Abstract
This study examined the pathological complete response (pCR) rate and safety of sequential gemcitabine-based combinations in breast cancer. We also examined gene expression profiles from tumour biopsies to identify biomarkers predictive of response. Indian women with large or locally advanced breast cancer received 4 cycles of gemcitabine 1200 mg m−2 plus doxorubicin 60 mg m−2 (Gem+Dox), then 4 cycles of gemcitabine 1000 mg m−2 plus cisplatin 70 mg m−2 (Gem+Cis), and surgery. Three alternate dosing sequences were used during cycle 1 to examine dynamic changes in molecular profiles. Of 65 women treated, 13 (24.5% of 53 patients with surgery) had a pCR and 22 (33.8%) had a complete clinical response. Patients administered Gem d1, 8 and Dox d2 in cycle 1 (20 of 65) reported more toxicities, with G3/4 neutropenic infection/febrile neutropenia (7 of 20) as the most common cycle-1 event. Four drug-related deaths occurred. In 46 of 65 patients, 10-fold cross validated supervised analyses identified gene expression patterns that predicted with ⩾73% accuracy (1) clinical complete response after eight cycles, (2) overall clinical complete response, and (3) pCR. This regimen shows strong activity. Patients receiving Gem d1, 8 and Dox d2 experienced unacceptable toxicity, whereas patients on other sequences had manageable safety profiles. Gene expression patterns may predict benefit from gemcitabine-containing neoadjuvant therapy.
Similar content being viewed by others
Main
Although the effectiveness of neoadjuvant chemotherapy vs adjuvant chemotherapy in locally advanced breast cancer remains controversial (Estévez and Gradishar, 2004; Cianfrocca and Gradishar, 2005), general findings indicate that both approaches result in equivalent disease-free survival and overall survival (Scholl et al, 1994; Fisher et al, 1998; van der Hage et al, 2001). Clinical trial results indicate that 60–80% of women who receive neoadjuvant chemotherapy have a clinical response, and 10–20% achieve a pathological complete response (pCR) (Cianfrocca and Gradishar, 2005). Because pCR predicts long-term survival (Fisher et al, 1998; Kuerer et al, 1999; Wolmark et al, 2001), ongoing research is focused on identifying regimens that improve the pCR rate and on identifying biomarkers that can predict response to neoadjuvant chemotherapy.
Anthracycline-based regimens are efficacious and widely used in neoadjuvant breast cancer therapy, but debate remains as to which combinations provide encouraging activity balanced by an acceptable safety profile. Gemcitabine has demonstrated single-agent activity in breast cancer (Heinemann, 2003), and is indicated as first-line therapy in combination with paclitaxel for patients with metastatic breast cancer after failure of prior anthracycline-containing adjuvant chemotherapy (Albain et al, 2004). In the neoadjuvant setting, gemcitabine has been combined with anthracyclines and/or taxanes in several phase II studies of locally advanced breast cancer, with clinical response rates and pCR rates ranging from 71–95% and 3–26%, respectively (Gomez et al, 2001; Silva et al, 2002; Estévez et al, 2004; Schneeweiss et al, 2004).
Gemcitabine plus cisplatin (Gem+Cis) combination therapy has also shown activity in metastatic or refractory breast cancer, with response rates from 26–50% (Heinemann, 2002). Given the tumour shrinking capability of the combination of gemcitabine and doxorubicin (Gem+Dox) (Pérez-Manga et al, 2000; Gomez et al, 2001), we hypothesised that additional cycles of combination chemotherapy may improve pCR rates. In this phase II study, we evaluated two combination regimens administered sequentially as neoadjuvant chemotherapy in Indian women with operable breast cancer: four cycles of Gem+Dox followed by four cycles of Gem+Cis.
Administering chemotherapy prior to surgical resection provides an opportunity for translational research. By collecting pretreatment biopsies, we attempted to identify biomarkers that could predict chemotherapy response, whereas collecting post-treatment tissue samples allowed us to examine gene expression changes in response to treatment. Recent studies have focused on using DNA microarrays to identify gene expression patterns predictive of chemotherapy response (Chang et al, 2003; Ayers et al, 2004; Bertucci et al, 2004; Hannemann et al, 2005; Iwao-Koizumi et al, 2005; Sørlie et al, 2006), and we performed similar analyses here.
Patients and methods
Eligibility criteria
Female patients 18 years or older with a histopathological or cytological diagnosis of breast carcinoma were eligible. Patients with T3, T4, N2, or T2 tumours ⩾3 cm were included. No prior treatments were permitted. Additional inclusion criteria were Karnofsky performance status ⩾70, adequate bone marrow reserve and organ function (hepatic and renal), and normal left ventricular ejection fraction (67±8%) by echocardiography. Patients with inflammatory breast cancer, a second primary malignancy, a previous cancer within the last 5 years, or active cardiac disease not controlled by therapy and/or an infarction within the preceding 6 months were excluded. Appropriate ethical review boards approved the protocol, patients provided written informed consent before enrolment, and the trial was conducted in accordance with the Declaration of Helsinki and good clinical practise standards.
