Abstract
Neurofibromatosis type 1 (NF1) is characterized by a high incidence of benign and malignant tumors attributed to loss of function of Nf1, which encodes neurofibromin, a tumor suppressor with Ras-GAP activity. Neurofibromin deficiency typically causes chronic activation of Ras, considered the major contributor to manifestation of NF1. Resistance to radio- and chemotherapy are typical of NF1-associated tumors, but the underlying mechanism is unknown. Here, we investigated interrelationships between neurofibromin expression, Ras activity, and sensitivity to apoptosis. Neurofibromin-deficient mouse embryonic fibroblasts (MEFs) and human NF1 tumor cells were more resistant than neurofibromin-expressing cells to apoptosis. Moreover, Nf1−/−, Nf1+/−, and Nf1+/+ MEFs exhibited gene-dosage-related resistance to apoptosis. Resistance of the Nf1-deficient cells was mediated by two survival pathways: a Ras-dependent pathway, and a Ras-independent pathway promoted by the lack of an NF1-GRD-independent proapoptotic action of neurofibromin. Therefore, besides its Ras-dependent growth inhibition, neurofibromin can exert tumor suppression via a proapoptotic effect.
Similar content being viewed by others
Main
Neurofibromatosis type 1 (NF1) is an autosomal dominant disorder affecting the nervous system. It is characterized by a high incidence of benign and malignant tumors. Nf1 encodes neurofibromin, a tumor suppressor with Ras-GTPase activating protein (GAP) activity1 that facilitates the hydrolysis of GTP by active Ras-GTP.2, 3, 4 Neurofibromin deficiency results in an increase in Ras-GTP and increased activation of its effectors.2, 3, 4, 5 These abnormalities contribute to cell transformation in NF1 patients.3, 4 Studies in Nf1-deficient mice,6, 7, 8 and Drosophila flies9 provide additional support for the significance of aberrant Ras-GTP in NF1. Notably, the GAP-related domain of neurofibromin (NF1-GRD) may not be the only neurofibromin domain underlying disease manifestations; for example, restoration of Ras signaling in Nf1 null mutant Drosophila flies does not correct the defect in body size,10 and expression of neurofibromin in melanoma and NIH 3T3 cells inhibits their growth without affecting Ras-GTP levels.11 Ras-GAP-independent functions of neurofibromin include regulation of adenylyl cyclase activity,10, 12 although neurofibromin can regulate adenylyl cyclase by a Ras-dependent pathway as well.13
In view of the tumor suppressor activity of neurofibromin and the role of other tumor suppressors such as p5314 and DAP-kinase15 in apoptosis and tumorigenesis, we postulate that neurofibromin, in addition to its role in controlling cell proliferation, also regulates cell susceptibility to apoptosis. The proposed proapoptotic function of neurofibromin could be mediated via its regulation of Ras, since Ras pathways reportedly elicit antiapoptotic effects.16, 17, 18 This notion is supported by the observations that various Nf1-deficient cells survive in the absence of trophic factors19 and that enhanced survival of Nf1-deficient sensory neurons is dependent on Ras.19, 20, 21 It is nevertheless possible that the proapoptotic action of neurofibromin is also exerted independently of Ras. The aim of the present study was to verify the existence of a proapoptotic action of neurofibromin, and – if confirmed – to determine the extent to which this putative action is dependent on Ras. Our results showed that neurofibromin-deficient cells are more resistant than neurofibromin-expressing cells to apoptosis. This resistance could be counteracted by inhibition of Ras activity in apoptosis induced by trophic factor deprivation but not by other triggers. Thus, neurofibromin confers sensitivity to apoptosis via Ras-dependent and -independent pathways.
Results
Characterization of SV40-immortalized MEFs comprised of the three Nf1 genotypes
Cell susceptibility to apoptosis was studied in both primary and SV40-immortalized mouse embryonic fibroblasts (MEFs) possessing the three Nf1 genotypes Nf1−/−, Nf1+/−, and Nf1+/+. This experimental system enables us to study the effects of different dosages of the Nf1 gene (Nf1−/−, Nf1+/−, and Nf1+/+) on cell susceptibility to apoptosis and on Ras signaling pathways in a defined genetic background.
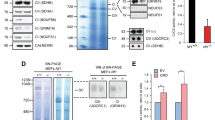
Nf1+/− mice22 were mated to obtain primary MEF cultures of the three Nf1 genotypes. These cultures were utilized to establish SV40-immortalized MEFs (hereafter termed SV40 MEFs). Expression levels of neurofibromin in the three Nf1 SV40 MEFs genotypes were consistent with their genotypes (Figure 1a). Furthermore, their signaling properties were consistent with those reported for their corresponding primary MEFs.23, 24 Accordingly, the steady-state levels of Ras-GTP, phosphorylated extracellular signal-regulated kinase (ERK), phosphorylated Akt/PKB, and phosphorylated mTOR in SV40 MEFs grown in 0.5% serum (hereafter termed serum-starved cells) were significantly higher in the Nf1−/− than in the Nf1+/+ genotype (Figure 1a and b). The Nf1+/− SV40 MEFs exhibited intermediate amounts of both active Ras and its downstream active targets (Figure 1a and b). Analysis of DNA synthesis in the three Nf1 SV40 MEF genotypes by the [3H] thymidine incorporation assay revealed that the rate of DNA synthesis was highest in the Nf1−/−, intermediate in the Nf1+/−, and lowest in the Nf1+/+ SV40 MEFs (Figure 1c). Thus, the three Nf1 SV40 MEF genotypes had retained the signaling properties of their primary MEF counterparts and could therefore be considered a suitable model system.
Effect of Nf1 gene dosage on the steady-state levels of Ras-GTP, phosphorylated ERK (pERK), phosphorylated AKT (pAKT), phosphorylated mTOR (pmTOR) (a and b) and on [3H] thymidine incorporation (c) in the three Nf1 SV40 MEF genotypes. (a) Nf1−/−, Nf1+/−, and Nf1+/+ SV40 MEFs were grown in medium supplemented with 0.5% serum for 18 h. Protein extracts prepared from these serum-starved cells were subjected to immunoblot analysis for Ras (Total Ras), pERK, ERK, pAKT, AKT, pmTOR, and mTOR expression or to a RAS-GTP pull-down assay followed by immunoblot analysis for Ras (Ras-GTP), as described in Materials and Methods. Neurofibromin expression was determined in the protein extracts prepared from the three Nf1 SV40 MEF genotypes grown in the presence of serum. Membranes of the neurofibromin immunoblots were reprobed with β-tubulin to assess the uniformity of sample loading. The results shown are from a representative experiment (one of three independent experiments). (b) Quantitative analysis of Ras-GTP, pERK, pAKT, and pmTOR. Immunoblots from each of the three independent experiments were scanned and the intensity of each of the Ras-GTP, pERK, pAKT, and pmTOR bands was quantified by densitometry and normalized relative to the expression of their corresponding total Ras, ERK, AKT, or mTOR proteins. The normalized Ras-GTP, pERK, pAKT, or pmTOR value for each Nf1 genotype is expressed as a percentage of the corresponding normalized value in Nf1+/+ SV40 MEFs. The values shown are means±S.D. (bars) (n=3). *P<0.05, **P<0.005, significantly different from Nf1+/+ MEFs. (c) [3H] thymidine incorporation in Nf1−/−, Nf1+/−, and Nf1+/+ SV40 MEFs. Cells were grown in medium supplemented with 0.5% serum. After 13 h the medium was replaced with medium supplemented with 1% serum and the cells were grown for an additional 1 h. [3H] thymidine was then added to the medium and the cells were grown for an additional 8 h. The amount of [3H] thymidine incorporated into the DNA was then determined as described in Materials and Methods. The values shown are means±S.D. (bars) (n=3). *P<0.05, significantly different from Nf1+/+ MEFs
Neurofibromin deficiency causes resistance to apoptosis
Next, we examined susceptibilities of the three Nf1 genotypes of the primary and SV40 MEFs to apoptosis induced by staurosporine, ultra-violet (UV) radiation, and the chemotherapeutic agent vincristine. The cells were incubated for 6.5 and 24 h with or without staurosporine, or incubated for 15 and 24 h with or without vincristine, or UV-irradiated or not irradiated and then maintained for an additional 15 h in culture. Cell viability was then determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The resistance of the Nf1−/− genotypes of both the primary and the SV40 MEFs to each of the three apoptotic stimuli were relatively high compared to the resistance of their respective Nf1+/+ primary and SV40 MEFs, while the Nf1+/− primary and SV40 MEFs exhibited an intermediate resistance (Figure 2a). We further assessed the effects of the different apoptotic stimuli on cell viability in the SV40 MEFs by the Trypan blue dye exclusion assay, which measures both live and dead cells. The percentage of dead cells observed in the Nf1−/− SV40 MEFs was significantly lower than that observed in the Nf1+/+ SV40 MEFs, and the percentage in the Nf1+/− SV40 MEFs was again intermediate between the two (Figure 2b). Combined, these results suggest that neurofibromin deficiency renders the cells resistant to the abovementioned apoptotic stimuli, and that the SV40 MEFs retained the neurofibromin-dependent responses to apoptosis exhibited by the primary MEFs. All subsequent experiments were therefore carried out with the SV40 MEFs.
