator caspases, evidence for a further downstream proteolytic cascade is limited. In particular, there is little evidence that cellular levels of caspase-3 that are activated via one pathway are sufficient to cleave and activate other initiator caspases. To address this issue, the ability of caspase-3, activated upon addition to cytosolic extracts of cytochrome c, to cause cleavage of caspase-2 was investigated. It was demonstrated that cleavage of caspase-2 follows, and is dependent upon, activation of caspase-3. Moreover, the activation of both caspases was inhibited by Bcl-2. Together, these data indicate that Bcl-2 can protect cells from apoptosis by acting at a point downstream from release of mitochondrial cytochrome c, thereby preventing a caspase-3 dependent proteolytic cascade.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Balch WE, Dunphy WG, Braell WA and Rothman JE. . 1984 Cell 39: 405–416.
Boldin MP, Goncharov TM, Goltsev YV and Wallach D. . 1996 Cell 85: 803–815.
Butt AJ, Harvey NL, Parasivam G and Kumar S. . 1998 J. Biol. Chem. 273: 6763–6768.
Chinnaiyan AM, O'Rourke K, Tewari M and Dixit VM. . 1995 Cell 81: 505–512.
Cohen GM. . 1997 Biochem. J. 326: 1–16.
Duan H and Dixit VM. . 1997 Nature 385: 86–89.
Faleiro L, Kobayashi R, Fearnhead H and Lazebnik Y. . 1997 EMBO J. 16: 2271–2281.
Harvey NL, Trapani JA, Fernandes-Alnemri T, Litwack G, Alnemri ES and Kumar S. . 1996 Genes Cells 1: 673–685.
Hockenbery D, Nunez G, Milliman C, Schreiber RD and Korsmeyer SJ. . 1990 Nature 348: 334–336.
Hsu H, Xiong J and Goeddel DV. . 1995 Cell 81: 495–504.
Li H, Bergeron L, Cryns V, Pasternack MS, Zhu H, Shi L, Greenberg A and Yuan J. . 1997a J. Biol. Chem. 272: 21010–21017.
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES and Wang X. . 1997b Cell 91: 479–490.
Liu X, Kim CN, Yang J, Jemmerson R and Wang X. . 1996 Cell 86: 147–158.
Martin SJ, Amarante-Mendes GP, Shi L, Chuang T-H, Casiano CA, O'Brien GA, Fitzgerald P, Tan EM, Bokoch GM, Greenberg AH and Green DR. . 1996 EMBO J. 15: 2407–2416.
Muzio M, Salvesen GS and Dixit VM. . 1997 J. Biol. Chem. 272: 2952–2956.
Ng FWH, Nguyen M, Kwan A, Branton PE, Nicholson DW, Cromlish JA and Shore GC. . 1997 J. Cell Biol. 139: 327–338.
Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, Munday NA, Raju SM, Smulson ME, Yamin TT, Yu VL and Miller DK. . 1995 Nature 376: 37–43.
Orth K, O'Rourke K, Salvesen GS and Dixit VM. . 1996 J. Biol. Chem. 271: 20977–20980.
Pan G, Humke EW and Dixit VM. . 1998a FEBS Lett. 426: 151–154.
Pan G, O'Rourke K and Dixit VM. . 1998b J. Biol. Chem. 273: 5841–5845.
Polverino AJ and Patterson SD. . 1997 J. Biol. Chem. 272: 7013–7021.
Reed JC. . 1997 Cell 91: 559–562.
Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B and Borner C. . 1998 Nature 391: 496–499.
Stanger BZ, Leder P, Lee T-H, Kim E and Seed B. . 1995 Cell 81: 513–523.
Takahashi A, Hirata H, Yonehara S, Imai Y, Lee K-K, Moyer RW, Turner PC, Mesner PW, Okazaki T, Sawai H, Kishi S, Yamamoto K, Okuma M and Sasada M. . 1997 Oncogene 14: 2741–2752.
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T-I, Jones DP and Wang X. . 1997 Science 275: 1129–1132.
Yang X, Chang HY and Baltimore D. . 1998 Mol. Cell 1: 319–325.
Zhivotovsky B, Orrenius S, Brustugun OT and Døskeland SO. . 1998 Nature 391: 449–450.
Zou H, Henzel WJ, Liu X, Lutschg A and Wang X. . 1997 Cell 90: 405–413.
Acknowledgements
This research is supported by the Medical Research Council (Grants G117/153 and G9533795MA to PGW). We are grateful to B Saulier-Le Drean for the gift of Xenopus PARP cDNA and to Stephen Green (Zeneca) for active caspase-3 and for caspase-2 cDNA.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Swanton, E., Savory, P., Cosulich, S. et al. Bcl-2 regulates a caspase-3/caspase-2 apoptotic cascade in cytosolic extracts. Oncogene 18, 1781–1787 (1999). https://doi.org/10.1038/sj.onc.1202490
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1202490
Keywords
This article is cited by
-
Downregulation of lncRNA TUG1 attenuates inflammation and apoptosis of renal tubular epithelial cell induced by ischemia-reperfusion by sponging miR-449b-5p via targeting HMGB1 and MMP2
Inflammation (2020)
-
Centaurea bruguierana inhibits cell proliferation, causes cell cycle arrest, and induces apoptosis in human MCF-7 breast carcinoma cells
Molecular Biology Reports (2020)
-
Knockdown of ribosomal protein S15A induces human glioblastoma cell apoptosis
World Journal of Surgical Oncology (2016)
-
Neurological Toxicity of Individual and Mixtures of Low Dose Arsenic, Mono and Di (n-butyl) Phthalates on Sub-Chronic Exposure to Mice
Biological Trace Element Research (2016)
-
Shikonin selectively induces apoptosis in human prostate cancer cells through the endoplasmic reticulum stress and mitochondrial apoptotic pathway
Journal of Biomedical Science (2015)