Abstract
Study design:
Controlled experimental human study.
Objectives:
To assess insulin resistance (IR) in tetraplegia and paraplegia, and the role of the spinal cord (SC) in glucose regulation.
Setting:
Laboratory of Spinal Research, Loewenstein Rehabilitation Hospital.
Methods:
Glucose and insulin levels and the heart rate variation spectral components LF (low frequency), HF (high frequency) and LF/HF were studied at supine rest, head-up tilt and after a standard meal in three groups: 13 healthy subjects, 7 patients with T4–T6 paraplegia and 11 patients with C4–C7 tetraplegia.
Results:
Glucose and insulin increased significantly after the meal in all groups (P<0.001). Glucose increased significantly more in the tetraplegia than in the other groups (P<0.01). Increases in insulin level tended to accompany increases in LF/HF after the meal in the tetraplegia and control groups but not in the paraplegia group.
Conclusion:
Post-prandial IR appears in C4–C7 but not in T4–T6 SC injury. The results of the study, combined with previously published findings, are consistent with the hypotheses that IR is related to activation of the sympathetic nervous system, and that below T4 the mid-thoracic SC is involved in the regulation of glucose and insulin levels.
Similar content being viewed by others
Introduction
Insulin resistance (IR) has been described in patients with spinal cord injury (SCI) and related to the apparently increasing risks of diabetes mellitus (DM) and cardiovascular disease in these patients, which may affect their survival.1, 2 The mechanism of IR after SCI is unknown, however, requiring investigation of the relationship between the spinal cord (SC) and insulin activities.
IR is a state in which a given concentration of insulin produces a less-than-expected biological effect. In patients, in this state, both insulin and glucose levels can increase more than in healthy people. IR is commonly associated with obesity, non-insulin-dependent DM and essential hypertension. The IR syndrome (also called metabolic syndrome) includes impaired insulin-stimulated glucose uptake, hyperinsulinemia, glucose intolerance, hypertension and dyslipidemia. Although IR could apply to any of the insulin effects, the term usually applies to the effect on tissue glucose uptake.3 The predominant peripheral tissue responsible for insulin-induced glucose uptake is muscle, and IR frequently manifests as impaired glucose uptake in muscle or fat tissue.1, 4
The primary factors responsible for the development of IR are unknown, but a variety of etiologies have been suggested, including a primary defect of the insulin molecule, insulin antibody formation, a decrease in the number of insulin receptors in the insulin target cells, a decrease in the receptor affinity for insulin, altered signal transduction at the level of the receptor, changes in cell membrane properties and post-receptor cytoplasmic defects that decrease glucose transport and glycogen synthesis. The cellular defects may be a primary manifestation of diseases such as type 2 DM, or may be secondary to diseases or conditions such as obesity.3, 4
Changes in autonomic nervous system (ANS) activity were also found to affect IR. Such changes can explain IR in patients with SCI, but the nature of ANS contribution to IR after SCI is not clear. Overall sympathetic nervous system (SNS) activity, which is the main SC ANS activity, is reduced after SCI, but most studies indicate that SNS activation did not reduce, but rather induced or exacerbated IR.5 There are studies, however, indicating that increased SNS activity is not essential for IR, and that insulin affects SNS activity.6
Some studies related IR to blood flow reduction following SNS activation and vasoconstriction, because reduction of blood flow can reduce tissue glucose uptake.5 It has also been proposed that increased sympathetic activity may promote IR by activating α1 adrenergic receptors in adipose tissue.7
Changes in the parasympathetic nervous system were also related to IR after SCI. Several animal studies suggested that normally vagal activation induces insulin sensitivity (IS). The suggested vagally-mediated IS induction was found to involve hypothalamic activation, afferent or efferent hepatic vagal branch activity8 and stimulation of hepatic receptors.6 Inhibition of vagal activity in response to reduction in sympathetic activity may be the cause of IR after SCI. But other studies showed that vagal stimulation may not induce IS, and that afferent hepatic vagal branch activation may contribute to IR.9
Additional studies suggested that SCI might affect the response to insulin because of alterations in body composition. The combination of higher fat content and reduced skeletal muscle mass, which are frequent in patients with chronic SCI, may result in pronounced competition between free fatty acids and glucose oxidation, leading to IR. In paralyzed regions, replacement of insulin-sensitive type IIA fibers with less insulin-sensitive type IIB fibers may also contribute to IR.2
Some studies indicate that IR in SCI may be affected by the level and severity of the injury. IR in SCI patients appeared more likely to occur in higher and complete injuries than in lower and incomplete lesions.1 Normal glucose metabolism was found in patients with T3–T4 paraplegia when controlling for heritage by comparison with siblings.10 There is no clear description, however, of IR investigation, in a group of patients with thoracic SCI above T3 or below T4.