Nf1−/− MEFs are more resistant than Nf1+/− or Nf1+/+ MEFs to cytotoxic drugs. SV40 (a–d) and primary (a) Nf1−/−, Nf1+/−, and Nf1+/+ MEFs were treated without or with staurosporine (Staur) (0.5 μM) or vincristine (Vinc) (0.07 μM) for the indicated times, or were UV-irradiated at the indicated intensities or not irradiated, and then grown for 15 h after which cell survival or death was determined. (a) Determination of cell viability in the different Nf1-genotype SV40 and primary MEFs by the MTT assay, performed as described in Materials and Methods. Survival was defined as the MTT value after each apoptotic treatment in each of the three Nf1 SV40 MEF genotypes, expressed as a percentage of the MTT values obtained in the absence of apoptotic treatment at the corresponding time points or UV irradiation intensities. The values shown are means±S.D. (bars) (n=3). *P<0.05, **P<0.005, significantly different from the corresponding treatment in Nf1+/+ MEFs. (b) Determination of cell death and viability in the three Nf1 SV40 MEF genotypes by the Trypan blue assay. After treatment with the apoptotic stimuli, blue (dead) and white (live) cells were counted under the microscope. Cell death was defined as the number of blue cells, expressed as a percentage of the total number of cells (blue and white) in each treatment. The values shown are means±S.D. (bars) (n=3). *P<0.05, **P<0.005, significantly different from the corresponding treatments in Nf1+/+ SV40 MEFs. (c) DEVDase activity in SV40 MEFs. The three Nf1 SV40 MEF genotypes were left untreated or were treated with Staur (0.5 μM) or Vinc (0.07 μM) for the indicated times, or were grown for 15 h with or without prior UV irradiation. DEVDase activity was assayed as described in Materials and Methods. The results are expressed as the ratio of DEVDase activity measured after each type of apoptotic treatment in each Nf1 genotype to the corresponding activity measured in the untreated cells. The values shown are from a representative experiment (n=3). (d) Determination of cell viability by colony formation. The three Nf1 SV40 MEF genotypes were treated with Staur (0.5 μM) for 6.5 h, and surviving cells were then allowed to form colonies as described in Materials and Methods. Colony formation for each Nf1 genotype was defined as the number of clones obtained after treatment with Staur, expressed as a percentage of the number obtained in untreated cultures. The values shown are means±S.D. (n=3). *P<0.05, **P<0.005, significantly different from Nf1+/+ SV40 MEFs. (e, f) Nf1−/−, Nf1+/−, and Nf1+/+ SV40 MEFs express similar amounts of apoptotic proteins. Nf1−/−, Nf1+/−, and Nf1+/+ SV40 MEFs were grown in medium supplemented with 10% serum. (e) Protein extracts prepared from these cells were subjected to immunoblot analysis for Bcl-xL, Mcl-1, Bax, Bak, caspase-8, -9, -3, and XIAP expression, as described in Materials and Methods. Membranes of immunoblots were reprobed with β-tubulin to assess the uniformity of sample loading. The results shown are from a representative experiment (one of two or three independent experiments). (f) Quantitative analysis of Bcl-xL, Mcl-1, Bax, Bak, caspase-8, -9, -3, and XIAP expression. Immunoblots from each of the different independent experiments were scanned and the intensity of each of the apoptotic bands was quantified by densitometry and normalized relative to the expression of their corresponding β-tubulin proteins. The normalized apoptotic proteins value for each Nf1 genotype is expressed as the fold increase of the corresponding normalized value in Nf1+/+ SV40 MEFs. Values shown are means±S.D. (bars) (n=3) or means±range (bars) (n=2)
We next examined the extent of caspase-associated apoptosis employing the DEVDase activity assay, which measures the activity of caspase-3 and -7. Each of the apoptotic stimuli induced an increase in DEVDase activity; the increase observed was significantly lower in the Nf1−/− than in the Nf1+/+ SV40 MEFs, and was intermediate in the Nf1+/− SV40 MEFs (Figure 2c). These results are in line with those obtained with the MTT and Trypan blue assays, and further demonstrate a positive correlation between neurofibromin deficiency and resistance to apoptosis. We also measured cell viability by the colony formation assay, in which the true number of cells that remained viable following the apoptotic stimulus is determined. This last assay was carried using staurosporine as a representative apoptotic stimulus. Staurosporine was removed after the treatment and the cells were then detached from the plates and replated at low density to allow colony formation. In agreement with the results obtained by the short-term cell-death and viability assays, the colony formation assay showed that the resistance to staurosporine was highest in the Nf1−/−, intermediate in the Nf1+/−, and lowest in the Nf1+/+ SV40 MEFs (Figure 2d).
These results suggest that the absence of neurofibromin promotes survival, and therefore that neurofibromin might act as a sensitizer of apoptosis. The resistance to apoptosis caused by neurofibromin deficiency might be attributable to an inherent decrease in the apoptotic potential of Nf1−/− cells resulting from a decrease in the expression of proapoptotic proteins or an increase in expression of antiapoptotic proteins or both. This did not appear to be the case since the basal expression levels of Bcl-2 family proteins (the antiapoptotic proteins Bcl-xL and Mcl-1 and the proapoptotic proteins Bax and Bak); caspases (the initiator caspases – caspase-8 and -9, and the effector caspase, caspase-3); and the X-linked inhibitor of apoptosis (XIAP) were similar in all the three Nf1 SV40 MEF genotypes (Figure 2e and f). Thus, the observed resistance of neurofibromin-deficient MEFs to apoptosis was not acquired as a result of changes in the expression levels of at least these key apoptotic components.
Effects of Ras inhibitors on the susceptibility of Nf1 SV40 MEF genotypes to apoptosis
The resistance of Nf1−/− MEFs to apoptosis could have been acquired as a result of an increase in Ras-mediated survival signaling, in which case the resistance to apoptosis would be abolished by inhibition of Ras activity. We therefore examined the impact of three independent Ras inhibitors (the Ras inhibitor FTS,25 the dominant-negative (DN) Ras mutant H-RasS17N and the NF1-GRD20 on susceptibility of the three Nf1 SV40 MEF genotypes to apoptosis.
To examine the effect of FTS, the cells were pretreated with FTS or with its vehicle for 24 h, and then subjected to the above-mentioned apoptotic stimuli in the continuous presence of FTS or its vehicle. Cell viability was determined after each treatment by the MTT assay. FTS had no effect on resistance to apoptosis of the three genotypes treated with staurosporine, UV irradiation, or vincristine (Figure 3a). A similar lack of effect of FTS was obtained when the cells were pretreated with FTS for 48 h (data not shown) or when cell viability was determined by the colony formation assay using treatment with staurosporine as a representative apoptotic stimulus (data not shown).