Study design
The primary objective of this multicentre, open-label, non-randomised, phase II study was to evaluate the pCR rate of sequential neoadjuvant Gem+Dox followed by Gem+Cis in patients with operable breast cancer. The secondary objectives of the study were to characterise toxicity, to examine gene expression profiles and identify biomarkers that might predict and/or characterise chemotherapy response, and to determine survival and progression-free survival rates after 5 years. Because patients remain in follow-up at the time of this publication, time-to-event parameters will be presented in a subsequent publication.
Treatment plan
Treatment consisted of four cycles (21 days) of gemcitabine 1200 mg m−2 plus doxorubicin 60 mg m−2 (Gem+Dox), four cycles of gemcitabine 1000 mg m−2 plus cisplatin 70 mg m−2 (Gem+Cis), and surgery. Gemcitabine, doxorubicin, and cisplatin were administered by intravenous infusion and cisplatin administration included standard hydration and antiemetic procedures. Full supportive care, including antibiotics, antiemetics, and granulocyte colony-stimulation factors, was administered as clinically indicated.
We administered three sequences of Gem+Dox during cycle 1 and collected tumour biopsies after the first dose of treatment via fine needle aspiration cytology to capture single-agent treatment effects and potential changes in molecular profiles. The first 20 patients received gemcitabine on days 1 and 8 plus doxorubicin on day 2 (Gem d1, 8; Dox d2). The next 20 patients received gemcitabine on days 1 and 8 plus doxorubicin on day 1 (Gem d1, 8; Dox d1). The remaining 25 patients received doxorubicin on day 1 plus gemcitabine on days 2 and 8 (Gem d2, 8; Dox d1). For cycles 2 through 4, all patients received Gem d1, 8 and Dox d1. For cycles 5 through 8, patients received gemcitabine on days 1 and 8 plus cisplatin on day 1 (Gem+Cis).
Day-1 doses for gemcitabine, doxorubicin, or cisplatin were delayed for 1 week if the absolute neutrophil count (ANC) was <1.5 × 109 l−1 and/or platelets were <100 × 109 l−1. Subsequent gemcitabine and cisplatin doses were decreased by 25% after any of the following: febrile neutropenia, grade 4 neutropenia lasting >7 days, grade 4 thrombocytopenia lasting >3 days, ⩾grade 2 bleeding with thrombocytopenia (any grade), or grade 3 non-haematologic toxicities (except nausea/vomiting). Grade 4 non-haematologic toxicities required either a 50% dose reduction of gemcitabine and cisplatin or a delay. Cisplatin doses were reduced by 50% for grade 2 peripheral neurotoxicity or creatinine clearance between 35 and 49 ml min−1; the cycle was delayed for grade 3 or 4 peripheral neurotoxicity or creatinine clearance <35 ml min−1. A decrease in ejection fraction below 45% or a net decrease in ejection fraction ⩾10% below baseline, or clinical congestive heart failure required patient discontinuation.
Gemcitabine day-8 doses were reduced by 25% if the ANC was between 0.5 and 0.99 × 109 l−1 or platelets were between 50 and 99 × 109 l−1. Gemcitabine day-8 doses were omitted if the ANC was <0.5 × 109 l−1 and platelets were >50 × 109 l−1, or for any ANC and platelets <50 × 109 l−1. Day-8 doses were reduced by 50% or omitted for grade 3 non-haematologic toxicities (except nausea/vomiting), and were omitted for grade 4 non-haematologic toxicities (except nausea/vomiting). Patients who required either a 6-week dose delay or three dose reductions were discontinued from treatment.
The extent and type of surgery that followed chemotherapy (breast conservation surgery or mastectomy with axillary lymph node dissection) was guided by the tumour size, physician, and/or patient decision. After surgery, patients who were oestrogen receptor- and/or progesterone receptor-positive were treated with tamoxifen.
Baseline and treatment assessments
Pretreatment biopsies (incisional) were taken for histopathology, prognostic marker evaluation (hormone receptor and HER2), and DNA microarray analysis. Hormone receptors (oestrogen receptor or progesterone receptor) and HER2 status were determined by immunohistochemistry. Two additional samples, one fine needle aspiration cytology collected before treatment on day 2 of cycle 1 and one needle core biopsy collected at the end of cycle 4, were collected for future correlative analyses.
Baseline radiological imaging studies (mammogram, abdominal ultrasound, and chest x-ray) were performed. Clinical response, evaluated by physical exam, was classified using the Southwest Oncology Group criteria (Green and Weiss, 1992) before each cycle for all patients who received at least one dose of chemotherapy. Pathologic response was assessed using the Miller and Payne classification system (Ogston et al, 2003), with a pCR defined as grade 5. Specifically, a grade 5 designation is characterised as no malignant cells in sections from the site of the previous tumour and allows for the presence of ductal carcinoma in situ. The pathologic response status was evaluated by a local pathologist and an independent reviewer. All patients who had surgery after chemotherapy were included in the assessment of pathologic response.
