Abstract
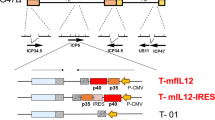
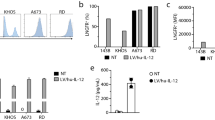
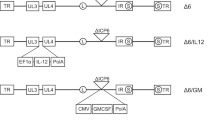
Synergy between interleukin-12 (IL-12) and B7-1 (CD80) for cancer immunotherapy has previously been demonstrated in animal models of breast cancer, lymphoma, and multiple myeloma. With a view to human clinical application, tricistronic retroviral and adenovirus vectors co-expressing IL-12 (IL-12p40 plus IL-12p35) and CD80 were constructed by utilizing two internal ribosome entry site (IRES) sequences to link the three cDNAs. A murine stem cell virus (MSCV)-based retroviral vector (MSCV-hIL12.B7) utilized distinct IRES sequences from the encephalomyocarditis virus (EMCV) and the foot-and-mouth disease virus (FMCV), whereas Ad5-based adenovirus vectors contained transcriptional units with two EMCV IRES sequences under the control of murine (AdMh12.B7) or human (AdHh12.B7) cytomegalovirus promoters. AdMh12.B7 was found to consistently direct higher levels of IL-12 and CD80 expression than AdHh12.B7 following infection of a number of human tumor cell lines. In preclinical studies, the human myeloma cell line U266 was infected with MSCV-hIL12.B7 and a resulting clonal cell line, U/MSCV-h12.B7, was generated with stable expression of CD80 and secreting IL-12 at 1 ng/24 h/106 cells. By comparison, following AdMh12.B7 infection, 81% of infected U266 cells (U/AdMh12.B7) expressed CD80 and secreted IL-12 at 25–50 ng/24 h/106 cells. Both engineered myeloma cell lines stimulated enhanced allogeneic mixed lymphocyte proliferation and provoked increases in cytotoxic T-lymphocyte responses and γ-interferon release from normal donor lymphocytes exposed to parental U266 cells. These results suggest potential clinical utility of AdMh12.B7 in immunotherapy strategies for the treatment of multiple myeloma and other cancers. Cancer Gene Therapy (2001) 8, 361–370
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Dranoff G . Cancer gene therapy: connecting basic research with clinical inquiry J Clin Oncol 1998 16: 2548–2556
Pardoll DM . Cancer vaccines Nat Med 1998 18: 1555–1564
Greten TF, Jaffee EM . Cancer vaccines J Clin Oncol 1999 17: 1047–1060
Dranoff G, Jaffee E, Lazenby A, et al . Vaccination with irradiated tumor cells engineered to secrete murine granulocyte macrophage colony-stimulating factor stimulates potent, specific and long-lasting antitumor immunity Proc Natl Acad Sci 1993 15: 3539–3543
Stewart AK, Lassam NJ, Quirt IC, et al . Adenovector mediated gene delivery of interleukin-2 in metastatic breast cancer and melanoma: results of a Phase 1 trial Gene Ther 1999 6: 350–363
Soiffer R, Lynch T, Mihm M, et al . Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma Proc Natl Acad Sci USA 1998 95: 13141–13146
Bowman L, Grossman M, Rill D, et al . IL-2 adenovector-transduced autologous tumor cells induce antitumor immune responses in patients with neuroblastoma Blood 1998 15: 1941–1949
Germain RN . MHC-dependent antigen processing and peptide presentation: providing ligands for T-lymphocyte activation Cell 1994 76: 287–299
Azuma M, Ito D, Yagita H, et al . B70 antigen is a second ligand for CTLA-4 and CD28 Nature 1993 366: 76–79
Schwartz RH . Co-stimulation of T lymphocytes: the role of CD28, CTLA-4 and B7/BB1 in interleukin-2 production and immunotherapy Cell 1992 71: 1065–1068
Linsey PS, Ledbetter JA . The role of CD28 receptor during T-cell responses to antigen Annu Rev Immunol 1993 11: 191–212
Gimmi CD, Freeman GJ, Gribben JG, et al . Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 co-stimulation Proc Natl Acad Sci USA 1993 90: 6586–6590
Wu TC, Huang AYC, Jaffee EM, et al . A reassessment of the role of B7-1 expression in tumor rejection J Exp Med 1995 182: 1415–1421
Trinchieri G, Scott P . Interleukin 12: basic principles and clinical applications Curr Top Microbiol Immunol 1999 238: 57–78