Effects of FTS on cell susceptibility to apoptosis, Ras-GTP levels, DNA synthesis, and cell growth in the three Nf1 SV40 MEF genotypes. (a) Effect of FTS on cell susceptibility to apoptosis. Nf1−/−, Nf1+/−, and Nf1+/+ SV40 MEFs were pretreated with 75 μM FTS or its vehicle (0.1% DMSO) for 24 h and were then left untreated or were treated with Staur or Vinc or were UV-irradiated, as described in Figure 2, in the continuous presence of FTS (+) or DMSO (−). Cell viability after treatments was determined by the MTT assay. Survival was defined as the MTT value after each apoptotic treatment in each of the Nf1 SV40 MEF genotypes, in the presence of DMSO or FTS, expressed as a percentage of the MTT value obtained in the corresponding Nf1 SV40 MEF genotype in the absence of the apoptotic treatment but in the presence of DMSO or FTS. Values are means±S.D. (bars) (n=3). *P<0.05, **P<0.005, significantly different from the corresponding treatment in Nf1+/+ MEFs. (b) Effect of FTS on steady-state Ras-GTP. The three Nf1 SV40 MEF genotypes were grown in medium supplemented with 0.5% serum for 18 h in the presence of 5 μM FTS (+) or its vehicle (0.1% DMSO) (−). Protein extracts were then prepared and the amount of Ras in each treatment was determined by immunoblot analysis. Ras-GTP was determined by the Ras pull-down assay. The results shown are from a representative experiment (one of three independent experiments). (c) Quantitative analysis of Ras-GTP. Intensities of the Ras-GTP bands in the different treatments were quantified by densitometry and normalized relative to total Ras expression for each Nf1 genotype. The normalized Ras-GTP value in each of the FTS-treated Nf1 SV40 MEF genotypes is expressed as a percentage of the normalized Ras-GTP value in the corresponding Nf1 SV40 MEF genotype treated with DMSO. Values shown are means±S.D. (bars) (n=3). *P<0.05, significantly different from the DMSO-treated MEFs. (d) Effect of FTS on [3H] thymidine incorporation in the three Nf1 SV40 MEF genotypes. Cells were grown in medium supplemented with 0.5% serum. After 13 h the medium was replaced with medium supplemented with 1% serum containing either 30 μM FTS or 0.1% DMSO and the cells were grown for an additional hour. [3H] thymidine was then added to the medium and the cells were grown for an additional 8 h in the continuous presence of FTS or DMSO. The amount of [3H] thymidine incorporated into the DNA was then determined as described in Materials and Methods. The values shown are means±S.D. (bars) (n=3). *P<0.05, **P<0.005, significantly different from DMSO-treated cells
Although FTS did not affect the susceptibility of the three Nf1 SV40 MEF genotypes to apoptosis it nevertheless inhibited growth and Ras activity of Nf1−/− SV40 MEFs. Accordingly, cell counts revealed that FTS (75 μM, 48 h) inhibited the growth of all three genotypes and that the effect was most pronounced in the Nf1−/− genotype (28±1.8, 23±0.5, and 15±0.4% inhibition in the Nf1−/−, Nf1+/−, and Nf1+/+ genotypes, respectively). In addition, DNA synthesis ([3H] thymidine incorporation) induced by 1% serum in 13 h serum-starved cells of all three genotypes was strongly inhibited by FTS (Figure 3b). Notably, the range of FTS concentrations used in these experiments and in all subsequent experiments in which cells were grown in low serum was 2.5–30 μM; FTS is more effective in low serum than in high serum because it binds to serum proteins; thus in low serum the concentration of free FTS is higher than in high serum.26, 27, 28 Last, FTS induced a reduction in steady-state Ras-GTP levels in the serum-starved Nf1 SV40 MEFs with no appreciable effect on total Ras levels (Figure 3c and d). The extent of inhibition correlated well with the Nf1 gene dosage and with the levels of Ras-GTP shown in Figure 1a and b: the lower the gene dosage, the higher the levels of Ras-GTP and the sensitivity to FTS.
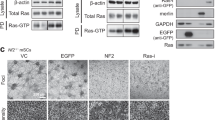
The effects of H-RasS17N and NF1-GRD on the susceptibility of Nf1−/− and Nf1+/+ SV40 MEFs to apoptosis were assayed in secreted alkaline phosphatase (SEAP)/H-RasS17N or SEAP/NF1-GRD co-transfectants. Empty vectors pcDNA3 or pac served, respectively, as controls. The cells were treated 18 h after transfection, with staurosporine for 6.5 or 24 h and cell viability was then determined by monitoring SEAP activity. Transfection with empty vectors did not alter the basic differences in the apoptotic responses of the two Nf1-genotype transfected SV40 MEFs; the Nf1−/− genotype remained more resistant than the Nf1+/+ genotype to staurosporine-induced apoptosis (Figure 4a). Similar results were obtained when the two Nf1 SV40 MEF genotypes were transfected with DN Ras or NF1-GRD; neither of these expression vectors altered the relatively higher resistance of the Nf1−/− than of the Nf1+/+ SV40 MEFs to staurosporine-induced apoptosis (Figure 4a). The inability of DN Ras or NF1-GRD to reverse the resistance of Nf1−/− and MEFs to apoptosis could not be attributed to a lack of expression or activity, since both DN Ras and NF1-GRD Nf1−/− SV40 MEF transfectants exhibited markedly smaller amounts of Ras-GTP and phosphorylated-ERK than those recorded in the vector-controlled Nf1−/− MEF transfectants (Figure 4b and c). Taken together, the results obtained by three independent methods of Ras inhibition strongly suggest that the enhanced resistance of Nf1−/− MEFs to apoptosis induced by the above-mentioned apoptotic triggers is not mediated by Ras-dependent survival signals. We therefore conclude that neurofibromin can sensitize MEFs to apoptosis by a mechanism that is independent of Ras.
Effects of DN Ras or NF1-GRD expression on cell viability, Ras-GTP levels, and ERK phosphorylation. Nf1−/− and Nf1+/+ SV40 MEFs were co-transfected with SEAP and with DN Ras, NF1-GRD, pcDNA3, or PAC expression vectors, as described in Materials and Methods. (a) After 18 h the transfected cultures were treated with Staur (0.5 μM) for 6.5 or 24 h or were left untreated, and viability of the transfected cells was determined by measurement of SEAP activity in the culture medium, as described in Materials and Methods. Cell survival was defined as SEAP activity in the Staur-treated transfected cultures and is expressed as a percentage of the SEAP activity in the Staur-untreated transfected cultures. (b) Protein extract was prepared from Staur-untreated transfected cultures 24 h after the addition of Staur to their sister cultures, and the expression levels of Ras-GTP, Ras, pERK, and ERK were determined as described in Figure 1. Values shown are from a representative experiment (n=3). (c) Ras-GTP and pERK were quantitatively analyzed by densitometry and were normalized relative to the expression levels of total Ras and ERK, as described in Figure 1. The normalized Ras-GTP or pERK values in each transfection are expressed as percentages of Ras-GTP or pERK values in cells transfected with the corresponding control empty vector (pcDNA3 or PAC). Values shown are means±S.D. (bars) (n=3). *P<0.05, **P<0.005, significantly different from pcDNA3- or PAC-transfected cells
Deficiency of neurofibromin causes Ras-dependent resistance of Nf1−/− SV40 MEFs to trophic factor deprivation
Studies have shown that various Nf1−/− cell types are more resistant than Nf1+/+ cells to apoptosis induced by trophic factor deprivation.19, 29 Therefore, we examined whether Nf1−/− SV40 MEFs would also be more resistant than Nf1+/+ and Nf1+/− SV40 MEFs to apoptosis induced by trophic factor deprivation, and if so, whether or not this effect was dependent on Ras. The three Nf1 SV40 MEF genotypes were deprived of serum for 15 h and the numbers of live and dead cells were then determined by counting. Resistance to serum deprivation was found to be relatively high in the Nf1−/−, relatively low in the Nf1+/+ and intermediate in the Nf1+/− SV40 MEFs (Figure 5a). These results thus show that the absence of neurofibromin also causes resistance to apoptosis induced by trophic factor deprivation in the Nf1−/− SV40 MEFs.
