Abstract
Objectives:
Uridine abrogates mitochondrial toxicities of nucleoside reverse transcriptase inhibitor in adipocyte cell culture. We aim to study the effect of uridine supplementation on human adipocyte mitochondrial DNA (mtDNA) levels in subjects with human immunodeficiency (HIV) lipoatrophy.
Methods:
Sixteen patients with lipoatrophy on stavudine-containing antiretroviral therapy were enrolled, and received NucleomaxX, a dietary supplement with a high bioavailability of uridine (36 g TID every other day for 16 weeks). Patients were then followed off-uridine for another 16 weeks. Highly active antiretroviral therapy remained unchanged during the trial.
Results:
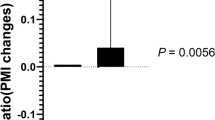
Fourteen patients completed the study. Two subjects dropped out before week 4 for study-unrelated reasons. No adverse events were noted throughout the study. HIV-1 RNA, CD4 counts, liver enzymes and hemoglobin remained unchanged. Body mass index, lactate, lipids, insulin and homeostasis model assessment of insulin resistance were unaltered. Fat and peripheral blood and mononuclear cell mtDNA levels did not correlate with each other and exhibited no changes throughout the study. Lipoatrophy scores by patients and physician improved significantly at weeks 16 and 32 compared to study entry.
Conclusion:
In this pilot study, NucleomaxX was safe, well tolerated without apparent deleterious effect on HIV indices. In contrast to in vitro data, NucleomaxX did not lead to changes in fat or blood mtDNA levels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bacchetti P, Gripshover B, Grunfeld C, Heymsfield S, McCreath H, Osmond D et al. Study of fat redistribution and metabolic change in HIV infection (FRAM) (2005) Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr 40, 119–120.
Bodnar AG, Cooper JM, Leonard JV, Schapira AH (1995). Respiratory-deficient human fibroblasts exhibiting defective mitochondrial DNA replication. Biochem J 305, 817–822.
Calabresi P, Falcone A, St Clair MH, Wiemann MC, Chu SH, Darnowski JW, For the trial to assess the regression of hyperlactatemia to evaluate the regression of established lipodystrophy in HIV-1-positive subjects (TARHEEL; ESS40010) Study Team (1990). Benzylacyclouridine reverses azidothymidine-induced marrow suppression without impairment of anti-human immunodeficiency virus activity. Blood 76, 2210–2215.
Fox RI, Herrmann ML, Frangou CG, Wahl GM, Morris RE, Strand V et al. (1999). Mechanism of action of leflunamide in rheumatoid arthritis. Clin Immunol 93, 198–208.
http://aactg.s-3.com/members/download/other/Metabolic/VenousLactateSOP.doc.
Joly V, Flandre P, Meiffredy V, Leturque N, Harel M, Aboulker JP et al. (2002). Increased risk of lipoatrophy under stavudine in HIV-1-infected patients: results of a substudy from a comparative trial. AIDS 16, 2447–2454.
Koch EC, Schneider J, Weiss R, Penning B, Walker UA (2003). Uridine excess does not interfere with the antiretroviral efficacy of nucleoside analogue reverse transcriptase inhibitors. Antivir Ther 8, 485–487.
Leyva A, van Groeningen CJ, Kraal I, Gall H, Peters GJ, Lankelma J, et al., the Rave Study Group (1984). Phase I and pharmacokinetic studies of high-dose uridine intended for rescue from 5-fluorouracil toxicity. Cancer Res 44, 5928–5933.
Mallal SA, John M, Moore CB, James IR, McKinnon EJ, the Adult AIDS Clinical Trials Group (2000). Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. AIDS 14 10, 1309–1316.
Manis FR, Cohn LB, McBride-Chang C, Wolff JA, Kaufman FR (1997). A longitudinal study of cognitive functioning in patients with classical galactosemia, including a cohort treated with oral uridine. J Inherit Metab Dis 20, 549–555.
Martin JL, Brown CE, Matthews-Davis N, Reardon JE (1994). Effects of antiviral nucleoside analogues on human DNA polymerases and mitochondrial DNA synthesis. Antimicrob Agents Chemother 38, 2743–2749.
Martin A, Smith D, Carr A, Ringland C, Amin J, Emery S et al. (2004). Reversibility of lipoatrophy in HIV-infected patients 2 years after switching from a thymidine analogue to abacavir: the MITOX Extension Study. AIDS 18, 1029–1036.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985). Homeostasis model assessment: insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419.
McComsey GA, Ward DJ, Hessenthaler SM, Sension MG, Shalit P, Lonergan TJ et al. (2004). Improvement in HAART-associated lipoatrophy in HIV-infected patients switched from stavudine to abacavir or zidovudine: The results of TARHEEL. Clin Infect Dis 38, 263–270.
McComsey GA, O’Riordan M, Storer N, Goldman S, Ganz J, Libutti DE et al. (2005). Clinical lipoatrophy assessment strongly correlates with DEXA-measured limb fat and subcutaneous fat mitochondrial DNA levels. Abstract 142 Program and Abstracts of the 13th Conference on Retroviruses and Opportunistic Infections 5–9 February 2005; Denver, Colorado.