Clinical laboratory tests (haematology and blood chemistries) were performed at baseline, before the day-8 dose of each cycle, within 4 days before the start of subsequent cycles, and on day 21 of cycle 8. All women who received at least one dose of chemotherapy were assessed for safety before each cycle using the National Cancer Institute Common Toxicity Criteria Scale, version 2.0 (National Cancer Institute, 1999).
Statistical considerations
Potential clinical prognostic factors (age, menopausal status, tumour size, clinical node status, hormone receptor status, and HER2 status) were assessed individually for their association with both clinical response and pCR using logistic regression analysis.
RNA isolation and microarray hybridisation
Pretreatment incisional biopsies were immediately placed into RNAlater® (Ambion, Foster City, CA, USA) and stored at −80 °C. Total RNA was prepared from biopsies using Qiagen RNAeasy kits (Qiagen, Valencia, CA, USA) and its quality was checked using an Agilent Bioanalyzer (Agilent, Santa Clara, CA, USA). Samples with ⩾1 μg of total RNA and discernable 18S and 28S peaks were used for microarray analysis (46 of 65 patients). Total RNA was amplified and labelled as previously described (Hu et al, 2005). Microarray hybridisations were performed on Agilent Human 1A (V2) microarrays using 2 μg of Cy3-labelled common reference sample (Novoradovskaya et al, 2004) and 2 μg of Cy5-labelled experimental sample. Microarrays were hybridised overnight, washed, dried, and scanned as previously described (Hu et al, 2005). Microarray image files were analysed with GenePix Pro 4.1 (Molecular Devices, Sunnyvale, CA, USA) and loaded into the UNC-CH Microarray Database (https://genome.unc.edu/), and are available in the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo) under the series numbers GSE8465.
Microarray analysis and prediction of response
Data from the microarray experiments were processed as previously described (Hu et al, 2005). Briefly, genes that did not have a signal intensity ⩾30 in both channels for ⩾70% of the experiments were excluded. To predict response, the gene expression data for the 46 pretreatment samples were analysed using the following as ‘supervising parameters’: clinical complete response (CR) after each cycle of chemotherapy (CR vs PR, SD, and PD combined), overall clinical complete response (CR vs non-CR, evaluated after the last successfully completed therapy cycle, not necessarily cycle 8), pCR, and clinical ER status (included as a positive control).
Four statistical classification methods were used to predict chemotherapy response using the pretreatment gene expression data: a k-nearest neighbour classifier (k-NN with k=1, 3, 5, or 7) with either Euclidean distance or one minus Spearman correlation as the distance function and a Class Nearest Centroid (CNC) classifier with either Euclidean distance or one minus Spearman correlation as the distance function, as described previously (Chung et al, 2004). To evaluate prediction accuracy, each of the four classification methods underwent 10-fold cross validation (CV); in each round of CV, each predictor using n genes (how the n genes were selected is described below) was trained on 90% of the samples and used to make predictions on the remaining 10%, with this procedure repeated nine more times such that every sample was ‘left out’ exactly once. The mean prediction accuracy for the 10 iterations was recorded for each classification method using n genes. Note that n was increased for subsequent rounds of CV. For each response variable, the set of n genes that gave the highest average prediction accuracy during CV was determined and reported for each classification method (accuracies were reported with associated binomial confidence intervals).
Each classification method required a gene/feature selection step to identify genes associated with each ‘class’ (i.e., CR vs PR+SD). For all four methods, we used a gene selection method described by Dudoit et al (2002); the genes were identified in the training set according to the ratio of between-class to within-class sums of squares. The top n-ranked genes were used during each round of CV. Because the number of cases in our study was relatively small (n=46), we did not break our data into training and test sets but instead performed 10-fold CV using the four statistical classification methods to avoid overfitting caused by using a single classification method or fortuitous training and test set randomisations.
Results
Patient characteristics
Sixty-five Indian women enroled between February 2003 and March 2004 at the All India Institute of Medical Sciences in New Delhi, the Christian Medical College and Hospital in Vellore, and the Hirabai Cowasji Jehangir Medical Research Institute, Jehangir Hospital, in Pune. Table 1 shows baseline patient and disease characteristics. Most patients had ductal breast cancer (61 of 65). Patients generally had large tumours, with 84% of primary tumours identified as T3 or larger, and the median size of the largest baseline lesion was 30 cm2 (range, 7.8–85.8 cm2).
Treatment administration
Fifty-three patients had post-treatment surgery, but only 40 of these completed all eight cycles of chemotherapy. Twenty-five patients (38.5%) discontinued before completing eight cycles, although 13 of these had surgery. Twelve of 25 patients who discontinued stopped on or before cycle 4. Reasons for discontinuing chemotherapy included: adverse event (5 of 25), death from study drug toxicity (4 of 25), unrelated death (1 of 25), disease progression (2 of 25), satisfactory response (9 of 25, and all but one of these had surgery), and other patient decision (4 of 25). For the subset of patients who discontinued without surgery (12 of 25), reasons for discontinuation included death (5 of 12), adverse event (2 of 12), and missing follow-up visits or other patient decision (5 of 12).