Ras inhibitors inhibit the enhanced resistance of Nf1−/− SV40 MEFs to apoptosis induced by serum deprivation. (a) Effect of FTS on cell viability. Nf1−/−, Nf1+/−, and Nf1+/+ SV40 MEFs were grown in serum-free medium for 15 h in the presence of DMSO (0.005%) or 2.5 μM FTS. Live and dead cells were counted using the Trypan blue assay and percentage cell death was defined as described in Figure 2b. Values shown are means±S.D. (bars) (n=3). **P<0.005, significantly different from the corresponding treatments in Nf1+/+ MEFs. (b, c) Effect of FTS on steady-state levels of Ras-GTP. Nf1−/−, Nf1+/−, and Nf1+/+ SV40 MEFs were grown in medium supplemented with 0.5% serum for 15 h in the presence of DMSO (0.005%) or 2.5 μM FTS. Protein extracts were then prepared and (a) the amounts of Ras and Ras-GTP in each treatment were determined as described in Figure 1. The results shown are from a representative experiment (one of three independent experiments). (c) Quantitative analysis of Ras-GTP. Intensities of the Ras-GTP bands obtained after the different treatments in the three independent experiments were quantified by densitometry and normalized relative to total Ras expression for each Nf1 genotype. The normalized Ras-GTP value in each of the FTS-treated Nf1 SV40 MEF genotypes is expressed as a percentage of the normalized Ras-GTP value in the corresponding DMSO-treated Nf1 SV40 MEF genotype. Values shown are means±S.D. (bars) (n=3). **P<0.005, significantly different from the DMSO-treated MEFs. (d) Effect of DN Ras on cell viability. Nf1−/− and Nf1+/+ SV40 MEFs were co-transfected with SEAP and with DN Ras or pcDNA3 expression vectors, as described in Materials and Methods. After 18 h, the transfected cells were deprived of serum as described in Materials and Methods, or were left in serum-supplemented medium. They were then allowed to grow for 24 h longer, and their viabilities were determined by measurement of SEAP activity in the culture medium, as described in Materials and Methods. Cell survival was defined as SEAP activity in the serum-deprived transfected cultures and is expressed as a percentage of the SEAP activity in the corresponding transfected cultures gown in the presence of serum. Values shown are means±S.D. (bars) (n=3). *P<0.05, **P<0.005, significantly different from pcDNA3-transfected cells
Next, we examined whether this survival effect is dependent on Ras activity. Examination of the effect of 2.5 μM FTS, which reduced Ras-GTP levels in all three serum-starved Nf1 SV40 MEF genotypes (Figure 5b and c), on cell death induced by the serum deprivation showed that FTS had overcome the relative resistance of both the Nf1−/− and the Nf1+/− MEFs to death (Figure 5a). This finding suggests that the increased resistance of neurofibromin-deficient MEFs to trophic factor deprivation is attributed to the increase in Ras activity. This contention was further supported by co-transfection experiments with DN Ras. Nf1−/−, Nf1+/−, and Nf1+/+ SV40 MEFs were each co-transfected with SEAP and the H-RasS17N expression vectors and 18 h later were deprived of serum for 24 h. The empty pcDNA3 vector served as a control. Cell viability was then determined by monitoring SEAP activity. Transfection with the empty vector did not alter the basic differences in sensitivity of the three genotypes of SV40 MEFs to serum deprivation: resistance to serum deprivation was found to be relatively high in the Nf1−/−, relatively low in the Nf1+/+ and intermediate in the Nf1+/− SV40 MEFs (Figure 5d). Transfection with DN Ras, however, overcame the relative resistance of both the Nf1−/− and the Nf1+/− MEFs to cell death induced by serum deprivation (Figure 5d). This latter finding indicated that DN Ras, like FTS, promotes serum-deprived cell death in both Nf1−/− and Nf1+/− MEFs but not in Nf1+/+ MEFs, further supporting the conclusion that the increased resistance of neurofibromin-deficient MEFs to trophic factor deprivation is attributable to the increase in Ras activity. These results taken together with those obtained with cytotoxic treatments suggest that neurofibromin promotes proapoptotic effects in MEFs via both Ras-dependent and Ras-independent pathways.
Ras-independent resistance of Nf1−/− MEFs to apoptosis is not mediated by the absence of cAMP
Neurofibromin reportedly promotes cAMP production by a domain distinct from the NF1-GRD.10, 12 These data, together with the reported evidence of cAMP-promoted apoptosis in T cells,30 led us to suspect that the resistance of Nf1−/− MEFs to apoptosis might be conferred by defective cAMP production. We therefore, examined whether an increase in cAMP in Nf1−/− MEFs can reduce the resistance of these cells to apoptosis. The three Nf1 SV40 MEF genotypes were pretreated for 1 h with forskolin (a direct activator of adenylyl cyclase) or with the cell-permeable cAMP analog 8-Br-cAMP, together with 3-isobutyl-1-methylxanthine (IBMX) (an inhibitor of phosphodiesterases, which degrade cAMP). The cells were then exposed to staurosporine for 6.5 or 13 h and cell viability was determined by the MTT assay. Neither forskolin nor 8-Br-cAMP affected the susceptibility of the three Nf1 SV40 MEF genotypes to apoptosis induced by staurosporine (Figure 6) although in all three Nf1 SV40 MEF genotypes, we observed a marked increase in cAMP levels (data not shown). These results suggest that the effect of neurofibromin on Ras-independent cell susceptibility to apoptosis is not mediated by cAMP.
Effect of forskolin (a) and 8-Br-cAMP (b) on the susceptibility of Nf1−/−, Nf1+/−, and Nf1+/+ SV40 MEFs to Staur. The three Nf1 SV40 MEF genotypes were pretreated for 1 h without (−) or with the indicated concentrations of forskolin or with 8-Br-cAMP (100 μM) and IBMX (200 μM), and were then treated with Staur (0.5 μM) or left untreated for the indicated times. Cell viability was determined by the MTT assay. Survival was defined as the MTT values obtained in cultures treated with Staur in the presence or absence of forskolin or 8-Br-cAMP and IBMX, expressed as a percentage of the MTT values obtained in the corresponding control cultures treated in the absence of Staur and in the presence or absence of forskolin or 8-Br-cAMP and IBMX. The values shown are means±S.D. (bars) (n=3). *P<0.05, **P<0.005, significantly different from the corresponding treatment in Nf1+/+ MEFs
Ras-dependent and Ras-independent survival of neurofibromin-deficient human MPNST cells
Next, we examined whether the differences in resistance to apoptosis observed between the three Nf1 MEF genotypes were also detectable between the MPNST ST88-14 (Nf1−/−), and the STS26T (Nf1+/+) schwannomas.27 ST88-14 and STS26T cells were treated with staurosporine or vincristine or exposed to UV irradiation and their viability was determined by the MTT assay. The Nf1-deficient ST88-14 schwannoma cells were significantly more resistant than the Nf1+/+ STS26T schwannoma cells to apoptosis induced by each of these triggers (Figure 7a). Similar results were obtained when we assessed the survival of staurosporine-treated ST88-14 and STS26T schwannomas using the colony-formation assay (Figure 7b).
