Abstract
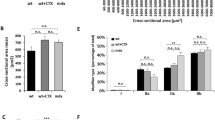
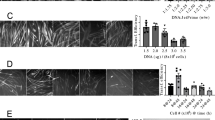
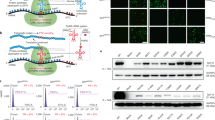
It is well established that mutations in specific structural elements of the motor protein myosin are directly linked to debilitating diseases involving malfunctioning striated muscle cells. A potential way to study the relationship between myosin structure and function is to express exogenous myosin in vivo and determine contractile properties of the transgenic muscle cells. However, in vivo expression of functional levels of contractile proteins using transient transgenesis in skeletal muscle has not been demonstrated. Presently, we used in vivo gene transfer to express high levels of full-length myosin light chain (MLC) in skeletal muscle fibers of Rana pipiens. Anterior tibialis (AT) muscles were injected with cardiotoxin to cause degeneration and then injected at various stages of regeneration with plasmid expression vectors encoding full-length MLC1f. In fibers from the most robustly transfected muscles 3 weeks after plasmid injections, trans-MLC1f expression averaged 22–43% of the endogenous MLC1f. Trans-MLC1f expression was the same whether a small epitope tag was placed on the C- or N-terminus and was highly variable along individual fibers. Confocal microscopy of skinned fibers showed correct sarcomeric incorporation of trans-MLC1f. The expression profile of myosin heavy chain isoforms 21 days after transfection was similar to normal AT muscle. These data demonstrate the feasibility of using in vivo gene transfer to probe the structural basis of contractile protein function in skeletal muscle. Based on these promising results, we discuss how further improvements in the level and consistency of myosin transgene expression may be achieved in future studies, and the therapeutic potential of plasmid gene transfer in regenerating muscle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Chamberlain JS . Gene therapy of muscular dystrophy. Hum Mol Genet 2002; 11: 2355–2362.
van Deutekom JC, van Ommen GJ . Advances in Duchenne muscular dystrophy gene therapy. Nat Rev Genet 2003; 4: 774–783.
Wolff JA et al. Direct gene transfer into mouse muscle in vivo. Science 1990; 247: 1465–1468.
Herweijer H, Wolff JA . Progress and prospects: naked DNA gene transfer and therapy. Gene Therapy 2003; 10: 453–458.
Vicat JM et al. Muscle transfection by electroporation with high-voltage and short-pulse currents provides high-level and long-lasting gene expression. Hum Gene Ther 2000; 11: 909–916.
Aihara H, Miyazaki J . Gene transfer into muscle by electroporation in vivo. Nat Biotechnol 1998; 16: 867–870.
Somiari S et al. Theory and in vivo application of electroporative gene delivery. Mol Ther 2000; 2: 178–187.
Mathiesen I . Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Therapy 1999; 6: 508–514.
Mir LM et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci USA 1999; 96: 4262–4267.
Vitadello M et al. Gene transfer in regenerating muscle. Hum Gene Ther 1994; 5: 11–18.
Bieber T et al. Intracellular route and transcriptional competence of polyethylenimine–DNA complexes. J Control Rel 2002; 82: 441–454.
Suh J, Wirtz D, Hanes J . Efficient active transport of gene nanocarriers to the cell nucleus. Proc Natl Acad Sci USA 2003; 100: 3878–3882.
Ow Sullivan MM, Green JJ, Przybycien TM . Development of a novel gene delivery scaffold utilizing colloidal gold–polyethylenimine conjugates for DNA condensation. Gene Therapy 2003; 10: 1882–1890.
Thomas M, Klibanov AM . Conjugation to gold nanoparticles enhances polyethylenimine's transfer of plasmid DNA into mammalian cells. Proc Natl Acad Sci USA 2003; 100: 9138–9143.
Houdusse A, Sweeney HL . Myosin motors: missing structures and hidden springs. Curr Opin Struct Biol 2001; 11: 182–194.
Spudich JA . The myosin swinging cross-bridge model. Nat Rev Mol Cell Biol 2000; 2: 387–392.
Schaub MC, Hefti MA, Zuellig RA, Morano I . Modulation of contractility in human cardiac hypertrophy by myosin essential light chain isoforms. Cardiovasc Res 1998; 37: 381–404.
Kohler J et al. Mutation of the myosin converter domain alters cross-bridge elasticity. Proc Natl Acad Sci USA 2002; 99: 3557–3562.
Lutz GJ, Bremner SN, Bade MJ, Lieber RL . Identification of myosin light chains in Rana pipiens skeletal muscle and their expression patterns along single fibres. J Exp Biol 2001; 204: 4237–4248.
Lutz GJ et al. Quantitative analysis of muscle fiber type and myosin heavy chain distribution in the frog hindlimb: implications for locomotory design. J Muscle Res Cell Motil 1998; 19: 717–731.
Lutz GJ et al. Influence of myosin isoforms on contractile properties of intact muscle fibers from Rana pipiens. Am J Physiol 2002; 282: C835–C844.