Wolf SF, Temple PA, Kobayashi M, et al . Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells J Immunol 1991 146: 3074–3081
Zitvogel L, Tahara H, Cai Q, et al . Construction and characterization of retroviral vectors expressing biologically active human interleukin-12 Hum Gene Ther 1994 5: 1493–1506
Fujiwara H, Hamaoka T . The antitumor effects of IL-12 involve enhanced IFN-gamma production by antitumor T cells, their accumulation to tumor sites andin situ IFN-gamma production Leukemia 1997 11: (Suppl 3) 570–571
Zitvogel L, Robbins PD, Storkus W, et al . Interleukin-12 and B7.1 co-stimulation cooperate in the induction of effective antitumor immunity and therapy of established tumors Eur J Immunol 1996 26: 1335–1341
Pizzoferrato E, Chu NR, Hawley TS, et al . Enhanced immunogenicity of B-cell lymphoma genetically engineered to express both B7-1 and interleukin-12 Hum Gene Ther 1997 8: 2217–2228
Hawley TS, Linsley PS, Hawley RG . Co-expression of B7-1 with interleukin-12 enhances vaccine-induced antitumor immunity in experimental myeloma Hematology 1998 3: 365–374
Pützer BM, Hitt M, Miller WJ, et al . Interleukin-12 and B7-1 co-stimulatory molecule expressed by an adenovirus vector act synergistically to facilitate tumor regression Proc Natl Acad Sci USA 1997 94: 10889–10894
Attal M, Harrousseau JL, Stoppa AM, et al . A prospective randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma N Engl J Med 1996 335: 91–97
Lokhorst HM, Schattenberg A, Cornelissen JJ, et al . Donor leukocyte infusions are effective in relapsed myeloma after allogeneic bone marrow transplantation Blood 1997 90: 4206–4211
Kwak LW, Taub DD, Duffey PL, et al . Transfer of myeloma idiotype-specific immunity from an actively immunized marrow donor Lancet 1995 345: 1016–1020
Treon SP, Mollick JA, Urashima M, et al . Muc-1 core protein is expressed on multiple myeloma cells and is induced by dexamethasone Blood 1999 93: 1287–1298
Teoh G, Tai YT, Greenfield EA, et al . Tumor rejection antigen 1 (GRP94) expression is induced by CD40 ligand activation of multiple myeloma cells and mediates allogeneic T-cell reactivity Blood 1998 92: (Suppl 1) 100a
Martı´nez-Salas E, Saiz JC, Davila M, et al . A single nucleotide substitution in the internal ribosome entry site of foot-and-mouth disease virus leads to enhanced cap-independent translationin vivo J Virol 1993 67: 3748–3755
Lieu FHL, Hawley TS, Fong AZC, et al . Transmissibility of murine stem cell virus-based retroviral vectors carrying both interleukin-12 cDNAs and a third gene: implications for immune gene therapy Cancer Gene Ther 1997 4: 167–175
Hawley RG, Lieu FHL, Fong AZC, et al . Retroviral vectors for production of interleukin-12 in the bone marrow to induce a graft-versus-leukemia effect Ann NY Acad Sci 1996 795: 341–345
Hawley RG, Lieu FHL, Fong AZC, et al . Versatile retroviral vectors for potential use in gene therapy Gene Ther 1994 1: 136–138
Miller AD, Garcia JV, von Suhr N, et al . Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus J Virol 1991 65: 2220–2224
Hitt MM, Addison CL, Graham FL . Human adenovirus vectors for gene transfer into mammalian cells Adv Pharmacol San Diego, CA: Academic Press 1997 137–206
Dessureault S, Graham F, Gallinger S . B7-1 gene transfer into human cancer cells by infection with an adenovirus-B7 (Ad-B7) expression vector Ann Surg Oncol 1996 3: 317–324
Bett AJ, Haddara W, Prevec L, et al . An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3 Proc Natl Acad Sci USA 1994 91: 8802–8806
Hitt M, Bett AJ, Prevec L, et al . In: Celis JE, ed.Cell Biology: A Laboratory Handbook San Diego: Academic 1994 479–490
Bramson J, Hitt M, Gallichan WS, et al . Construction of a double recombinant adenovirus vector expressing a heterodimeric cytokine:in vitro andin vivo production of biologically active interleukin 12 Hum Gen Ther 1996 10: 333–342