NF1 MPNST cells exhibit Ras- and cAMP-independent and Ras-associated survival effects. (a) ST88-14 cells are more resistant than STS26T cells to apoptosis induced by Staur, UV, or Vinc. ST88-14 cells and STS26T cells were treated with Staur or Vinc or were left untreated for the indicated times, or were UV-irradiated at the indicated intensities and then grown for 15 h. Viability of the treated cells was determined by the MTT assay. Survival was defined as the MTT value after each apoptotic treatment, expressed as a percentage of the MTT value in the absence of apoptotic treatment at the corresponding time point. The values shown are means±S.D. (bars) (n=3). *P<0.05, **P<0.005, significantly different from the corresponding treatment in STS26T cells. (b) Colony formation assay of ST88-14 and STS26T cells treated with Staur (0.5 μM) for 6.5 h. Cells that survived the apoptotic treatment were allowed to form colonies, as described in Materials and Methods. Colony formation was defined as the number of clones obtained after treatment with Staur and expressed as a percentage of the number obtained in untreated cultures. The values shown are means±S.D. (n=3). **P<0.005, significantly different from STS26T cells. (c) Effect of FTS on the susceptibility of ST88-14 and STS26T cells to apoptosis induced by Staur. ST88-14 cells and STS26T cells were pretreated with 75 μM FTS or its vehicle (0.1% DMSO) for 24 h and were then treated with Staur or left untreated, as described in Figure 2, in the continuous presence of FTS or DMSO. Cell viability after treatments was determined by the MTT assay. Survival was defined as the MTT value after the apoptotic treatment in each cell line, in the presence of DMSO or FTS, expressed as a percentage of the MTT value obtained in the absence of apoptotic treatment but in the presence of DMSO or FTS. The values shown are means±S.D. (bars) (n=3). *P<0.05, **P<0.005, significantly different from the corresponding treatment in STS26T cells. (d) Effects of DN Ras or NF1-GRD expression on the susceptibility of ST88-14 and STS26T cells to apoptosis induced by Staur. ST88-14 cells and STS26T cells were co-transfected with SEAP and with DN Ras, NF1-GRD, pcDNA3, or PAC expression vectors, as described in Materials and Methods. After 18 h, the transfected cultures were treated with Staur as described in Figure 2 or were left untreated, and viability of the transfected cells was determined by measurement of SEAP activity in the culture medium, as described in Materials and Methods. Cell survival was defined as SEAP activity in the Staur-treated transfected cultures and is expressed as a percentage of the SEAP activity in the Staur-untreated transfected cultures. (e) cAMP does not affect the susceptibility of ST88-14 or STS26T cells to apoptosis. ST88-14 cells and STS26T cells were pretreated with forskolin (20 or 50 μM) or 8-Br-cAMP (100 μM) and IBMX (200 μM) for 1 h and were then treated with Staur (0.5 μM) or left untreated for the indicated times. Cell viability was determined by the MTT assay and survival was defined as described in Figure 6. The values shown are means±S.D. (bars) (n=3). *P<0.05, **P<0.005, significantly different from the corresponding treatments in STS26T cells. Ras inhibitors sensitize ST88-14 cells to apoptosis stimulated by trophic factor withdrawal (f, g). (f) Effect of FTS on apoptosis stimulated by serum starvation. ST88-14 cells and STS26T cells were grown in medium supplemented with 0.5% serum for 15 h, and 20 μM FTS or 0.1% DMSO was then added and the cells were grown for 24 h longer. Cell death was then determined by the Hoechst 33258 dye-exclusion assay, as described in Materials and Methods. Cell death in the treated cultures was defined as the number of cells stained with the Hoechst 33258 dye and expressed as a percentage of the total number of cells. The values shown are means±S.D. (bars) (n=3). **P<0.005, significantly different from the corresponding treatments in STS26T cells. (g) Effect of DN Ras and NF1-GRD on apoptosis induced by serum deprivation. ST88-14 cells and STS26T cells were co-transfected with SEAP and with DN Ras, NF1-GRD, pcDNA3, or PAC expression vectors, as described in Materials and Methods. After 18 h the transfected cultures were deprived of serum for 5 or 24 h, as described in Materials and Methods, or were left in medium supplemented with serum. Viability of the transfected cells was determined by measurement of SEAP activity in the culture medium, as described in Materials and Methods. Cell survival was defined as SEAP activity in the serum-deprived transfected cultures and is expressed as a percentage of the SEAP activity in the corresponding transfected cultures gown in the presence of serum. *P<0.05, **P<0.005, significantly different from the corresponding empty vector transfectants
Next, we investigated the role of Ras and of cAMP on the increased resistance of the Nf1-deficient ST88-14 schwannoma cells to apoptosis by examining the effects of FTS, DN Ras, NF1-GRD, forskolin, and 8-Br-cAMP on the susceptibility of ST88-14 and STS26T schwannomas to staurosporine-induced apoptosis, as described above for the MEFs. The results showed that the increased resistance to staurosporine-induced apoptosis of ST88-14 cells was not abolished by FTS (Figure 7c), DN Ras, or NF1-GRD (Figure 7d), nor by forskolin or 8-Br-cAMP (Figure 7e). Thus, as in the case of the MEFs, the human Nf1-deficient schwannoma cells exhibited enhanced resistance to apoptosis induced by staurosporine, vincristine, or UV irradiation, and this resistance appeared to be independent of both Ras and cAMP at least in the case of apoptosis induced by staurosporine.
Next, we examined whether, by analogy to the SV40 MEFs, the Nf1-deficient ST88-14 cells exhibit enhanced Ras-dependent survival when challenged by trophic factor deprivation. Recently, we showed that FTS effectively reduces steady-state levels of Ras-GTP and inhibits growth of ST88-14 cells.27 We therefore treated serum-starved ST88-14 and STS26T cells with FTS or with its vehicle for 24 h, and then determined the numbers of live and dead cells. In the absence of FTS, the serum-starved ST88-14 and STS26T cells showed similar viability (Figure 7f) possibly because the survival of these two schwannoma cell lines under these conditions is dependent on numerous factors in addition to the presence of neurofibromin. Nonetheless, the ST88-14 cells were more sensitive than the STS26T cells to the effects of FTS (Figure 7f). The effect of Ras on the viability of ST88-14 and STS26T cells under conditions of trophic factor deprivation was further examined in serum-deprived ST88-14 and STS26T cells transfected with DN Ras, NF1-GRD, or empty vectors (Figure 7g). Compared to the empty vector, DN Ras, or NF1-GRD significantly reduced the viability of serum-starved ST88-14 cells. No such reductions were observed in serum-starved STS26T-transfected cells. These results thus suggest that for survival under conditions of trophic factor deprivation, the Ras dependence of the neurofibromin-deficient ST88-14 cells is greater than that of neurofibromin-expressing STS26T cells.
Thus, neurofibromin promotes sensitivity to trophic factor deprivation-induced apoptosis by inhibiting Ras in human MPNST cells.
Discussion
The results of this study show that neurofibromin can promote sensitivity to apoptosis and that the apoptotic effect is mediated both by Ras-dependent and by Ras- and cAMP-independent pathways. This conclusion is based on the following findings: (1) In neurofibromin-deficient cells, resistance to apoptosis was enhanced, and (in the case of the MEFs) was dependent on gene dosage. (2) The enhanced resistance of neurofibromin-deficient SV40 MEFs and MPNST cells to staurosporine, UV irradiation, and vincristine was independent of Ras and cAMP, as demonstrated by the inability of Ras inhibitors or agents that elevate cAMP levels to counteract it. (3) Survival of the neurofibromin-deficient SV40 MEFs and MPNST cells in the absence of or incomplete support by growth factors was significantly dependent on Ras, as demonstrated by the ability of Ras inhibitors to increase or promote cell death under these conditions. Detection of the proapoptotic effects of neurofibromin in both MEFs and human MPNST cells suggests that they represent a general rather than a species- or cell-type-specific feature of the protein.
