Abstract
Stable isotopes of hydrogen and oxygen (δ2H, δ18O and δ17O) serve as powerful tracers in hydrological investigations. To our knowledge, daily precipitation isotope record especially 17O-excess is rare in the mid-latitudes. To fill such knowledge gap, daily precipitation samples (n=446) were collected from June 2014 to May 2018 in Indianapolis, Indiana, U.S. A Triple Water Vapor Isotope Analyzer (T-WVIA) based on Off-Axis Integrated Cavity Output Spectroscopy (OA-ICOS) technique was used to concurrently measure precipitation isotopic variations (δ2H, δ18O and δ17O). Meanwhile, 17O-excess and d-excess as second-order isotopic variables were calculated to provide additional information on precipitation formation and transport mechanisms. This study presents a four-year daily precipitation isotope dataset for mid-latitudes, and makes it available to researchers around the world who may use it as a reference for site comparisons and for assessing global hydrological models.
Design Type(s) | source-based data analysis objective • data collection and processing objective |
Measurement Type(s) | isotope |
Technology Type(s) | laser absorption spectroscopy |
Factor Type(s) | temporal_instant |
Sample Characteristic(s) | Town of Zionsville • city |
Machine-accessible metadata file describing the reported data (ISA-Tab format)
Similar content being viewed by others
Background & Summary
Stable isotopes of hydrogen and oxygen (δ2H and δ18O) are widely used as natural tracers in ecohydrological and hydroclimatic studies1–5. In recent years, with the development of high-precision analytical methods6,7, 17O (the least natural abundant (0.038%) oxygen isotope)8, becomes a new tracer to probe hydrological and meteorological processes.
Stable isotopic compositions of precipitation are affected by complex meteorological and geographical factors, such as atmospheric conditions at the moisture source and precipitation site, moisture transport trajectories, altitude of condensation and latitude5,9–11. There are two types of mass-dependent fractionation process (i.e., equilibrium fractionation and kinetic fractionation) during the precipitation formation. The individual stable isotopes (δ2H, δ18O and δ17O) demonstrate different sensitivities to equilibrium and kinetic fractionation processes12. Two second-order isotopic variables, deuterium excess (d-excess=δ2H − 8×δ18O)13 and 17O-excess (17O-excess=ln (δ17O + 1)−0.528×ln (δ18O + 1))14, can be utilized to provide additional constraints. The d-excess is sensitive to the kinetic fractionation processes due to the elimination of the 2H and 18O co-variation during the equilibrium fractionation9,15. The d-excess of precipitation is influenced by both moisture source temperature and relative humidity (hereafter RH). Similar to d-excess, 17O-excess is also sensitive to the kinetic fractionation (e.g., evaporation and condensation in supersaturation condition)16,17. However, theoretically 17O-excess is mainly sensitive to the RH due to the canceled temperature effect on 18O and 17O14,18,19. 17O-excess therefore could serve as a new tracer to better understand hydrological and meteorological processes. 17O-excess in polar ice cores has been used to reconstruct past climate over glacial-interglacial cycles12,20–22. The evolution of 17O-excess reflects the different microphysical processes along the squall line and is sensitive to convective processes in African precipitation23. Recent studies show that the relationship between 18O and 17O can be used to differentiate drought type (e.g., synoptic drought vs. local drought)24 and differentiate fog and dew formations at the Namib Desert25. Thus far, there are few studies on precipitation 17O-excess in the middle latitude regions11,26. δ17O measurements with acceptable precision has been challenging because of its low natural abundance. The traditional Isotope Ratio Mass Spectrometry (IRMS) technique is one of the most widely used approaches to measure δ17O. However, it is complicated, expensive and time-consuming, and can only be carried out in a small number of laboratories worldwide6,19,27. In recent years, laser absorption spectroscopy (LAS) techniques including Cavity Ring Down Spectroscopy (CRDS) and Off-Axis Integrated Cavity Output Spectroscopy (OA-ICOS) technique have been developed for δ17O analysis. Based on the recent assessments, the precision of CRDS and OA-ICOS δ17O and 17O-excess measurements are lower than traditional IRMS technique, but almost comparable6,7,11,26,28.