The median relative dose intensity was 97% for doxorubicin, 96% for gemcitabine in cycles 1 through 4, 86% for gemcitabine in cycles 5 through 8, and 95% for cisplatin. There were 49 dose reductions (37 gemcitabine, seven doxorubicin, and five cisplatin) due to adverse events, with neutropenia (25 of 49) and febrile neutropenia (15 of 49) being the most prevalent reasons for adjustment. There were 34 gemcitabine dose omissions attributed to adverse events, most of which were due to neutropenia (13 of 34), fatigue (6 of 34), or mucositis (3 of 34). Of the 33 cycle delays attributed to adverse events, most were due to neutropenia, leukopenia, or mucositis.
Clinical results
Efficacy
Patient outcomes are summarised in Table 2. The overall clinical response rate was 81.5% (with 22 CRs), and all of the responders showed the first signs of response on or before cycle 4. After completion of therapy, 35 patients had modified radical mastectomy and 18 had breast conservation surgery. Of the 53 patients who had surgery, 13 (24.5%) showed a pCR.
Prognostic factor analyses conducted on all enroled patients showed that age, menopausal status, hormone receptor status, HER2 status, tumour size, and clinical node status were not associated with either clinical response (CR or PR) or pCR.
Toxicity
Although subgroup analyses of cohorts that had alternate cycle-1 dosing sequences were not preplanned, unforeseen safety issues prompted a more detailed evaluation. Patients who received Gem d1, d8 and Dox d2 during cycle 1 showed higher rates of toxicity. This was unexpected since the three patient cohorts received the same treatment schedule for Gem+Dox in cycles 2 through 4 (Gem d1, 8; Dox d1) and for Gem+Cis in cycles 5 through 8. Supplementary Table 1 lists the incidence of toxicity by cycle for each cycle-1 cohort. Eighty per cent of the CTC grade 3 or 4 (G3/4) toxicities seen in cycle 1 occurred in the patients who received Gem d1, 8 and Dox d2 (33 of 41). The most common cycle-1 toxicities for this cohort were febrile neutropenia or infection with G3/4 neutropenia in 35% of patients (three patients with G3 and four patients with G4) and G3/4 vomiting in 25% of patients (three patients with G3 and two patients with G4). G3 dehydration (3 of 20), G3 diarrhoea (3 of 20), and G3 thrombocytopenia (3 of 20) each occurred in 15% of patients in cycle 1.
A summary of G3/4 toxicity reported throughout the entire study is shown in Table 3. Neutropenia was prevalent in all three cohorts, with an incidence of 23.1% for grade 3 (15 of 65) and 18.5% (12 of 65) for grade 4. Both G3 mucositis and G3/4 anaemia were somewhat more prevalent in the Gem d1, 8 and Dox d2 cohort, but the incidence for each cohort was no higher at the beginning than at the end of the study.
Five patients died on-study and four deaths were attributed to treatment-related toxicity. These deaths did not appear to be directly related to the alternate dosing schedules used in cycle 1. For one patient, the doses for Gem+Dox were incorrectly calculated in cycle 1 such that the patient received a 20% higher dose for gemcitabine and a 25% higher dose for doxorubicin. The patient was hospitalised for neutropenic sepsis several days after the day-8 dose of gemcitabine and died. The second patient experienced neutropenia with infection, hypoglycemia, and anaemia during cycle 3 and died of cardiac arrest. The third patient died after 8 days of hospitalisation in cycle 8 for convulsions, vomiting, diarrhoea, and neutropenic sepsis. The fourth patient was hospitalised during cycle 8 for severe diarrhoea and died of cardiac arrest secondary to severe hypokalemia. A fifth patient died of acute myocardial infarction, which was not attributed to study treatment.
Gene expression analysis and prediction of response
Analysis of tumour samples using the breast intrinsic gene set
We assayed 46 pretreatment biopsy samples using Agilent human microarrays covering over 17 000 genes (19 of 65 samples failed to give usable RNA). To investigate the expression data, we first hierarchically clustered (Eisen et al, 1998) the 46 pretreatment samples using a 1300-gene breast intrinsic gene set developed by Hu et al (2006), which can identify the intrinsic tumour subtypes (luminal A, luminal B, basal-like, HER2+/ER− and normal breast-like) that show significant differences in patient outcome (Sørlie et al, 2001, 2003). The main intrinsic subtypes of luminal, basal-like, and HER2+/ER− (Supplementary Figure 1) were identifiable in this Indian patient dataset, which is consistent with previous studies showing that the breast tumour intrinsic subtypes are conserved across ethnic groups (Yu et al, 2004; Carey et al, 2006).
Association of response with breast intrinsic subtype
We examined response rates within the breast intrinsic subtypes as assigned from Supplementary Figure 1. The basal-like subtype had the highest pCR rate (7 of 14, 50%), which is consistent with findings of previous studies (Rouzier et al, 2005; Carey et al, 2007), and luminal tumours had the lowest pCR rate (3 of 15, 20%), but the association was not statistically significant (Table 4, P=0.23).
