Abstract
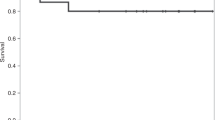
There is a significant amount of morbidity and mortality following myeloablative umbilical cord blood transplantation (UCBT). Reduced intensity (RI) conditioning offers an alternative to myeloablative conditioning before UCBT. We investigated RI-UCBT in 21 children and adolescents with malignant (n=14), and non-malignant diseases (n=7). RI conditioning consisted of fludarabine (150–180 mg/m2) with either busulfan (⩽8 mg/kg)+rabbit antithymocyte globulin (R-ATG; n=16) or cyclophosphamide+R-ATG±etoposide (n=5). Human leukocyte antigen match: 4/6 (n=13), 5/6 (n=5) and 6/6 (n=3). The median total nucleated cell and CD34+ cell dose per kilogram were 3.58 × 107 and 2.54 × 105, respectively. The median time for neutrophil and platelet engraftment was 17.5 and 52 days, respectively. There were six primary graft failures (chronic myelogenous leukemia (CML), β-thalassemia, hemophagocytic lymphohistiocytosis (HLH) and myelodysplastic syndrome (MDS)). The probability of developing grade II to grade IV acute graft-versus-host disease (GVHD) and chronic GVHD was 28.6 and 16.7%, respectively. Incidence of transplant-related mortality (TRM) was 14%. The 5 years overall survival (OS) in all patients was 59.8%. The 5 years OS for patients with average versus poor-risk malignancy was 77.8 versus 22.2% (P=0.03). RI-UCBT may result in graft failure in specific high-risk chemo-naïve patients (CML, β-thalassemia, HLH and MDS), but in more heavily pretreated pediatric and adolescent recipients results in rapid engraftment and may be associated with decreased severe GVHD and TRM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kurtzberg J, Laughlin M, Graham ML, Smith C, Olson JF, Halperin EC et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med 1996; 335: 157–166.
Wagner JE, Rosenthal J, Sweetman R, Shu XO, Davies SM, Ramsay NK et al. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood 1996; 88: 795–802.
Cairo MS, Wagner JE . Placental and/or umbilical cord blood: an alternative source of hematopoietic stem cells for transplantation. Blood 1997; 90: 4665–4678.
Rocha V, Wagner Jr JE, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and international bone marrow transplant registry working committee on alternative donor and stem cell sources. N Engl J Med 2000; 342: 1846–1854.
Barker JN, Davies SM, DeFor T, Ramsay NK, Weisdorf DJ, Wagner JE . Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood 2001; 97: 2957–2961.
Rocha V, Cornish J, Sievers EL, Filipovich A, Locatelli F, Peters C et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood 2001; 97: 2962–2971.
Michel G, Rocha V, Chevret S, Arcese W, Chan KW, Filipovich A et al. Unrelated cord blood transplantation for childhood acute myeloid leukemia: a Eurocord Group analysis. Blood 2003; 102: 4290–4297.
Locatelli F, Rocha V, Reed W, Bernaudin F, Ertem M, Grafakos S et al. Related umbilical cord blood transplantation in patients with thalassemia and sickle cell disease. Blood 2003; 101: 2137–2143.
Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P et al. Cord-blood transplants from unrelated donors in patients with Hurler's syndrome. N Engl J Med 2004; 350: 1960–1969.
Styczynski J, Cheung YK, Garvin J, Savage DG, Billote GB, Harrison L et al. Outcomes of unrelated cord blood transplantation in pediatric recipients. Bone Marrow Transplant 2004; 34: 129–136.
Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med 2001; 344: 1815–1822.
Gluckman E, Rocha V, Chevret S . Results of unrelated umbilical cord blood hematopoietic stem cell transplant. Transfus Clin Biol 2001; 8: 146–154.
Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood 2002; 100: 1611–1618.
Moscardo F, Sanz GF, Sanz MA . Unrelated-donor cord blood transplantation for adult hematological malignancies. Leuk Lymphoma 2004; 45: 11–18.
Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med 2004; 351: 2265–2275.
Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med 2004; 351: 2276–2285.