In the present experiments, which were part of a larger study,11, 12, 13, 14 we compared glucose and insulin responses to a standard meal and head-up tilt (HUT) in a group of patients with mid-thoracic, below T4, SCI, with responses of patients with cervical SCI and of healthy subjects. We also compared the insulin response to meal with post-prandial changes in low frequency (LF)/high frequency (HF) ratio, the LF/HF heart rate (HR) variations power values. These are obtained by spectral analysis that shows the sinusoidal components of the ultradian HR fluctuations and computes the power (or amplitude square) of each component. Plotting the power of HR fluctuations against their frequency displays the typical peaks around 0.15 Hz (LF) and 0.4 Hz (HF). The HF fluctuations are vagally mediated, the LF fluctuations are mediated by both sympathetic and parasympathetic tone, and the LF/HF ratio represents the sympato-vagal balance.15 These comparisons were performed to infer the role of the mid-thoracic SC and of the ANS in inducing IR.
Subjects and methods
Subjects
A total of 13 healthy control subjects and 18 patients with SCI of 3 months to 31 years duration were included in the present experiments. Seven patients had T4–T6 paraplegia and 11 had C4–C7 tetraplegia. The control subjects were nine men and four women of age 34±13 years old. The patients with paraplegia were six men and one woman, 33±10 years old, with an American Spinal Injury Association impairment scale (AIS) grade16 of A. The patients with tetraplegia were 11 men, 42±8 years old, eight with AIS grade A and three with AIS grade B. Age differences between the groups were nonsignificant (P>0.05). Subjects did not present any medical conditions that may affect the results, such as febrile disease, heart failure, renal failure, DM, obesity or an additional neurological impairment.
Procedure
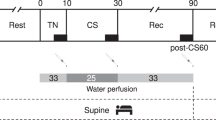
The ethics committee of Loewenstein Rehabilitation Hospital approved the study, and all participants signed an informed consent. In the morning, after a 12-h fast, each subject lay supine on an electric tilt-bed, in a relatively quiet hospital environment with an ambient temperature of about 22 °C. Subjects were tied to the bed by wide bands around the chest, pelvis and knees, avoiding pressure to the abdomen.
A 30-min period of supine rest was followed by 10 min of 35° tilting (HUT 1) and 15 min of supine rest, after which, at minute 55 of the experiment, subjects were given a standard meal (Table 1). At 45 min after the start of the meal, the bed was tilted again to 35° for 10 min (HUT 2) and then returned to the horizontal position.
Blood samples for glucose and insulin were drawn from the intravenous catheter at minutes 25, 35, 70, 85, 95, 105, 115 and 145 of the experiment (before the meal, 15, 30, 40, 60 and 90 min after the meal and 5 min after starting each HUT).
Blood was drawn slowly and cautiously to prevent hemolysis. The first 0.5 ml of each drawing was eliminated to remove the heparin that rinsed the intravenous catheter. After inserting the blood samples into appropriate tubes, the catheter was re-rinsed with diluted heparin. The tubes with the blood samples were stored in crushed ice within a cooled thermally insulated case during the experiment, and then delivered to the laboratory. Within 90 min after the experiment the samples underwent cooled centrifugation. The separated plasma was analyzed immediately, or frozen and analyzed later.
Glucose plasma levels were determined by an automatic hospital analyzer that uses the glucose oxidase method. Insulin level was determined by radioimmunoassays, which are based on substance-specific antibodies and labeled substance that competes for antibody sites.
Continuous ECG traces were obtained for HR recording using surface electrodes and a preamplifier A/D system (BIOPAC Systems, Santa Barbara, CA, USA). ECG signals were sampled online at 500 Hz. The digitized signal was converted offline into a continuous beat-to-beat HR signal and re-sampled offline at 10 Hz. A spectral analysis of HRV was performed after applying a median filter with a 251-sample length. A discrete Fourier transform was used in combination with the Welch Periodogram method to compute the power (amplitude squared) of the sampled HR signal fluctuations.17 The integrals of the power values between 0 and 0.17 Hz (LF) and between 0.17 and 0.5 Hz (HF) were calculated to obtain the LF and HF components of the power spectrum. The ratios of HRV power values (LF/HF) were calculated for the following time intervals: 5 min before HUT 1 and the meal, 15 min from the start of the meal, 15–30 min from the start of the meal, 30–45 min from the start of the meal, 10 min during HUT 2 and 85–90 min from the start of the meal.