Prediction of neoadjuvant chemotherapy response
We performed supervised analyses on the pretreatment gene expression data and determined the 10-fold CV error rates for predicting (1) pCR, (2) overall clinical complete response (CR vs non-CR), and (3) CR vs non-CR after each cycle (results presented here are only for CR vs non-CR after cycle 8). Supplementary Tables 2 through 4 show that the 10-fold CV analyses using the CNC and k-NN classification methods yielded gene expression profiles/predictors that accurately classified tumours according to (1) pCR vs no pCR (73% (95% CI: 0.57–0.85) −78% [95% CI: 0.63–0.89] accuracy), (2) overall CR vs non-CR (83% [95% CI: 0.69–0.92] −88% [95% CI: 0.75–0.95] accuracy), and (3) CR vs non-CR after cycle 8 (75% [95% CI: 0.57–0.87] −89% [95% CI: 0.73–0.96] accuracy) for the 46 tumour samples that yielded pretreatment microarray data. Each of the four prediction methods achieved similar accuracies when used to predict a given response variable. Prediction of ER status was included as a positive control for our gene expression-based predictors; it represents the upper threshold of how good a predictor can be (87% [95% CI: 0.74–0.94] −89% [95% CI: 0.76–0.95] accuracy) on this dataset, providing a benchmark against which the chemotherapy response predictors can be judged (Supplementary Table 5).
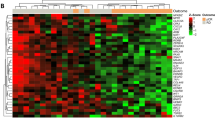
Using the gene lists identified in the 10-fold CV analysis as being predictive of pCR (Supplementary Table 2, Euclidian Nearest Centroid), we hierarchically clustered (Eisen et al, 1998) the pretreatment biopsy samples to better understand the predictive genes and their relationships to each other. Figure 1 shows the hierarchical clustering of tumours using the 94-gene set predictive of pCR (76% accuracy in the 10-fold CV analysis). (Note: the classification of samples into clusters and the associated accuracies shown in Figures 1 and 2 and Supplementary Figure 2 are different from those observed in the 10-fold CV analysis and are for illustrative purposes only.) Using the program EASE (Hosack et al, 2003), the Gene Ontology (GO) (http://www.geneontology.org) categories ‘nucleotide/nucleic acid metabolism’ and ‘DNA metabolism’ were over-represented relative to chance in the gene set highly expressed in tumours with pCR (Figure 1, top gene dendrogram branch).
Figure 2 shows the hierarchical clustering of tumours using the 71-gene set predictive of clinical response at treatment cycle 8 (86% accuracy in the 10-fold CV analysis). According to EASE, the GO categories ‘nucleotide/nucleic acid metabolism,’ ‘RNA metabolism,’ ‘DNA repair,’ and ‘response to stress’ were over-represented relative to chance in the gene set highly expressed in tumours with CR at cycle 8 (Figure 2, bottom gene dendrogram branch). Interestingly, the complete responders at cycle 8 showed high expression of NUDT2, which is not only involved in nucleotide metabolism but may also promote apoptosis (Vartanian et al, 1999; Swarbrick et al, 2005). Supplementary Figure 2 shows the hierarchical clustering of tumours using the 66-gene set predictive of overall CR (88% accuracy in the 10-fold CV analysis). Interestingly, the responders represented in Supplementary Figure 2 showed high expression of FADD, which also promotes apoptosis (Chinnaiyan et al, 1995).
Discussion
This phase II study of sequential Gem+Dox and Gem+Cis as neoadjuvant therapy in breast cancer showed strong activity (an overall clinical response rate of 81.5% and a pCR rate of 24.5%), but also revealed unforeseen safety issues related to the administration of an alternate schedule of Gem+Dox in cycle 1. To facilitate tissue sampling for biomarker analyses in the present trial, we used three dosing sequences in cycle 1 only. Dosing sequences were Gem d1, d8 and Dox d2 or Gem d2, d8 and Dox d1 in two of the three patient cohorts, and the more standard dosing sequence of Gem d1, d8 and Dox d1 for the third cohort in cycle 1 and for all patients in cycles 2 through 4. Patients who received Gem d1, d8 and Dox d2 during cycle 1 showed higher rates of toxicity during cycle 1 (Supplementary Table 1), with febrile neutropenia, neutropenia with infection, and vomiting being the most prevalent toxicities. Toxicity profiles for the other two cohorts were more manageable (Table 3), and the incidence and types of adverse events were similar to those in previous phase II studies of gemcitabine plus doxorubicin in patients with breast cancer (Pérez-Manga et al, 2000; Gomez et al, 2001; El Serafy et al, 2003; Bensalem and Bouzid, 2006).