Takahashi S, Iseki T, Ooi J, Tomonari A, Takasugi K, Shimohakamada Y et al. Single-institute comparative analysis of unrelated bone marrow transplantation and cord blood transplantation for adult patients with hematologic malignancies. Blood 2004; 104: 3813–3820.
Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med 1997; 337: 373–381.
Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med 1998; 339: 1565–1577.
Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA, Kiem HP et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood 1997; 89: 3048–3054.
Kean LS, Durham MM, Adams AB, Hsu LL, Perry JR, Dillehay D et al. A cure for murine sickle cell disease through stable mixed chimerism and tolerance induction after nonmyeloablative conditioning and major histocompatibility complex-mismatched bone marrow transplantation. Blood 2002; 99: 1840–1849.
Bradley MB, Sattler RM, Raftopoulos H, Ward M, Grossman IR, Townes TM et al. Correction of phenotype in a thalassemia mouse model using a nonmyeloablative marrow transplantation regimen. Biol Blood Marrow Transplant 2002; 8: 453–461.
Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998; 91: 756–763.
Carella AM, Cavaliere M, Lerma E, Ferrara R, Tedeschi L, Romanelli A et al. Autografting followed by nonmyeloablative immunosuppressive chemotherapy and allogeneic peripheral-blood hematopoietic stem-cell transplantation as treatment of resistant Hodgkin's disease and non-Hodgkin's lymphoma. J Clin Oncol 2000; 18: 3918–3924.
Childs R, Chernoff A, Contentin N, Bahceci E, Schrump D, Leitman S et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med 2000; 343: 750–758.
Horwitz ME, Barrett AJ, Brown MR, Carter CS, Childs R, Gallin JI et al. Treatment of chronic granulomatous disease with nonmyeloablative conditioning and a T-cell-depleted hematopoietic allograft. N Engl J Med 2001; 344: 881–888.
Giralt S, Thall PF, Khouri I, Wang X, Braunschweig I, Ippolitti C et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood 2001; 97: 631–637.
Khoury H, Adkins D, Brown R, Pence H, Vij R, Goodnough LT et al. Low incidence of transplantation-related acute complications in patients with chronic myeloid leukemia undergoing allogeneic stem cell transplantation with a low-dose (550 cGy) total body irradiation conditioning regimen. Biol Blood Marrow Transplant 2001; 7: 352–358.
Dreger P, Brand R, Hansz J, Milligan D, Corradini P, Finke J et al. Treatment-related mortality and graft-versus-leukemia activity after allogeneic stem cell transplantation for chronic lymphocytic leukemia using intensity-reduced conditioning. Leukemia 2003; 17: 841–848.
Lee CK, Badros A, Barlogie B, Morris C, Zangari M, Fassas A et al. Prognostic factors in allogeneic transplantation for patients with high-risk multiple myeloma after reduced intensity conditioning. Exp Hematol 2003; 31: 73–80.
Gomez-Almaguer D, Ruiz-Arguelles GJ, Tarin-Arzaga Ldel C, Gonzalez-Llano O, Jaime-Perez JC, Lopez-Martinez B et al. Reduced-intensity stem cell transplantation in children and adolescents: the Mexican experience. Biol Blood Marrow Transplant 2003; 9: 157–161.
Del Toro G, Satwani P, Harrison L, Cheung YK, Brigid Bradley M, George D et al. A pilot study of reduced intensity conditioning and allogeneic stem cell transplantation from unrelated cord blood and matched family donors in children and adolescent recipients. Bone Marrow Transplant 2004; 33: 613–622.
Jacobsohn DA, Duerst R, Tse W, Kletzel M . Reduced intensity haemopoietic stem-cell transplantation for treatment of non-malignant diseases in children. Lancet 2004; 364: 156–162.
Rao K, Amrolia PJ, Jones A, Cale CM, Naik P, King D et al. Improved survival after unrelated donor bone marrow transplantation in children with primary immunodeficiency using a reduced-intensity conditioning regimen. Blood 2005; 105: 879–885.
Satwani P, Harrison L, Morris E, Del Toro G, Cairo MS . Reduced-intensity allogeneic stem cell transplantation in adults and children with malignant and nonmalignant diseases: end of the beginning and future challenges. Biol Blood Marrow Transplant 2005; 11: 403–422.
Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE . Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood 2003; 102: 1915–1919.
Miyakoshi S, Yuji K, Kami M, Kusumi E, Kishi Y, Kobayashi K et al. Successful engraftment after reduced-intensity umbilical cord blood transplantation for adult patients with advanced hematological diseases. Clin Cancer Res 2004; 10: 3586–3592.
Chao NJ, Koh LP, Long GD, Gasparetto C, Horwitz M, Morris A et al. Adult recipients of umbilical cord blood transplants after nonmyeloablative preparative regimens. Biol Blood Marrow Transplant 2004; 10: 569–575.
Yuji K, Miyakoshi S, Kato D, Miura Y, Myojo T, Murashige N et al. Reduced-intensity unrelated cord blood transplantation for patients with advanced malignant lymphoma. Biol Blood Marrow Transplant 2005; 11: 314–318.
Petersdorf EW, Smith AG, Haase AM, Martin PJ, Hansen JA . Polymorphism of HLA-DRw52-associated DRB1 genes as defined by sequence-specific oligonucleotide probe hybridization and sequencing. Tissue Antigens 1991; 38: 169–177.
Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, Migliaccio AR et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA 1995; 92: 10119–10122.
Fraser JK, Cairo MS, Wagner EL, McCurdy PR, Baxter-Lowe LA, Carter SL et al. Cord Blood Transplantation Study (COBLT): cord blood bank standard operating procedures. J Hematother 1998; 7: 521–561.
Cairo MS, Wagner EL, Fraser J, Cohen G, van de Ven C, Carter SL et al. Characterization of banked umbilical cord blood hematopoietic progenitor cells and lymphocyte subsets and correlation with ethnicity, birth weight, sex, and type of delivery: a Cord Blood Transplantation (COBLT) Study report. Transfusion 2005; 45: 856–866.
Kurtzberg J, Cairo MS, Fraser JK, Baxter-Lowe L, Cohen G, Carter SL et al. Results of the cord blood transplantation (COBLT) study unrelated donor banking program. Transfusion 2005; 45: 842–855.
Osunkwo I, Bessmertny O, Harrison L, Cheung YK, Van de Ven C, del Toro G et al. A pilot study of tacrolimus and mycophenolate mofetil graft-versus-host disease prophylaxis in childhood and adolescent allogeneic stem cell transplant recipients. Biol Blood Marrow Transplant 2004; 10: 246–258.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation 1974; 18: 295–304.
Militano O, Boskoff B, Del Toro G, Van de Ven C, Satwani P, Bradley B et al. Sequential administration of sargramostim (GM-CSF) and filgrastim (G-CSF) in pediatric allogeneic stem cell transplant (AlloSCT) recipients undergoing myeloablative (MA) conditioning: cost-effective and more rapid platelet recovery in UCB recipients. Biol Blood Marrow Transplant 2006; 12: 43.
Roman E, Osunkwo I, Militano O, Cooney E, Garvin J, Bradley MB et al. Liposomal amphotericin B prophylaxis is effective in prevention of invasive mold infections following allogeneic stem cell transplantation in pediatric patients. Pediatric Blood and Cancer 2005; 45: 418–419.
Shereck EB, Cooney E, van de Ven C, Della-Lotta P, Cairo MS . A pilot phase II study of alternate day ganciclovir and foscarnet in preventing cytomegalovirus (CMV) infections in at-risk pediatric and adolescent allogeneic stem cell transplant recipients. Pediatr Blood Cancer 2007 (E-pub 2006; doi:10-002/PBC.21043).
National Cancer Institute Common Terminology Criteria for Adverse Events v.3 (CTCAE) (http://ctep.cancer.gov/reporting/ctc.html).
National Cancer Institute Common Toxicity Criteria version 2.0 (http://ctep.cancer.gov/reporting/ctc.html).
Kaplan EL, Meier P . Non-parametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Locatelli F, Rocha V, Chastang C, Arcese W, Michel G, Abecasis M et al. Factors associated with outcome after cord blood transplantation in children with acute leukemia. qEurocord-Cord Blood Transplant Group. Blood 1999; 93: 3662–3671.