McComsey GA, Paulsen DM, Lonergan TJ, Hessenthaler SM, Hoppel CL, Williams VC et al. (2005). Improvements in lipoatrophy, mitochondrial DNA content and adipose tissue apoptosis levels after replacement of stavudine with either abacavir or zidovudine. AIDS 19, 15–23.
Moyle GJ, Sabin CA, Cartledge J, Johnson M, Wilkins E, Churchill D et al. RAVE (Randomized Abacavir versus Viread Evaluation) Group UK (2006). a randomized comparative trial of tenofovir DF or abacavir as replacement for a thymidine analogue in persons with lipoatrophy. AIDS 20, 2043–2050.
Nolan D, Hammond E, Martin A, Taylor L, Hermann S, McKinnon E et al. (2003). Mitochondrial DNA depletion and morphologic changes in adipocytes associated with nucleoside reverse transcriptase inhibitor therapy. AIDS 17, 1329–1338.
Setzer B, Schlesier M, Walker UA (2005). Effects of didanosine-related depletion on mtDNA in human T lymphocytes. J Infect Dis 191, 848–855.
Sommadossi JP, Carlisle R, Schinazi RF, Zhou Z (1988). Uridine reverses the toxicity of 3′-azido-3′ deoxythymidine in normal human granulocyte-macrophage progenitor cells in vitro without impairment of antiretroviral activity. Antimicrob Agents Chemother 32, 997–1001.
Sutinen J, Walker UA, Sevastianova K, Hakkinen AM, Ristola M, Yki-Jarvinen H (2005). Uridine supplementation increases subcutaneous fat in patients with HAART-associated lipodystrophy: a randomized placebo controlled trial. Abstract 7 The 7th Workshop on Adverse Drug Reactions and Lipodystrophy in HIV 13–16 November 2005, Dublin, Ireland.
Tebas P, Zhang J, Yarasheski K, Evans S, Fischl M, Shevitz A et al. (2005). Switch to a protease inhibitor-containing/nucleoside reverse transcriptase inhibitor-sparing regimen increases appendicular fat and serum lipid levels without affecting glucose metabolism or bone mineral density. The results of a prospective randomized trial, ACTG 5125s. Program and abstracts of the 12th Conference on Retroviruses and Opportunistic Infections 22–25 February 2005; Boston, Massachusetts. Abstract 40.
van Groeningen CJ, Leyva A, Kraal I, Peters GJ, Pinedo HM (1986). Clinical and pharmacokinetic studies of prolonged administration of high-dose uridine intended for rescue from 5-FU toxicity. Cancer Treat Rep 70, 745–750.
Venhoff N, Zilly M, Lebrecht D, Schirmer D, Klinker H, Thoden J et al. (2005). Uridine pharmacokinetics of mitocnol a sugar cane extract. AIDS 19, 739–740.
Walker UA, Auclair M, Lebrecht D, Kornprobst M, Capeau J, Caron M (2006). Uridine abrogates the adverse effects of antiretroviral pyrimidine analogues on adipose cell functions. Antivir Ther 11, 25–34.
Walker UA, Bickel M, Lütke Volksbeck SI, Ketelsen UP, Schöfer H, Setzer B et al. (2002). Evidence of nucleoside analogue reverse transcriptase inhibitor-associated genetic and structural defects of mitochondria in adipose tissue of HIV-infected patients. J Acquir Immune Defic Syndr 29, 117–121.
Walker UA, Langmann P, Miehle N, Zilly M, Klinker H, Petschner F (2004). Beneficial effects of oral uridine in mitochondrial toxicity. AIDS 18, 1085–1086.
Walker UA, Venhoff N (2005). Uridine in the prevention and treatment of NRT1-induced mitochondrial toxicity. Antivir Ther 10, M117–M123.
Walker UA, Venhoff N, Koch E, Olschweski M, Schneider J, Setzer B (2003). Uridine abrogates mitochondrial toxicity related to nucleoside analogue reverse transcriptase inhibitors in HepG2 cells. Antivir Ther 8, 463–470.
Webster DR, Simmonds HA, Potter CF, Becroft DM (1979). Purine and pyrimidine metabolism in hereditary orotic aciduria during a 15 year follow up study. Adv Exp Med Biol 122B, 203–208.
Acknowledgements
This work was supported by NIAID AI-060484 (GM), and was partially funded by Bristol Myers Squibb Co. This study was presented at the 7th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV, 13–17 November 2005, Dublin, Ireland.
Role of the funding source: The funding source had no role in the collection, analysis or interpretation of the data or in the decision to submit the paper for publication. Dr McComsey serves as consultant for Bristol Myers Squibb Co. Dr UA Walker has applied for patents regarding the use of uridine or its precursors in lipodystrophy. He also serves as a consultant for the company that produces NucleomaxX.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McComsey, G., O'Riordan, M., Setzer, B. et al. Uridine supplementation in HIV lipoatrophy: pilot trial on safety and effect on mitochondrial indices. Eur J Clin Nutr 62, 1031–1037 (2008). https://doi.org/10.1038/sj.ejcn.1602793
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602793
Keywords
This article is cited by
-
Antiretroviral Therapy–Induced Mitochondrial Toxicity: Potential Mechanisms Beyond Polymerase-γ Inhibition
Clinical Pharmacology & Therapeutics (2014)
-
Pathogenesis and management of lipoatrophy
Current HIV/AIDS Reports (2008)