This study also demonstrates that Nf1−/−, Nf1+/−, and Nf1+/+ SV40 MEFs collectively serve as a useful model system for studying cellular aspects of NF1. The Nf1−/− SV40 MEFs like human NF1 MPNST cells exhibit upregulation of Ras-GTP and its signaling pathways as well as enhanced resistance to apoptosis. Furthermore, this system allows us to study the effects of different gene dosages of neurofibromin on cell growth and apoptosis in a defined genetic background. This is an important feature, as comparison between NF1 and non-NF1 tumors from different individuals might show different effects that could stem from differences in their genetic backgrounds. In addition, fibroblasts are among the key cell types that constitute neurofibromas.31
Our finding indicating that neurofibromin promotes NF1-GRD-independent proapoptotic effects is in line with the suggestion that domain(s) other than the NF1-GRD can promote the tumor suppressor function of neurofibromin.11 Thus, neurofibromin seems to exert its tumor suppressor effect by two pathways, encoded within two different domains of the gene. One pathway is associated with cell growth inhibition and is encoded by the NF1-GRD, while the other is associated with apoptosis and is encoded via NF1-GRDs and non NF1-GRDs. Interestingly, proapoptotic action was also attributed to merlin, the tumor suppressor gene product that underlies neurofibromatosis type 2.32, 33
To the best of our knowledge the NF1-GRD- and cAMP-independent proapoptotic action of neurofibromin is a novel, hitherto unknown function of this protein. This new function has important potential for extending our understanding of NF1 and hence facilitating its treatment. The enhanced resistance of neurofibromin-deficient cells to apoptosis might underlie, at least in part, the lack of response of NF1 patients to cytotoxic drugs and could have significant implications for NF1 therapy. First, it is necessary to re-evaluate the use and efficacy of cytotoxic drugs for the treatment of NF1-associated tumors. Secondly, treatment of NF1-associated tumors with Ras inhibitors alone might not be sufficient to sensitize the cells to commonly used cytotoxic drugs. It seems that treatment, to be beneficial, should include the combined utilization of a Ras inhibitor and a cytotoxic drug capable of bypassing the proapoptotic step affected by neurofibromin. The design of such drugs requires understanding of the mechanism whereby neurofibromin deficiency confers resistance to apoptosis. Our finding that the expression levels of key apoptotic components were similar in neurofibromin-expressing and -deficient MEFs suggested that the increased resistance to apoptosis of neurofibromin-deficient cells is mediated not by an inherent enhancement in their apoptotic threshold due to changes in the expression levels of key apoptotic proteins (at least those examined in the present study), but rather by the lack of a more direct proapoptotic action of neurofibromin. The mechanism, as well as the specific neurofibromin domain within which this proapoptotic activity is contained, have yet to be determined. Such studies are currently in progress in our laboratory.
Materials and Methods
Materials
All reagents were purchased from Sigma (St Louis, MO, USA) unless otherwise stated. Ac-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (Ac-DEVD-7AMC) was from Biomol Research Laboratories (Plymouth Meeting, PA, USA). Farnesylthiosalicyic acid (FTS) was prepared as previously described in detail.34
Antibodies
Rabbit anti-neurofibromin polyclonal antibody (Ab) (BL956) was purchased from Bethyl Laboratories (Montgomery, TX, USA). Rabbit anti-Bcl-x, mouse anti-caspase-9, rabbit anti-Akt, rabbit anti-phospho Akt (S473), rabbit anti-mTOR, and rabbit anti-phospho mTOR (S2448) Abs were purchased from Cell Signaling Technology (Danvers, MA, USA). Rabbit anti-caspase-3 and rabbit anti-ERK (c-14) Abs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse anti-phospho ERK monoclonal antibody (mAb) was purchased from Sigma. Mouse anti-Pan Ras mAb (Ab-3) was purchased from Calbiochem (La Jolla, CA, USA). Rabbit anti-Bax-NT and rabbit anti-Bak-NT Abs were purchased from Upstate Cell Signaling Solutions (Charlottesville, VA, USA). Rat anti-caspase-8 was purchased from Alexis Biochemicals (San Diego, CA, USA). Mouse anti-XIAP was purchased from BD Transduction Laboratories (Lexington, KY, USA).
Cell culture
Human NF1 ST88-14 MPNST, non-NF1 STS26T schwannoma, and the different primary and SV40-immortalized Nf1 genotypes (Nf1+/+, Nf1+/−, and Nf1−/−) of MEFs were grown in high-glucose Dulbecco's-modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum (both from Biological Industries, Beit Ha Emek, Israel) with 0.0004% β-mercaptoethanol added to the MEF growth medium only. In serum-starvation or serum-deprivation experiments the cells were detached from the plates, centrifuged, washed once with PBS, resuspended in DMEM without serum or with 0.5% serum, respectively, and then seeded in 96-well or six-well plates in the presence or absence of DMSO or FTS.
Transgenic Nf1 mice and preparation of primary mouse embryonic fibroblasts
C57BL/6J mice with a targeted Nf1 gene allele were obtained from Dr N Ratner (University of Cincinnati Medical School, Cincinnati, OH, USA).22 To obtain the three Nf1 genotypes (Nf1+/+, Nf1+/−, and Nf1−/−), Nf1+/− mice were mated and checked for the presence of a copulatory plug. To obtain embryos, pregnant mice were killed after 11.5 days (the day the plug was observed was designated as day 0.5). Embryos were transferred to a sterile dish, washed in PBS, their internal organs were removed, and the rest of the embryo was chopped into small pieces that were then incubated with trypsin/EDTA for 40 min at 37°C and 8% CO2, followed by centrifugation at 1000 × g. The resulting pellets were resuspended in growth medium and the cells were seeded in 10-cm plates. After 4 h the medium was changed and the attached surviving fibroblasts were allowed to grow further. The genotypes of the different embryos were determined by PCR using the appropriate primer set as described.22
Generation of SV40-immortalized MEFs
The three primary Nf1 MEF genotypes were immortalized by transfection with SV40 whole genome DNA (a gift from Dr Atan Gross, The Weizmann Institute of Science) using the lipofectamine transfection reagent (InvitroGen Life Technologies, Paisley, UK). At 1 day before transfection, primary MEFs were seeded at a density of 2 × 105 cells per well in six-well plates. To each dish, we added 1 ml of DNA-lipofectamine mixture, 2 μg DNA (1 μg SV40 whole genome DNA and 1 μg pcDNA3 DNA) and 10 μg of lipofectamine in 1 ml of OptiMEM (InvitroGen), according to the manufacturer's instructions. The cells were split 24 h after transfection and seeded in 10-cm2 dishes at a density of 1 × 103 to 1 × 104 cells per dish. Immortalized clones from each Nf1 genotype were collected 14 days after transfection. For each of the three genotypes at least three different clones, obtained from different batches of primary cultures, were analyzed for their Ras signaling pathways and susceptibility to apoptosis, and all yielded similar results. For further analysis, clones c1 (Nf1−/−), c7 (Nf1+/−), and c7 (Nf1+/+) were chosen as representative clones for the immortalized Nf1 MEF genotypes and were designated SV40 Nf1−/− MEF, SV40 Nf1+/− MEF, and SV40 Nf1+/+ MEF, respectively.
Plasmids
The expression vectors pEGFP, pcDNA3, pCMV-SEAP, and pRSV-H-Ras(S17N) (DN-Ras) have been described elsewhere.35, 36 The MSCV-NF1GRD vector and its empty puromycin resistance gene (Pac) vector were obtained from Dr. D Wade Clapp (Indiana University School of Medicine).
Transfection
At 1 day before transfection, the three Nf1 SV40 MEF genotypes and the schwannoma cell lines were seeded at a density of 2 × 105 cells per well in six-well plates or in 35-mm dishes. The SV40 MEFs were transfected by the lipofectamine reagent. To each well or dish, we added 1 ml of DNA−lipofectamine mixture (InvitroGen) as described above. The cells were co-transfected with SEAP and GFP using either the DN-RAS or the NF1-GAP expression vectors. After incubation of the cells with the DNA−lipofectamine mixture for 18 h, DMEM supplemented with 10% serum with or without staurosporine was added, and incubation was continued for 6.5 or 24 h. The schwannoma cell lines were transfected by the FuGENE 6 reagent (Roche Diagnostics, Mannheim, Germany). To each dish, we added 1 ml of DNAFuGENE mixture, 2 μg DNA, and 3 μl of FuGENE 6 in 1 ml of OptiMEM, according to the manufacturer's instructions. The ratios of the DNA vectors used for transfection were 0.43 : 0.43 : 1 : 1 for DN-Ras/pEGFP/SEAP/pcDNA3 plasmids, 1 : 0.3 : 0.7 for pcDNA3/pEGFP/SEAP plasmids, 1 : 0.3 : 0.7 for NF1-GAP/pEGFP/SEAP plasmids, and 1 : 0.3 : 0.7 for Pac/pEGFP/SEAP plasmids.