The objective of this article is to provide a four-year (June 2014 to May 2018) isotope (δ2H, δ18O, δ17O, d-excess and 17O-excess) dataset of daily precipitation from Indianapolis, Indiana of the central United States (39.88°N, 86.27°W; 258 m above sea level). Influencing factors of the precipitation formation at the site is relatively complicated and caused by different water vapor sources (Continental, Pacific, Atlantic, Gulf of Mexico, and Arctic)29–31. We provided detailed description of the instrument operation (δ2H, δ18O and δ17O) using Triple Water Vapor Isotope Analyzer (T-WVIA-45-EP; Los Gatos Research Inc. (LGR), Mountain View, CA, USA) based on OA-ICOS technique. Then, detailed 17O-excess data filter method was described which was found to be useful to quality control the dataset as demonstrated in our recent work26. It is the first publicly available daily precipitation isotope dataset (δ2H, δ18O, δ17O, d-excess and 17O-excess) from the central United States, which would provide valuable information for scientists for site comparisons and assessing global hydrological models.
Methods
Sample collections
The sampling location is Zionsville (Indianapolis), Indiana of the central United States (39.88°N, 86.27 °W). The sampling device is placed on the ground with a diameter of ~35 cm and volume of ~6000ml. We collected 446 daily precipitation samples from June 2014 to May 2018. To reduce evaporation effects on isotopes, samples were immediately transferred from the precipitation collector to sealed glass vials (Qorpak Bottles, Fisher Scientific Co. Germany) except for those occurring after midnight. In those cases, they were collected at the earliest possible time in the morning. Snowfall samples were first melted in sealed plastic bags and then poured into the vials. All of the samples were stored at 4 °C until isotope analysis. Notably, samples containing impurities were filtered with 0.45 μm syringe filters (Cellulose Nitrate Membrane Filters, GE Healthcare Co. UK) or centrifuged (Iec Centra CL2 Centrifuge, Thermo Electron Co. USA) depending on the size of the impurities before being measured. The meteorological data during the study period were obtained from the Zionsville meteorological station (https://www.wunderground.com).
Isotope measurements
A Triple Water Vapor Isotope Analyzer (T-WVIA-45-EP; Los Gatos Research Inc. (LGR), Mountain View, CA, USA), based on Off-Axis Integrated Cavity Output Spectroscopy (OA-ICOS) technique, was used to concurrently measured three isotopic ratios (δ2H, δ18O and δ17O) of water vapor. Water Vapor Isotope Standard Source (WVISS, LGR, Mountain View, CA, USA) is a vaporization device without inducing isotope fractionation during the transformation of liquid water into water vapor. Through the combined operation of the WVISS and T-WVIA instruments, 2H/1H, 18O/16O and 17O/16O ratios of all the precipitation samples were continually and simultaneously measured at IUPUI (Indiana University-Purdue University Indianapolis) Ecohydrology Lab, as described in our previous studies28,32. Typically a minimum of 0.5 ml sample is needed to ensure the data quality. The water isotopic ratios were expressed in δ-notation as a deviation from a reference ratio:
where R is the atomic ratio (e.g., 2H/H, 18O/16O or 17O/16O) of the sample, and RVSMOW is the respective isotope ratio of the international standard Vienna Standard Mean Ocean Water (hereafter VSMOW).
To achieve high precision, the following procedure was followed as described in our earlier work28,32. The internal temperature of WVISS was preheated to 80 oC to ensure complete vaporization of the liquid sample. The process usually takes about 2 h when the ambient temperature is about 25 oC. The T-WVIA was also turned on about 2 h before the measurements to ensure ideal measuring conditions with chamber temperature and gas pressure being around 50 oC and 40 Torr during measurements. Pipe-heating cable was used to heat the Teflon tubing connecting the WVISS and T-WVIA to avoid condensation of water vapor.
To avoid memory effects from residual water, the WVISS nebulizer was first purged for at least 2 min, and then the “stabilize” option of the device was turned on for 2 min to expel residual air inside the vaporizing chamber. The vapor concentration was adjusted by the “dilution control” knob through controlling the flow rates of dry air and the liquid water sample. All the samples were measured under 13000 ppm with higher precision based on our previous work26,28. Each sample was measured for 2 min, and the data output frequency was 1 Hz, which means 120 data points were generated for each sample.
Isotope calibration and normalization
To routine checking the instrument performance, five commercially available working standards from LGR with known isotopic composition (Table 1) were analyzed as reference waters after every five precipitation samples.
Additionally, in order to reduce inter-laboratory difference using different technique and calibration methods, all of the isotope ratios were normalized using two International Atomic Energy Agency (IAEA) standards VSMOW and Standard Light Antarctic Precipitation (SLAP) as calibration materials. “Measured” δ value with respect to VSMOW was first calculated using the formula below described by Steig et al.7:
where δ is the δ2H, δ18O or δ17O, and “raw” value is directly derived from the ratio of measured isotopologue abundance.