Statistical analysis
The effects of supine rest, meal and HUT, within and between groups (paraplegia, tetraplegia and healthy subjects), and the effects of the groups themselves on the values of glucose and insulin were examined by analysis of variance with repeated measurements. Post hoc comparisons between groups and conditions were performed to determine the specific influence of SCI on glucose and insulin levels. Data were analyzed using SPSS for Windows (SPSS Inc., Chicago, IL, USA).
Results
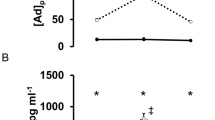
There were no significant differences in premeal glucose and insulin levels (Figures 1 and 2) between the subject groups (P>0.05).
Glucose levels (mean+s.d.; mg per 100 ml) at various time points after the onset of the experiment. The liquid meal ingestion started at minute 55, head-up tilt (HUT) at minutes 30 and 100. Before the meal, glucose levels were similar in all groups. After the meal, glucose level increased in all patients, in patients with tetraplegia significantly more than in other subjects, despite a concomitant increase in insulin level (Figure 2). Tilting did not affect glucose levels.
Insulin levels (mean+s.d.; μU ml−1) at various time points after the onset of the experiment. The liquid meal ingestion started at minute 55, tilting at minutes 30 and 100. Before the meal insulin levels were similar in all groups. After the meal, insulin increased in all groups, but mean insulin levels increased in the tetraplegia group more than in other groups. Tilting (HUT) did not affect insulin level.
After the meal, glucose increased significantly (P<0.001) in the patients with tetraplegia, and more than in other subjects (P<0.01) (Figure 1). Insulin showed a significant increase after meal (P<0.001), irrespective of group (P>0.37). The mean post-prandial insulin level, however, was always higher in tetraplegia than in the other groups, and the difference in mean insulin levels between tetraplegia and paraplegia increased almost steadily after the meal (Figure 2).
Increases in insulin levels tended to accompany increases in LF/HF after the meal in the tetraplegia and control groups but not in the paraplegia group (Figure 3). A significant correlation, however, was not found between the changes in insulin levels and LF/HF, 15-90 min after starting the meal. HUT effect on glucose and insulin levels (Figures 1 and 2) was not found to be significant, irrespective of group (P>0.05).
The relationship of the increase in insulin level (d insulin) with the increase in LF/HF (d LF/HF) 40 min after starting the liquid meal, in control subjects (C, n=12, r=0.4), in patients with tetraplegia (T, n=11, r=0.56) and paraplegia (P, n=7, r=0.02). Increases in insulin levels tended to accompany increases in LF/HF after meal in the tetraplegia and control groups, although the changes in insulin levels and LF/HF were not significantly correlated in any group.
Discussion
In the patients with tetraplegia but not with mid-thoracic paraplegia, glucose levels increased after the meal significantly more than they did in healthy control subjects, eliciting a higher increase in mean insulin levels. Peak post-prandial levels in tetraplegia reached values of 173 mg per 100 ml for glucose and 135 μU ml−1 for insulin. This indicates post-prandial IR (at least relative to control subjects) in patients with tetraplegia but not in patients with mid-thoracic paraplegia. The IR evident in tetraplegia may result from damage to cervical SC neurons that transmit IS inducing signals, or from other contributing factors, such as the combination of higher fat content and reduced skeletal muscle mass after cervical SCI.2 We suggest, however, that it may result from damage to cervical SC neurons that suppress signals generated in the thoracic SC, which have the capability of reducing IS. Damage that eliminates such signals could be the reason for the lack of IR in patients with mid-thoracic paraplegia in the present experiment. IR induction as a result of such damage indicates a role for the mid-thoracic SC in glucose regulation.
Results also show an apparent relationship between LF/HF values and post-prandial insulin elevation in patients with cervical SCI and in healthy subjects. The apparent relationship between LF/HF and insulin level may indicate a relationship between ANS activity (responsible for the LF/HF changes) and IR after SCI. A relationship of this type supports a role for the thoracic SC in glucose regulation because only thoracic SC neurons could transmit the ANS signals (as described in the same patients with tetraplegia in our previous publication),11 increasing LF and LF/HF following food ingestion trough SNS activation. The source of these signals must be below the cervical SC, and probably in the thoracic SC, because the SNS pathways from the brain to the heart are damaged in tetraplegia and the brain stem cannot transmit the SNS signals that affect HRV.