Slattery JT, Clift RA, Buckner CD, Radich J, Storer B, Bensinger WI et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood 1997; 89: 3055–3060.
Iannone R, Casella JF, Fuchs EJ, Chen AR, Jones RJ, Woolfrey A et al. Results of minimally toxic nonmyeloablative transplantation in patients with sickle cell anemia and beta-thalassemia. Biol Blood Marrow Transplant 2003; 9: 519–528.
de Lima M, Anagnostopoulos A, Munsell M, Shahjahan M, Ueno N, Ippoliti C et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood 2004; 104: 865–872.
Gluckman E, Rocha V, Eurocord Study. The impact of number and type of HLA incompatibilities and cell dose on outcomes of unrelated cord blood transplants for patients with malignant and non-malignant disorders. Biol Blood Marrow Transplant 2006; 12: 1221–1222.
Chao NJ, Emerson SG, Weinberg KI . Stem cell transplantation (cord blood transplants). Hematology (Am Soc Hematol Educ Program) 2004; 1: 354–371.
Matsumura T, Narimatsu H, Kami M, Yuji K, Kusumi E, Hori A et al. Cytomegalovirus infections following umbilical cord blood transplantation using reduced intensity conditioning regimens for adult patients. Biol Blood Marrow Transplant 2007; 13: 577–583.
Couriel DR, Saliba RM, Giralt S, Khouri I, Andersson B, de Lima M et al. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant 2004; 10: 178–185.
Mielcarek M, Martin PJ, Leisenring W, Flowers ME, Maloney DG, Sandmaier BM et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood 2003; 102: 756–762.
Roman E, Cooney E, Harrison L, Militano O, Wolownik K, Hawks R et al. Preliminary results of the safety of immunotherapy with gemtuzumab ozogamicin following reduced intensity allogeneic stem cell transplant in children with CD33+ acute myeloid leukemia. Clin Cancer Res 2005; 11: 7164s–7170s.
Yamashiro D, Bradley MB, Harrison L, Lee A, Glade-Bender J, Bessmertny O et al. A pilot study of sequential myeloablative autologous stem cell transplantation (MA-AutoSCT) and immunotherapy with reduced intensity allogeneic stem cell transplant (RI-AlloSCT) for high-risk neuroblastoma. Pediatric Blood and Cancer 2004; 43: 458.
Acknowledgements
We thank Leslie Disla for her assistance with the preparation of this manuscript. We also thank the nurses of the inpatient and outpatient units for their expert care and most importantly to the patients and their families who participated in these clinical research studies. This paper was presented in part at the American Society of Blood and Marrow Transplant, 2007 Annual Meeting, Keystone, Colorado. This work was supported in part by the Pediatric Cancer Research Foundation, National Institute of Arthritis and Musculoskeletal and Skin Diseases (R21AR49330) (MSC), National Cancer Institute (5P30CA13697) (MSC), Marisa Fund, Sonia Scaramella Fund, Bevanmar Foundation and Brittany Barron Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bradley, M., Satwani, P., Baldinger, L. et al. Reduced intensity allogeneic umbilical cord blood transplantation in children and adolescent recipients with malignant and non-malignant diseases. Bone Marrow Transplant 40, 621–631 (2007). https://doi.org/10.1038/sj.bmt.1705785
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705785
Keywords
This article is cited by
-
Hematopoietic stem cell transplantation in children with Griscelli syndrome type 2: a single-center report on 35 patients
Bone Marrow Transplantation (2020)
-
Disease-specific hematopoietic stem cell transplantation in children with inherited bone marrow failure syndromes
Annals of Hematology (2017)
-
Cellular engineering and therapy in combination with cord blood allografting in pediatric recipients
Bone Marrow Transplantation (2016)
-
Sequential myeloablative autologous stem cell transplantation and reduced intensity allogeneic hematopoietic cell transplantation is safe and feasible in children, adolescents and young adults with poor-risk refractory or recurrent Hodgkin and non-Hodgkin lymphoma
Leukemia (2015)
-
Reduced-toxicity myeloablative conditioning consisting of 8-Gy total body irradiation, cyclophosphamide and fludarabine for pediatric hematological malignancies
Scientific Reports (2014)