Although several different dosing regimens of Gem+Dox were applied in previous studies, they were all similar in that gemcitabine and doxorubicin were both given on day 1 and, in some cases, were given together again on day 8 (Pérez-Manga et al, 2000; Gomez et al, 2001; El Serafy et al, 2003; Bensalem and Bouzid, 2006). In these previous studies, haematologic toxicities were the most common, but alopecia and mucositis were also prevalent. We know of no other studies that have evaluated the administration of gemcitabine and doxorubicin sequentially on days 1 and 2, and these results suggest that this schedule induces unacceptable toxicity. The number of treatment-related deaths was also high in this study, but the affected patients were not disproportionately associated with any single cohort. The details of each case were evaluated thoroughly, and we were unable to identify unifying characteristics that indicated why these individuals were more vulnerable to the serious side effects of chemotherapy. Notably, while the standard Gem+Dox dose schedule produced a toxicity profile that was similar to previous studies with Gem+Dox, the toxic death rate was higher than that in previous trials (Pérez-Manga et al, 2000; Gomez et al, 2001; El Serafy et al, 2003; Bensalem and Bouzid, 2006). These events highlight the need for appropriate patient education, careful screening, and the use of additional supportive services in developing countries like India, especially in areas where health-care access may be limited.
The identification of molecular predictors of patient outcomes will help us design safer and more effective regimens that are tailored to individual patients. In the present study, we determined that pretreatment gene expression patterns could predict response to gemcitabine-containing neoadjuvant therapy. The accuracy rates and associated confidence intervals achieved were encouraging and similar to those achieved by predictors developed for other neoadjuvant regimens (Chang et al, 2003; Ayers et al, 2004). pCR is strongly correlated with improved long-term disease-free and overall survival (Fisher et al, 1998; Kuerer et al, 1999; Wolmark et al, 2001). There is evidence that clinical complete response at the end of neoadjuvant chemotherapy is also correlated with improved long-term survival (Ferrière et al, 1998; Chang et al, 1999; Pierga et al, 2003; Cleator et al, 2005). Thus, the gene expression-based predictors obtained in this study for pCR, overall clinical complete response, and clinical complete response after cycle 8 have the potential to be clinically useful if further validated. As might be expected from gemcitabine's mechanism of action, nucleotide metabolism signatures were found to be associated with response.
To conclude, sequential neoadjuvant gemcitabine plus doxorubicin followed by gemcitabine plus cisplatin was effective for downstaging large breast tumours in patients with operable breast cancer, thereby improving treatment options for patients who desired breast conservation surgery instead of mastectomy. Future results for overall and disease-free survival will determine if these high response rates will translate into improved long-term efficacy. Significant toxicity was observed in a subgroup of patients who received Gem d1, d8 and Dox d2, and this dosing sequence is not recommended. The gene expression-based predictors identified here may provide a method for selecting patients most likely to benefit from gemcitabine-containing neoadjuvant therapy and, thus, warrant further validation using additional datasets as they emerge.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Albain KS, Nag S, Calderillo-Ruiz G, Jordaan JP, Llombart A, Pluzanska A, Pawlicki M, Melemed AS, O’Shaughnessy J, Reyes JM (2004) Global phase III study of gemcitabine plus paclitaxel (GT) vs paclitaxel (T) as frontline therapy for metastatic breast cancer (MBC): first report of overall survival. J Clin Oncol 22 (14S): 510 (abstract)
Ayers M, Symmans WF, Stec J, Damokosh AI, Clark E, Hess K, Lecocke M, Metivier J, Booser D, Ibrahim N, Valero V, Royce M, Arun B, Whitman G, Ross J, Sneige N, Hortobagyi GN, Pusztai L (2004) Gene expression profiles predict complete pathologic response to neoadjuvant paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide chemotherapy in breast cancer. J Clin Oncol 22: 2284–2293
Bensalem A, Bouzid K (2006) Gemcitabine in metastatic breast cancer. J Clin Oncol 24 (18S): 10735 (abstract)
Bertucci F, Finetti P, Rougemont J, Charafe-Jauffret E, Nasser V, Loriod B, Camerlo J, Tagett R, Tarpin C, Houvenaeghel G, Nguyen C, Maraninchi D, Jacquemier J, Houlgatte R, Birnbaum D, Viens P (2004) Gene expression profiling for molecular characterization of inflammatory breast cancer and prediction of response to chemotherapy. Cancer Res 64: 8558–8565
Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM (2007) The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 13: 2329–2334
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295: 2492–2502