Ras-GTP assays
Cells were lysed with Ras-binding domain (RBD) lysis buffer containing 50 mM Tris-HCl (pH 7.6), 20 mM MgCl2, 200 mM NaCl, 0.5% NP40, 1 mM DTT, 1 mM antiproteases, and 0.1 mM orthovanadate. Lysates containing 1 mg protein were analyzed for Ras-GTP by the glutathione S-transferase–Ras-binding domain pull-down assay followed by Western immunoblotting with pan anti-Ras antibodies, as described previously.36
Immunoblotting
Total extracts (100 μg protein) from each treatment were separated by 7.5 and 12.5% SDS-PAGE and electroblotted onto supported nitrocellulose. Each blot was blocked for 1 h in 10 mM Tris base, 150 mM NaCl, and 0.05% Tween-20 (TBST) containing 5% fat-free milk, and then incubated with a primary antibody overnight at 4°C. The membranes were then washed three times (10 min each) with TBST and this was followed by incubation for 1 h at room temperature with the appropriate secondary Ab (goat anti-mouse; donkey anti-rat, or goat anti-rabbit IgG peroxidase conjugate; Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The blots were developed using the Supersignal West Pico chemiluminescence kit (Pierce Biotechnology, Rockford, IL, USA). Each blot was reprobed with β-tubulin, ERK, mTOR, or AKT to verify that protein was uniformly loaded across the gel.
Assessment of cell survival
Trypan blue
Cells were detached from the plate and resuspended in 0.4% Trypan blue solution. White and blue cells were then viewed and were counted microscopically using a hemacytometer.
Hoechst 33258 dye exclusion
After treatments, Hoechst 33258 (1 μg/ml) was added to the culture medium and light and fluorescence images were collected on a fluorescence microscope (Olympus, × 10 objective lens) connected to a CCD camera for imaging. Cells whose nuclei were stained with the dye were counted as dead cells. The number of Hoechst 33258-positive cells was expressed as a percentage of the total number of cells.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
The numbers of live cells in the 96-well plates (5 × 103 cells per well) were determined by the MTT assay, as described elsewhere.37 Briefly, MTT was dissolved in PBS at a concentration of 5 mg/ml. From this stock solution, 10 μl per 100 μl of medium was added to each well, after which the plates were incubated at 37°C for 4 h. Acid-isopropanol (100 μl of 0.04 M HCl in isopropanol) was then added to the well and mixed in. After 15 min at room temperature, the plates were read on a microELISA reader at a test wavelength of 540 nm and a reference wavelength of 690 nm.
SEAP activity
The three Nf1 SV40 MEF genotypes and the schwannoma cell lines were co-transfected with GFP and SEAP and with DN-Ras, NF1-GAP, pcDNA3, or the Pac expression vector, as described above. Viability of the transfected cells was determined both by measuring the activity of SEAP in the medium of the transfected cells and by estimating the number of GFP-positive cells visualized by fluorescence microscopy (data not shown). SEAP activity reflects the viability of a pool of transfected cells, whereas the number of GFP-positive cells reflects viability on a single-cell basis. In the experiments in which staurosporine was used as the apoptotic inducer the efficiency of transfection was determined by measuring SEAP activity in the medium 18 h after transfection, and then replacing the transfection medium with fresh medium with or without staurosporine and allowing the cells to grow for an additional 24 h. SEAP activity was measured in each transfection 6.5 and 24 h after the medium was changed. These SEAP values were normalized relative to the SEAP activity measured 18 h after transfection (which indicated the efficiency of transfection) in each case. In the serum-deprivation experiments, the efficiency of transfection was determined by measuring SEAP activity in the medium 18 h after transfection. The cells were then collected by trypsinization and washed with PBS by centrifugation at 1000 × g. The resulting pellets were resuspended in medium supplemented with or without serum and then the cells were replated and allowed to grow for an additional 24 h. SEAP activity was measured in each transfection 5 and 24 h after replating. These SEAP values were normalized relative to the SEAP activity measured 18 h after transfection (which indicated the efficiency of transfection) in each case. SEAP activity was assayed as described elsewhere.35 Briefly, 200 μl from the culture medium of the transfected cells were collected and spun for 2 min at 10 000 × g. The supernatant was incubated at 65°C for 10 min, and aliquots (30 μl) from each treatment were then incubated with 200 μl of SEAP buffer (1 M diethanolamine, 0.5 mM MgCl2, 10 mM L-homo-arginine, and 5 mg/ml p-nitrophenyl phosphate; at 37°C for about 4 h. Assays were performed in quadruplicate for each treatment. The plates were read on a microELISA reader at a wavelength of 405 nm.
Assay for DEVDase activity
The activity of caspase-3/-7 was measured in terms of the assayed DEVDase activity (cleavage of the fluorescent synthetic peptide Ac-DEVD-7-AMC). The three Nf1 SV40 MEF genotypes (1 × 106 cells) were treated with staurosporine or vincristine or were left untreated for the indicated times or were UV-irradiated and then grown for an additional 15 h. At each time point the cells were collected, centrifuged, washed with PBS, and then frozen at −70°C. Each cell pellet was resuspended in 100 μl of extraction buffer (50 mM Tris-HCl, pH 7.4, 1 mM EDTA, and 10 mM EGTA) and then lysed by three rounds of freezing and thawing. The extracts were centrifuged for 5 min at 20 000 × g. Supernatants (150 μg protein) were measured for DEVDase activity using a reaction buffer containing AC-DEVD-7-AMC (50 μM), 50 mM Tris-HCl pH 7.5, 1 mM DTT, and 2 mM MgCl2. After 30 min at 37°C, fluorescence was measured at 360 nm for excitation and 460 nm for emission.
Colony-formation assay
SV40 MEFs, ST88, or STS26T cells (2 × 105 cells grown in six-well plates) were treated with 0.5 μM staurosporine or left untreated in the presence or absence of FTS. After 6.5 h the cells were detached from the plates, spun, and replated in 10-cm dishes and grown under regular growth conditions for 7–10 days. The resulting colonies were fixed with 10% acetic acid in PBS, stained with 0.4% crystal violet (dissolved in 10% acetic acid in PBS), and counted.
Incorporation of [3H] thymidine
SV40 MEFs (3 × 104 cells grown in 12-well plates) were serum starved for 13 h, after which 1% serum was added to the cells in the presence or absence of 30 μM FTS or DMSO. After incubation for 1 h, 1 μCi/ml [3H] thymidine was added to the cultures and the cells were grown for an additional 8 h. The cells were then washed with ice-cold PBS, and this was followed by incubation for 15 min in 100% methanol at −20°C. The cells were placed on ice and incubated with 10% trichloroacetic acid for an additional 10 min. This treatment was repeated twice. Finally, the cells were solubilized with 300 μl per sample with 0.1 N NaOH containing 0.1% SDS. Each solubilized sample was added to 4 ml of scintillation fluid, and radioactivity (incorporation of [3H] thymidine into DNA) was measured in quadruplicate on a liquid scintillation counter.
Abbreviations
- Ac-DEVD-7AMC:
-
Ac-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin Ac-DEVD-7AMC
- ERK:
-
extracellular signal-regulated kinase
- DMEM:
-
Dulbecco's-modified Eagle's medium
- DN:
-
dominant negative
- FTS:
-
farnesylthiosalicylic acid
- GAP:
-
GTPase activating protein
- GFP:
-
green fluorescent protein
- GRD:
-
GAP-related domain
- IBMX:
-
3-isobutyl-1-methylxanthine
- MEF:
-
mouse embryonic fibroblast
- MPNST:
-
malignant peripheral nerve sheath tumor
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NF1:
-
neurofibromatosis type 1
- PI3-kinase:
-
phosphatidylinositol-3-kinase
- RBD:
-
Ras-binding domain
- SEAP:
-
secreted alkaline phosphatase
- TBS:
-
Tris-buffered saline
- UV:
-
ultra-violet
- XIAP:
-
X-linked inhibitor of apoptosis
References
Rubin JB, Gutmann DH . Neurofibromatosis type 1 – a model for nervous system tumour formation? Nat Rev Cancer 2005; 5: 557–564.