Then, normalization to the VSMOW-SLAP scale was following the procedure described in Schoenemann et al.27:
where δ is the δ2H, δ18O or δ17O, and the assigned values of SLAP is showed in Table 1. Here, SLAP2 is used as the replacement water standard for SLAP, which is not significantly different from SLAP for isotope values33. Therefore, SLAP2 is still referred as SLAP hereafter. The two international standards (VSMOW and SLAP) were measured once during each day of the measurements.
17O-excess data processing
Significant 17O-excess error is influenced by small peculiarities in either δ18O or δ17O due to small order of magnitude for 17O-excess (per meg, i.e., 0.001‰)20. To minimize sources of error, two types of quality control filters were used to check each individual data point. One is regression coefficient (λ = ln (δ17O + 1)/ln (δ18O + 1)), which will be the same as mass-dependent fractionation coefficient (θ) during the isotopic fractionation processes of liquid-vapor equilibrium and in water vapor diffusion in air2,19. The fractionation coefficient of oxygen isotope was found to be 0.511 ± 0.005 for kinetic transport effects2 and 0.529±0.001 for equilibrium effects19. The other restriction is 17O-excess value. Almost all of the 17O-excess values of global precipitation (e.g., rainfall, snowfall, and ice) fall within the range of −100 to +100 per meg11,17,23,34–36. Therefore, to attain better precision of 17O-excess, any measurements outside the 0.506 and 0.530 range, as well as outside the observed range (−100 to +100 per meg), were removed from the analysis. The final 17O-excess value for every precipitation sample was given as the mean value of quality-controlled data. To check the precision of our measurements, SLAP and the five working standards from LGR as mentioned above were used to calculate the precision. Additionally, Greenland Ice Sheet Precipitation (GISP), another international standard, was also measured to check the stability of our instrument precision.
Code Availability
No custom code was used in this work.
Data Records
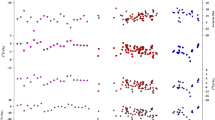
Daily precipitation isotope database is archived in PANGAEA in a single table including 446 rows and 6 columns (Data Citation 1). Each row presents a daily precipitation event, and each column corresponds to an isotope variable including three individual stable isotopes (δ2H, δ18O and δ17O) and two second-order isotopic variables (d-excess and 17O-excess) (Table 2). Figure 1 shows a summary of the 4-year isotope record (2014 to 2018). The δ2H values varied from −236.75‰ to 17.64‰ with an average of −39.06‰ (Table 3). The δ18O values varied from −31.54‰ to 3.23‰ with an average of −6.25‰. The δ17O values varied from −16.77‰ to 1.68‰ with an average of −3.27‰. The d-excess values varied from −25.8‰ to 29.6‰ with an average of 9.3‰. The 17O-excess values varied from −26 to 69 per meg with an average of 31 per meg. The local meteoric water line (LMWL) between δ18O and δ2H based on the 446 precipitation samples in the four years was δ2H=7.73 (±0.07)×δ18O + 7.39 (±0.62) (R2=0.96, p<0.001), which is close to the Global Meteoric Water Line (GMWL, δ2H=8×δ18O + 10) (Fig. 2). The local meteoric water line (LMWL) between δ18O and δ17O was ln (δ17O + 1) = 0.5275 (±0.0001) × ln (δ18O+1) + 0.000028 (±0.000001) (R2=1, p<0.001), similar to the GMWL for oxygen (ln (δ17O + 1)=0.528×ln (δ18O + 1) + 0.000033 (R2=0.99999)36 (Fig. 2).
Technical Validation
Multiple standards were used to validate our measurements and our measurement precision was compared with reported values in the literature (Tables 4 and 5). The precision of SLAP in our measurements was 0.79‰, 0.04‰, 0.02‰ and 3 per meg for δ2H, δ18O, δ17O and 17O-excess, respectively (Table 4). The precision of GISP was 0.12‰, 0.02‰, 0.02‰ and 7 per meg for δ2H, δ18O, δ17O and 17O-excess, respectively. The precision range for five working standards was between 0.07‰ to 0.80‰ for δ2H, 0.01‰ to 0.06‰ for δ18O, 0.02‰ to 0.03‰ for δ17O, and 2 to 12 per meg for 17O-excess.
Therefore, the 17O-excess precision of our OA-ICOS technique (2 to 12 per meg) is comparable with IRMS technique (0.1 to 16 per meg)17,21,27,34–36, as well as for CRDS method (7 to 10 per meg)7,11 and another type of OA-ICOS water analyzer (10 to 18 per meg)6 (Table 5). Meanwhile, the precisions of the three individual isotopes (δ2H, δ18O and δ17O) were also acceptable compared with the previous studies (Table 5).