Lu QL, Bou-Gharios G, Partridge TA . Non-viral gene delivery in skeletal muscle: a protein factory. Gene Therapy 2003; 10: 131–142.
Bigey P, Bureau MF, Scherman D . In vivo plasmid DNA electrotransfer. Curr Opin Biotechnol 2002; 13: 443–447.
Horsley V et al. Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. J Cell Biol 2001; 153: 329–338.
Seale P, Rudnicki MA . A new look at the origin, function and ‘stem-cell’ status of muscle satellite cells. Dev Biol 2000; 218: 115–124.
Prockop DJ . Further proof of the plasticity of adult stem cells and their role in tissue repair. J Cell Biol 2003; 160: 807–809.
Hill M, Goldspink G . Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol 2003; 549: 409–418.
Goldring K, Partridge T, Watt D . Muscle stem cells. J Pathol 2002; 197: 457–467.
Launay T et al. Expression and neural control of myogenic regulatory factor genes during regeneration of mouse soleus. J Histochem Cytochem 2001; 49: 887–899.
Zak R, Martin AF, Prior G, Rabinowitz M . Comparison of turnover of several myofibrillar proteins and critical evaluation of double isotope method. J Biol Chem 1977; 252: 3430–3435.
Corrie JET et al. Dynamic measurement of myosin light-chain-domain tilt and twist in muscle contraction. Nature 1999; 400: 425–430.
Rayment I et al. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 1993; 261: 50–58.
Huxley HE . The mechanism of muscle contraction. Science 1969; 164: 1356–1366.
Holmes KC, Geeves MA . The structural basis of muscle contraction. Philos Trans R Soc Lond B Biol Sci 2000; 355: 419–431.
Goldman YE . Wag the tail: structural dynamics of actomyosin. Cell 1998; 93: 1–4.
Uyeda TQP, Abramson PD, Spudich JA . The neck region of the myosin motor domain acts as a lever arm to generate movement. Proc Natl Acad Sci 1996; 93: 4459–4464.
Lowey S, Waller GS, Trybus KM . Function of skeletal muscle myosin heavy chain and light chain isoforms by an in vitro motility assay. J Biol Chem 1993; 268: 20414–20418.
Lowey S, Waller GS, Trybus KM . Skeletal muscle myosin light chains are essential for physiological speeds of shortening. Nature 1993; 365: 454–456.
Root DD . The dance of actin and myosin: a structural and spectroscopic perspective. Cell Biochem Biophys 2002; 37: 111–139.
Dominguez R, Freyzon Y, Trybus KM, Cohen C . Crystal structure of a vertebrate smooth muscle myosin motor domain and its complex with the essential light chain: visualization of the pre-power stroke state. Cell 1998; 94: 559–571.
Ho G, Chisholm RL . Substitution mutations in the myosin essential light chain lead to reduced actin-activated ATPase activity despite stoichiometric binding to the heavy chain. J Biol Chem 1997; 272: 4522–4527.
Terrak M et al. Two distinct myosin light chain structures are induced by specific variations within the bound IQ motifs-functional implications. EMBO J 2003; 22: 362–371.
Houdusse A, Cohen C . Target sequence recognition by the calmodulin superfamily: implications from light chain binding to the regulatory domain of scallop myosin. Proc Natl Acad Sci USA 1995; 92: 10644–10647.
Lutz GJ, Razzaghi S, Lieber RL . Cloning and characterization of the S1 domain of four myosin isoforms from functionally divergent fiber types in adult Rana pipiens skeletal muscle. Gene 2000; 250: 97–107.
Julian FJ, Rome LC, Stephenson DG, Striz S . The maximum speed of shortening in living and skinned frog muscle fibres. J Physiol 1986; 370: 181–199.
Lutz GJ, Cuizon DB, Ryan AF, Lieber RL . Four novel myosin heavy chain transcripts define a molecular basis for muscle fibre types in Rana pipiens. J Physiol 1998; 508.3: 667–680.
Lutz GJ, Lieber RL . Myosin isoforms in anuran skeletal muscle: their influence on contractile properties and in vivo muscle function. Microsc Res Tech 2000; 50: 443–457.
Shah SB, Lieber RL . Simultaneous imaging and functional assessment of cytoskeletal protein connections in passively loaded single muscle cells. J Histochem Cytochem 2003; 51: 19–29.
Acknowledgements
We are grateful to Dr Richard Lieber for helpful discussions and support. We thank Dr Jifeng Zhang for technical support and helpful discussions. This study was supported by NIH Grants AR45631 and AR46469 to GJ Lutz, and NIH Grant AR40050 and a grant from the Department of Veterans Affairs to Richard L Lieber.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robinson, D., Bremner, S., Sethi, K. et al. In vivo expression of myosin essential light chain using plasmid expression vectors in regenerating frog skeletal muscle. Gene Ther 12, 347–357 (2005). https://doi.org/10.1038/sj.gt.3302411
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302411