Chang J, Powles TJ, Allred DC, Ashley SE, Clark GM, Makris A, Assersohn L, Gregory RK, Osborne CK, Dowsett M (1999) Biologic markers as predictors of clinical outcome from systemic therapy for primary operable breast cancer. J Clin Oncol 17: 3058–3063
Chang JC, Wooten EC, Tsimelzon A, Hilsenbeck SG, Gutierrez MC, Elledge R, Mohsin S, Osborne CK, Chamness GC, Allred DC, O’Connell P (2003) Gene expression profiling for the prediction of therapeutic response to docetaxel in patients with breast cancer. Lancet 362: 362–369
Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM (1995) FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81: 505–512
Chung CH, Parker JS, Karaca G, Wu J, Funkhouser WK, Moore D, Butterfoss D, Xiang D, Zanation A, Yin X, Shockley WW, Weissler MC, Dressler LG, Shores CG, Yarbrough WG, Perou CM (2004) Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell 5: 489–500
Cianfrocca M, Gradishar WJ (2005) Controversies in the therapy of early stage breast cancer. Oncologist 10: 766–779
Cleator SJ, Makris A, Ashley SE, Lal R, Powles TJ (2005) Good clinical response of breast cancers to neoadjuvant chemoendocrine therapy is associated with improved overall survival. Ann Oncol 16: 267–272
Dudoit S, Fridlyand J, Speed TP (2002) Comparison of discrimination methods for the classification of tumors using gene expression data. J Am Stat Assoc 97: 77–87
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95: 14863–14868
El Serafy M, El Khodary A, El Zawahry H, Mansour O, Gaballah H (2003) Gemcitabine plus doxorubicin as first-line treatment in advanced or metastatic breast cancer (MBC). Proc Am Soc Clin Oncol 22: 199 (abstract)
Estévez LG, Gradishar WJ (2004) Evidence-based use of neoadjuvant taxane in operable and inoperable breast cancer. Clin Cancer Res 10: 3249–3261
Estévez LG, Sánchez-Rovira P, Dómine M, León A, Calvo I, Jaén A, Casado V, Rubio G, Daz M, Miró C, Lobo F (2004) Biweekly docetaxel and gemcitabine as neoadjuvant chemotherapy in stage II and III breast cancer patients: preliminary results of a phase II and pharmacogenomic study. Semin Oncol 32 (2 Suppl 5): 31–36
Ferrière JP, Assier I, Curé H, Charrier S, Kwiatkowski F, Achard JL, Dauplat J, Chollet P (1998) Primary chemotherapy in breast cancer: correlation between tumor response and patient outcome. Am J Clin Oncol 21: 117–120
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz Jr AB, Hoehn JL, Lees AW, Dimitrov NV, Bear HD (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16: 2672–2685
Gomez H, Kahatt C, Falcon S, Santillana S, de Mendoza FH, Valdivia S, Vallejos C, Otero J, Pen DL (2001) A phase II study of neoadjuvant gemcitabine plus doxorubicin in stage IIIB breast cancer: a preliminary report. Semin Oncol 28 (3 Suppl 10): 57–61
Green S, Weiss GR (1992) Southwest oncology group standard response criteria, end point definitions and toxicity criteria. Invest New Drugs 10: 239–253
Hannemann J, Oosterkamp HM, Bosch CA, Velds A, Wessels LF, Loo C, Rutgers EJ, Rodenhuis S, van de Vijver MJ (2005) Changes in gene expression associated with response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 23: 3331–3342
Heinemann V (2002) Gemcitabine plus cisplatin for the treatment of metastatic breast cancer. Clin Breast Cancer 3 (Suppl 1): 24–29
Heinemann V (2003) Role of gemcitabine in the treatment of advanced and metastatic breast cancer. Oncology 64: 191–206
Hosack DA, Dennis G, Sherman BT, Lane HC, Lempicki RA (2003) Identifying biological themes within lists of genes with EASE. Genome Biol 4: R70
Hu Z, Fan C, Oh DS, Marron JS, He X, Qaqish BF, Livasy C, Carey LA, Reynolds E, Dressler L, Nobel A, Parker J, Ewend MG, Sawyer LR, Wu J, Liu Y, Nanda R, Tretiakova M, Ruiz Orrico A, Dreher D, Palazzo JP, Perreard L, Nelson E, Mone M, Hansen H, Mullins M, Quackenbush JF, Ellis MJ, Olopade OI, Bernard PS, Perou CM (2006) The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics 7: 96
Hu Z, Troester M, Perou CM (2005) High reproducibility using sodium hydroxide-stripped long oligonucleotide DNA microarrays. Biotechniques 38: 121–124
Iwao-Koizumi K, Matoba R, Ueno N, Kim SJ, Ando A, Miyoshi Y, Maeda E, Noguchi S, Kato K (2005) Prediction of docetaxel response in human breast cancer by gene expression profiling. J Clin Oncol 23: 422–431
Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, Theriault RL, Singh G, Binkley SM, Sneige N, Buchholz TA, Ross MI, McNeese MD, Buzdar AU, Hortobagyi GN, Singletary SE (1999) Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 17: 460–469
National Cancer Institute (1999) Common Toxicity Criteria (version 2). http://ctep.cancer.gov/reporting/ctc.html (accessed 02 May 2007)
Novoradovskaya N, Whitfield ML, Basehore LS, Novoradovsky A, Pesich R, Usary J, Karaca M, Wong WK, Aprelikova O, Fero M, Perou CM, Botstein D, Braman J (2004) Universal reference RNA as a standard for microarray experiments. BMC Genomics 5: 20
Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, Schofield A, Heys SD (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12: 320–327