Additional information
How to cite this article: Tian, C. and Wang, L. Stable isotope variations of daily precipitation from 2014–2018 in the central United States. Sci. Data. 6:190018 https://doi.org/10.1038/sdata.2019.18 (2019).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
References
Jameel, Y. et al. Tap water isotope ratios reflect urban water system structure and dynamics across a semiarid metropolitan area. Water Resour. Res. 52, 5891–5910 (2016).
Angert, A., Cappa, C. D. & DePaolo, D. J. Kinetic 17O effects in the hydrologic cycle: Indirect evidence and implications. Geochim. Cosmochim. Acta 68, 3487–3495 (2004).
Wang, L. et al. Partitioning evapotranspiration across gradients of woody plant cover: Assessment of a stable isotope technique. Geophys. Res. Lett. 37, L09401 (2010).
Zhao, L. et al. Factors controlling spatial and seasonal distributions of precipitation δ18O in China. Hydrol. Process. 26, 143–152 (2012).
Crawford, J., Hughes, C. E. & Parkes, S. D. Is the isotopic composition of event based precipitation driven by moisture source or synoptic scale weather in the Sydney Basin, Australia? J. Hydrol. 507, 213–226 (2013).
Berman, E. S., Levin, N. E., Landais, A., Li, S. & Owano, T. Measurement of δ18O, δ17O and 17O-excess in water by Off-Axis Integrated Cavity Output Spectroscopy and Isotope Ratio Mass Spectrometry. Anal. Chem. 85, 10392–10398 (2013).
Steig, E. et al. Calibrated high-precision 17O-excess measurements using cavity ring-down spectroscopy with laser-current-tuned cavity resonance. Atmos. Meas. Tech. 7, 2421–2435 (2014).
Coplen, T. B. et al. Isotope-abundance variations of selected elements. Pure and Applied Chemistry 74, 1987–2017 (2002).
Guan, H., Zhang, X., Skrzypek, G., Sun, Z. & Xu, X. Deuterium excess variations of rainfall events in a coastal area of South Australia and its relationship with synoptic weather systems and atmospheric moisture sources. J. Geophys. Res. 118, 1123–1138 (2013).
Jouzel, J. et al. Water isotopes as tools to document oceanic sources of precipitation. Water Resour. Res. 49, 7469–7486 (2013).
Affolter, S., Häuselmann, A. D., Fleitmann, D., Häuselmann, P. & Leuenberger, M. Triple isotope (δD, δ17O, δ18O) study on precipitation, drip water and speleothem fluid inclusions for a Western Central European cave (NW Switzerland). Quat. Sci. Rev. 127, 73–89 (2015).
Risi, C. et al. Understanding the 17O-excess glacial-interglacial variations in Vostok precipitation. J. Geophys. Res. 115, D10112 (2010).
Dansgaard, W. Stable isotopes in precipitation. Tellus 16, 436–468 (1964).
Barkan, E. & Luz, B. Diffusivity fractionations of H216O/H217O and H216O/H218O in air and their implications for isotope hydrology. Rapid Commun. Mass Spectrom. 21, 2999–3005 (2007).
Masson-Delmotte, V. et al. A review of Antarctic surface snow isotopic composition: observations, atmospheric circulation, and isotopic modeling. Journal of Climate 21, 3359–3387 (2008).
Landais, A., Ekaykin, A., Barkan, E., Winkler, R. & Luz, B. Seasonal variations of 17O-excess and d-excess in snow precipitation at Vostok station, East Antarctica. J. Glaciol 58, 725–733 (2012).
Li, S., Levin, N. E. & Chesson, L. A. Continental scale variation in 17O-excess of meteoric waters in the United States. Geochim. Cosmochim. Acta 164, 110–126 (2015).
Jacob, H. & Sonntag, C. An 8-year record of the seasonal variation of 2H and 18O in atmospheric water vapour and precipitation at Heidelberg, Germany. Tellus B 43, 291–300 (1991).
Barkan, E. & Luz, B. High precision measurements of 17O/16O and 18O/16O ratios in H2O. Rapid Commun. Mass Spectrom. 19, 3737–3742 (2005).
Winkler, R. et al. Deglaciation records of 17O-excess in East Antarctica: reliable reconstruction of oceanic normalized relative humidity from coastal sites. Clim. Past 8, 1–16 (2012).
Schoenemann, S. W., Steig, E. J., Ding, Q., Markle, B. R. & Schauer, A. J. Triple water-isotopologue record from WAIS Divide, Antarctica: Controls on glacial-interglacial changes in 17O-excess of precipitation. J. Geophys. Res. 119, 8741–8763 (2014).