Martin GA, Viskochil D, Bollag G, McCabe PC, Crosier WJ, Haubruck H et al. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell 1990; 63: 843–849.
Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J . Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature 1992; 356: 713–715.
DeClue JE, Papageorge AG, Fletcher JA, Diehl SR, Ratner N, Vass WC et al. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell 1992; 69: 265–273.
Guha A, Lau N, Huvar I, Gutmann D, Provias J, Pawson T et al. Ras-GTP levels are elevated in human NF1 peripheral nerve tumors. Oncogene 1996; 12: 507–513.
Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA . Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nat Genet 1994; 7: 353–361.
Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME et al. Mouse models of tumor development in neurofibromatosis type 1. Science 1999; 286: 2172–2176.
Bajenaru ML, Zhu Y, Hedrick NM, Donahoe J, Parada LF, Gutmann DH . Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol Cell Biol 2002; 22: 5100–5113.
Guo HF, Tong J, Hannan F, Luo L, Zhong Y . A neurofibromatosis-1-regulated pathway is required for learning in Drosophila. Nature 2000; 403: 895–898.
The I, Hannigan GE, Cowley GS, Reginald S, Zhong Y, Gusella JF et al. Rescue of a Drosophila NF1 mutant phenotype by protein kinase A. Science 1997; 276: 791–794.
Johnson MR, DeClue JE, Felzmann S, Vass WC, Xu G, White R et al. Neurofibromin can inhibit Ras-dependent growth by a mechanism independent of its GTPase-accelerating function. Mol Cell Biol 1994; 14: 641–645.
Dasgupta B, Dugan LL, Gutmann DH . The neurofibromatosis 1 gene product neurofibromin regulates pituitary adenylate cyclase-activating polypeptide-mediated signaling in astrocytes. J Neurosci 2003; 23: 8949–8954.
Hannan F, Ho I, Tong JJ, Zhu Y, Nurnberg P, Zhong Y . Effect of neurofibromatosis type I mutations on a novel pathway for adenylyl cyclase activation requiring neurofibromin and Ras. Hum Mol Genet 2006; 15: 1087–1098.
Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M . Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 1991; 352: 345–347.
Raveh T, Kimchi A . DAP kinase-a proapoptotic gene that functions as a tumor suppressor. Exp Cell Res 2001; 264: 185–192.
Dee K, Freer M, Mei Y, Weyman CM . Apoptosis coincident with the differentiation of skeletal myoblasts is delayed by caspase 3 inhibition and abrogated by MEK-independent constitutive Ras signaling. Cell Death Differ 2002; 9: 209–218.
Guerrero S, Casanova I, Farre L, Mazo A, Capella G, Mangues R . K-ras codon 12 mutation induces higher level of resistance to apoptosis and predisposition to anchorage-independent growth than codon 13 mutation or proto-oncogene overexpression. Cancer Res 2000; 60: 6750–6756.
Cox AD, Der CJ . The dark side of Ras: regulation of apoptosis. Oncogene 2003; 22: 8999–9006.
Donovan S, See W, Bonifas J, Stokoe D, Shannon KM . Hyperactivation of protein kinase B and ERK have discrete effects on survival, proliferation, and cytokine expression in Nf1-deficient myeloid cells. Cancer Cell 2002; 2: 507–514.
Hiatt K, Ingram DA, Huddleston H, Spandau DF, Kapur R, Clapp DW . Loss of the nf1 tumor suppressor gene decreases fas antigen expression in myeloid cells. Am J Pathol 2004; 164: 1471–1479.
Klesse LJ, Parada LF . p21 ras and phosphatidylinositol-3 kinase are required for survival of wild-type and NF1 mutant sensory neurons. J Neurosci 1998; 18: 10420–10428.
Brannan CI, Perkins AS, Vogel KS, Ratner N, Nordlund ML, Reid SW et al. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev 1994; 8: 1019–1029.
Cichowski K, Santiago S, Jardim M, Johnson BW, Jacks T . Dynamic regulation of the Ras pathway via proteolysis of the NF1 tumor suppressor. Genes Dev 2003; 17: 449–454.
Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K . The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci USA 2005; 102: 8573–8578.
Kloog Y, Cox AD . Prenyl-binding domains: potential targets for Ras inhibitors and anti-cancer drugs. Semin Cancer Biol 2004; 14: 253–261.
Kloog Y, Cox AD, Sinensky M . Concepts in Ras-directed therapy. Exp Opin Invest Drugs 1999; 8: 2121–2140.
Barkan B, Starinsky S, Friedman E, Stein R, Kloog Y . The Ras inhibitor farnesylthiosalicylic acid as a potential therapy for neurofibromatosistype 1. Clin Cancer Res 2006; 12: 5533–5542.
Blum R, Nakdimon I, Goldberg L, Elkon R, Shamir R, Rechavi G et al. E2F1 identified by promoter and biochemical analysis as a central target of glioblastoma cell-cycle arrest in response to Ras inhibition. Int J Cancer 2006; 119: 527–538.
Dasgupta B, Gutmann DH . Neurofibromin regulates neural stem cell proliferation, survival, and astroglial differentiation in vitro and in vivo. J Neurosci 2005; 25: 5584–5594.
Jondal M, Xue Y, McConkey DJ, Okret S . Thymocyte apoptosis by glucocorticoids and cAMP. Curr Top Microbiol Immunol 1995; 200: 67–79.
Rosenbaum T, Boissy YL, Kombrinck K, Brannan CI, Jenkins NA, Copeland NG et al. Neurofibromin-deficient fibroblasts fail to form perineurium in vitro. Development 1995; 121: 3583–3592.
Schulze KM, Hanemann CO, Muller HW, Hanenberg H . Transduction of wild-type merlin into human schwannoma cells decreases schwannoma cell growth and induces apoptosis. Hum Mol Genet 2002; 11: 69–76.
Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C et al. The tumour-suppressor genes NF2/Merlin and expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol 2006; 8: 27–36.
Marom M, Haklai R, Ben Baruch G, Marciano D, Egozi Y, Kloog Y . Selective inhibition of Ras-dependent cell growth by farnesylthiosalisylic acid. J Biol Chem 1995; 270: 22263–22270.
Lindenboim L, Yuan J, Stein R . Bcl-xS and Bax induce different apoptotic pathways in PC12 cells. Oncogene 2000; 19: 1783–1793.
Paz A, Haklai R, Elad G, Ballan E, Kloog Y . Galectin-1 binds H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene 2001; 20: 7486–7493.
Lindenboim L, Haviv R, Stein R . Inhibition of drug-induced apoptosis by survival factors in PC12 cells. J Neurochem 1995; 64: 1054–1063.
Acknowledgements
We are grateful to Ms Shirley Smith for excellent editorial assistance. We thank Dr. N Ratner for providing us with the C57BL/6J mice with a targeted Nf1 gene allele. This work was supported by DOD Grant NF 030009 (YK and RS), the Israel Ministry of Health (YK and RS), and the Recanati Foundation for Research in Medicine (YK and RS). Yoel Kloog is the incumbent of the Jack H Skirball Chair in Applied Neurobiology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by J Cleveland
Rights and permissions
About this article
Cite this article
Shapira, S., Barkan, B., Fridman, E. et al. The tumor suppressor neurofibromin confers sensitivity to apoptosis by Ras-dependent and Ras-independent pathways. Cell Death Differ 14, 895–906 (2007). https://doi.org/10.1038/sj.cdd.4402057
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4402057
Keywords
This article is cited by
-
Tumor growth of neurofibromin-deficient cells is driven by decreased respiration and hampered by NAD+ and SIRT3
Cell Death & Differentiation (2022)
-
Advancement in research and therapy of NF1 mutant malignant tumors
Cancer Cell International (2020)
-
CRAF mutations in lung cancer can be oncogenic and predict sensitivity to combined type II RAF and MEK inhibition
Oncogene (2019)
-
RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers
Nature Cell Biology (2018)
-
Bone mineral metabolism in patients with neurofibromatosis type 1 (von Recklingausen disease)
Archives of Dermatological Research (2012)