Pérez-Manga G, Lluch A, Alba E, Moreno-Nogueira JA, Palomero M, García-Conde J, Khayat D, Rivelles N (2000) Gemcitabine in combination with doxorubicin in advanced breast cancer: final results of a phase II pharmacokinetic trial. J Clin Oncol 18: 2545–2552
Pierga JY, Mouret E, Laurence V, Diéras V, Savigioni A, Beuzeboc P, Dorval T, Palangié T, Jouve M, Pouillart P (2003) Prognostic factors for survival after neoadjuvant chemotherapy in operable breast cancer. The role of clinical response. Eur J Cancer 39: 1089–1096
Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L (2005) Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 11: 5678–5685
Schneeweiss A, Huober J, Sinn HP, von Fournier D, Rudlowski C, Beldermann F, Krauss K, Solomayer E, Hamerla R, Wallwiener D, Bastert G (2004) Gemcitabine, epirubicin and docetaxel as primary systemic therapy in patients with early breast cancer: results of a multicentre phase I/II study. Eur J Cancer 40: 2432–2438
Scholl SM, Fourquet A, Asselain B, Pierga JY, Vilcoq JR, Durand JC, Dorval T, Palangié T, Jouve M, Beuzeboc P, Garcio-Giralt E, Salmon RJ, De la Rochefordière A, Campana F, Pouillart P (1994) Neoadjuvant vs adjuvant chemotherapy in premenopausal patients with tumors considered too large for breast conserving surgery: preliminary results of a randomised trial: S6. Eur J Cancer 30A: 645–652
Silva A, Gonzalez H, Perez M, Rivera S, Lira-Puerto V, Castaneda N, Morales F, Calderillo G, Alcedo J, Onate L, Gallardo D, de La Garza J (2002) Neoadjuvant gemcitabine plus epirubicin in locally advanced breast cancer: evidence of activity in a phase II study. Proc Am Soc Clin Oncol 21: 2015 (abstract)
Sørlie T, Perou CM, Fan C, Geisler S, Aas T, Nobel A, Anker G, Akslen LA, Botstein D, Børresen-Dale AL, Lønning PE (2006) Gene expression profiles do not consistently predict the clinical treatment response in locally advanced breast cancer. Mol Cancer Ther 5: 2914–2918
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lønning P, Børresen-Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98: 10869–10874
Sørlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lønning PE, Brown PO, Børresen-Dale AL, Botstein D (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100: 8418–8423
Swarbrick JD, Buyya S, Gunawardana D, Gayler KR, McLennan AG, Gooley PR (2005) Structure and substrate-binding mechanism of human Ap4A hydrolase. J Biol Chem 280: 8471–8481
van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L (2001) Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 19: 4224–4237
Vartanian A, Alexandrov I, Prudowski I, McLennan A, Kisselev L (1999) Ap4A induces apoptosis in human cultured cells. FEBS Lett 456: 175–180
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B (2001) Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 2001: 96–102
Yu K, Lee CH, Tan PH, Tan P (2004) Conservation of breast cancer molecular subtypes and transcriptional patterns of tumor progression across distinct ethnic populations. Clin Cancer Res 10: 5508–5517
Acknowledgements
We thank doctors GK Rath, O Nair, R Bhamrah, A Varghese, S John, U Dhar, A Amin, J Abhyanker, and A Seth for assisting with patient care. Allen Melemed, Debasish Roychowdhury, Bente Frimodt-Moller, David Chonzi, Sue Sutton, Noelle Gasco, Shilpi Ray, and Donna Miller made contributions toward study design, interpretation of clinical data, study coordination, scientific writing collaboration, and article preparation. This research was supported by Eli Lilly and Company.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Presented in part at the 27th Annual San Antonio Breast Cancer Symposium, December 8–11, 2004, San Antonio, TX, USA and at the 42nd Annual Meeting of the American Society of Clinical Oncology, June 2–6, 2006, Atlanta, GA, USA.
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Julka, P., Chacko, R., Nag, S. et al. A phase II study of sequential neoadjuvant gemcitabine plus doxorubicin followed by gemcitabine plus cisplatin in patients with operable breast cancer: prediction of response using molecular profiling. Br J Cancer 98, 1327–1335 (2008). https://doi.org/10.1038/sj.bjc.6604322
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604322
Keywords
This article is cited by
-
Meta-analysis on the association between pathologic complete response and triple-negative breast cancer after neoadjuvant chemotherapy
World Journal of Surgical Oncology (2014)
-
The value of platinum agents as neoadjuvant chemotherapy in triple-negative breast cancers: a systematic review and meta-analysis
Breast Cancer Research and Treatment (2014)
-
A phase 2 study of sequential neoadjuvant chemotherapy with gemcitabine and doxorubicin followed by gemcitabine and cisplatin in patients with large or locally advanced operable breast cancer: results from long-term follow-up
Breast Cancer (2013)
-
Therapeutic potential for phenytoin: targeting Nav1.5 sodium channels to reduce migration and invasion in metastatic breast cancer
Breast Cancer Research and Treatment (2012)
-
Building prognostic models for breast cancer patients using clinical variables and hundreds of gene expression signatures
BMC Medical Genomics (2011)