Landais, A., Barkan, E. & Luz, B. Record of δ18O and 17O-excess in ice from Vostok Antarctica during the last 150,000 years. Geophys. Res. Lett. 35, L02709 (2008).
Landais, A. et al. Combined measurements of 17O-excess and d-excess in African monsoon precipitation: Implications for evaluating convective parameterizations. Earth Planet. Sci. Lett. 298, 104–112 (2010).
Kaseke, K. F. et al. Precipitation Origins and Key Drivers of Precipitation Isotope (18O, 2H, and 17O) Compositions Over Windhoek. J. Geophys. Res. 123, 7311–7330 (2018).
Kaseke, K. F., Wang, L. & Seely, M. K. Nonrainfall water origins and formation mechanisms. Science Advances 3, e1603131 (2017).
Tian, C., Wang, L., Kaseke, K. F. & Bird, B. W. Stable isotope compositions (δ2H, δ18O and δ17O) of rainfall and snowfall in the central United States. Sci. Rep. 8, 6712 (2018).
Schoenemann, S. W., Schauer, A. J. & Steig, E. J. Measurement of SLAP2 and GISP δ17O and proposed VSMOW-SLAP normalization for δ17O and 17O-excess. Rapid Commun. Mass Spectrom. 27, 582–590 (2013).
Tian, C., Wang, L. & Novick, K. A. Water vapor δ2H, δ18O and δ17O measurements using an off-axis integrated cavity output spectrometer-sensitivity to water vapor concentration, delta value and averaging-time. Rapid Commun. Mass Spectrom. 30, 2077–2086 (2016).
Andresen, J., Hilberg, S., Kunkel, K. & Center, M. R. C. Historical climate and climate trends in the Midwestern USA. US National Climate Assessment Midwest Technical Input Report (2012).
Bird, B. W., Wilson, J. J., Gilhooly, W. P. III, Steinman, B. A. & Stamps, L. Midcontinental Native American population dynamics and late Holocene hydroclimate extremes. Sci. Rep. 7, 41628 (2017).
Sjostrom, D. J. & Welker, J. M. The influence of air mass source on the seasonal isotopic composition of precipitation, eastern USA. J. Geochem. Explor. 102, 103–112 (2009).
Wang, L., Caylor, K. K. & Dragoni, D. On the calibration of continuous, high-precision δ18O and δ2H measurements using an off-axis integrated cavity output spectrometer. Rapid Commun. Mass Spectrom. 23, 530–536 (2009).
Lin, Y., Clayton, R. N. & Gröning, M. Calibration of δ17O and δ18O of international measurement standards-VSMOW, VSMOW2, SLAP, and SLAP2. Rapid Commun. Mass Spectrom. 24, 773–776 (2010).
Pang, H. et al. Spatial distribution of 17O-excess in surface snow along a traverse from Zhongshan station to Dome A, East Antarctica. Earth Planet. Sci. Lett. 414, 126–133 (2015).
Steen-Larsen, H. et al. What controls the isotopic composition of Greenland surface snow? Clim. Past 10, 377–392 (2014).
Luz, B. & Barkan, E. Variations of 17O/16O and 18O/16O in meteoric waters. Geochim. Cosmochim. Acta 74, 6276–6286 (2010).
IAEA: Reference Sheet for VSMOW2 and SLAP2 international measurement standards International Atomic Energy Agency (IAEA) (2009).
Data Citations
Tian, C., & Wang, L. PANGAEA https://doi.org/10.1594/PANGAEA.895300 (2018)
Acknowledgements
Funding for this work was made available from the Indiana University-Purdue University Indianapolis Research Support Funds Grant and U.S. National Science Foundation (IIA-1427642 and EAR-1554894).
Author information
Authors and Affiliations
Contributions
C.T. analyzed the samples and wrote the manuscript. L.W. conceived the idea, collected the samples and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
ISA-Tab metadata
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ The Creative Commons Public Domain Dedication waiver http://creativecommons.org/publicdomain/zero/1.0/ applies to the metadata files made available in this article.
About this article
Cite this article
Tian, C., Wang, L. Stable isotope variations of daily precipitation from 2014–2018 in the central United States. Sci Data 6, 190018 (2019). https://doi.org/10.1038/sdata.2019.18
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/sdata.2019.18
This article is cited by
-
Stable isotope variations of dew under three different climates
Scientific Data (2022)
-
Triple isotope variations of monthly tap water in China
Scientific Data (2020)