Foreman KE, Wrone-Smith T, Krueger AE, et al . Expression of co-stimulatory molecules CD80 and/or CD86 by a Kaposi's sarcoma tumor cell line induces differential T-cell activation and proliferation Clin Immunol 1999 91: 345–353
Guinan EC, Gribben JG, Boussiotis VA . Pivotal role of the B7:CD28 pathway in transplantation, tolerance and tumor immunity Blood 1994 84: 3261–3282
Wu TC, Huang AYC, Jaffee EM, et al . A reassessment of the role of B7-1 expression in tumor rejection J Exp Med 1995 182: 1415–1421
Chen L, McGowan P, Ashe S, et al . Tumor immunogenicity determines the effect of B7 co-stimulation on T-cell–mediated tumor immunity J Exp Med 1994 179: 523–532
Desai BB, Quinn PM, Wolitzky AG, et al . IL-12 receptor: II. Distribution and regulation of receptor expression J Immunol 1992 148: 3125–3132
Brunda MJ . Role of IL-12 as an antitumor agent: current status and future directions Res Immunol 1995 1466: 423–431
Sgadari C, Angiolillo AL, Tosata G . Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein-10 Blood 1996 87: 3877–3883
Carrol MW, Overwijk WW, Surman DR, et al . Construction and characterization of a triple recombinant vaccinia virus encoding B7-1, interleukin 12, and a model tumor antigen J Natl Cancer Inst 1998 90: 1881–1887
Addsion CL, Hitt M, Kunksen D, et al . Comparison of the humanversus murine cytomegalovirus immediate early gene promoters for transgene expression by adenoviral vectors J Gen Virol 1997 78: 1653–1661
Colombo MP, Vagliani M, Spreafico F, et al . Amount of interleukin 12 available at the tumor site is critical for tumor regression Cancer Res 1996 56: 2531–2534
Shtil AA, Turner JG, Durfee J, et al . Cytokine-based tumor cell vaccine is equally effective against parental and isogenic multi-drug resistant myeloma cells: the role of cytotoxic T lymphocytes Blood 1999 93: 1831–1837
Kopantzev E, Rochke V, Rudikoff S . Interleukin 2–mediated modulation of plasma cell tumor growth in a model of multiple myeloma Hum Gene Ther 1998 9: 13–19
Yi Q, Dabadghao S, Osterborg A, et al . Myeloma bone marrow plasma cells: evidence for their capacity as antigen-presenting cells Blood 1997 90: 1960–1967
Wendtenr C-M, Nolte A, Mangold E, et al . Gene transfer of co-stimulatory molecules B7-1 and B7-2 into human multiple myeloma cells by recombinant adeno-associated virus enhances the cytolytic T-cell response Gene Ther 1997 4: 726–735
Reichardt VL, Okada CY, Liso A, et al . Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma — a feasibility study Blood 1999 93: 2411–2419
Osterborg A, Yi Q, Henriksson L, et al . Idiotypic immunization combined with granulocyte macrophage colony-stimulating factor in myeloma patients–induced type I, major histocompatibility complex restricted CD8- and CD4-specific T-cell responses Blood 1998 91: 2459–2466
Acknowledgements
Support for these studies was obtained from the Medical Research Council of Canada, the Myeloma Research Fund, the McCarty Cancer Foundation, Nelson Arthur Hyland Foundation, and the ABC Group.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wen, XY., Mandelbaum, S., Li, Z. et al. Tricistronic viral vectors co-expressing interleukin-12 (1L-12) and CD80 (B7-1) for the immunotherapy of cancer: Preclinical studies in myeloma. Cancer Gene Ther 8, 361–370 (2001). https://doi.org/10.1038/sj.cgt.7700321
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700321
Keywords
This article is cited by
-
Gene and virotherapy for hematological malignancies
International Journal of Hematology (2016)
-
Pharmacological‐based translational induction of transgene expression in mammalian cells
EMBO reports (2004)
-
Decreased tumorigenic potential of EphA2-overexpressing breast cancer cells following treatment with adenoviral vectors that express EphrinA1
Cancer Gene Therapy (2004)