Glucose and insulin levels were within the normal range in all study groups at premeal supine-rest. Results indicating IR were evident in SCI only after food ingestion, consistent with the earlier findings.1 This implies that the IR is relatively mild in patients with SCI, reflecting the fact that most of them do not suffer from overt DM.1 Nevertheless, IR in SCI patients is dangerous: isolated post-challenge hyperglycemia may represent an increased risk of DM, cardiovascular disease, and mortality.18
The main limitations of this study are the relatively small number of examined subjects, the multiplicity of tests and analyses and the clinical but not anatomical classification of SCI completeness.
The relatively small number of participants resulted only in a tentative impression for the relationship of IR with ANS activity. Future studies with larger groups, which will also compare mid-thoracic with upper-thoracic SCI, may confirm or refute these findings and the hypothetic involvement of the mid-thoracic SC in IR regulation.
Multiple statistical analyses were required to avoid losing important information collected for additional tests performed on the same subjects, as part of the larger study. The additional tests, detailed elsewhere,11, 12, 13, 14 include the HUT examinations performed before and after the meal. The HUT could have confounded the effect of the liquid meal, but it was not found to influence glucose or insulin levels, before or after the meal, irrespective of group, consistent with former observations in healthy men.19 Because the multiplicity of analyses increased the chance of incidental findings, only P-values below 0.01 were considered significant for the main inferences.
We used AIS grading for the classification of injury completeness, and therefore the exact anatomical extent and boundaries of the injuries are not well defined, although all of the injuries were complete or almost complete according to the clinical definitions. For this reason we cannot completely exclude the sparing of autonomic nerve fibers at the lesion sites, which could affect the findings. But this is an inherent drawback in most human SC studies, and the probability of its interference with the findings in groups with almost-complete lesions is small.
Furthermore, additional factors may also contribute to the lower IR probability in paraplegia than in tetraplegia. Among them, the smaller muscle mass reduction, as suggested in an earlier publication,1 the younger mean age that was found to be associated with lower peak serum glucose concentration after SCI,1 the higher activity level that was found to be related to glucose tolerance after SCI20 and a possible lower level of stress. These imply that various mechanisms can be attributed to IR in SCI, and the mechanism suggested in this paper is only one of the hypotheses deserving further investigation. There is no data, however, contradicting the suggested contribution of mid-thoracic sympathetic damage, which is supported by our findings, and is in line with previous publications.
Conclusions
Results suggesting post-prandial IR were found in C4–C7 but not in T4−T6 SCI. Increases in insulin levels after meal are apparently related to concomitant changes in sympatho-vagal balance. Combined with previously published findings, these changes support the hypotheses that IR is related to activation of the SNS, and that a portion of the SC below T4 is involved in the regulation of glucose and insulin levels.
References
Bauman WA, Adkins RH, Spungen AM, Waters RL . The effect of residual neurological deficit on oral glucose tolerance in persons with chronic spinal cord injury. Spinal Cord 1999; 37: 765–771.
Karlsson AK . Insulin resistance and sympathetic function in high spinal cord injury. Spinal Cord 1999; 37: 494–500.
Hunter SJ, Garvey WT . Insulin action and insulin resistance: diseases involving defects in insulin receptors, signal transduction, and the glucose transport effector system. Am J Med 1998; 105: 331–345.
Sell H, Eckel J, Dietz-Schroeder D . Pathways leading to muscle insulin resistance: the muscle–fat connection. Arch Physiol Biochem 2006; 112: 105–113.
Jamerson KA, Smith SD, Amerena JV, Grant E, Julius S . Vasoconstriction with norepinephrine causes less forearm insulin resistance than a reflex sympathetic vasoconstriction. Hypertension 1994; 23: 1006–1011.
Landsberg L . Role of the sympathetic adrenal system in the pathogenesis of the insulin resistance syndrome. Ann NY Acad Sci 1999; 892: 84–90.
McCarty MF . Elevated sympathetic activity may promote insulin resistance syndrome by activating alpha-1 adrenergic receptors on adipocytes. Med Hypotheses 2004; 62: 830–838.
Uno K, Katagiri H, Yamada T, Ishigaki Y, Ogihara T, Imai J et al. Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science 2006; 312: 1656–1659.
Bernal-Mizrachi C, Xiaozhong L, Yin L, Knutsen RH, Howard MJ, Arends JJA et al. An afferent vagal nerve pathway links hepatic PPARα activation to glucocorticoid-induced insulin resistance and hypertension. Cell Metab 2007; 5: 91–102.
Karlsson AK, Attvall S, Jansson PA, Sullivan L, Lonnroth P . Influence of the sympathetic nervous system on insulin sensitivity and adipose tissue metabolism: a study in spinal cord-injured subjects. Metabolism 1995; 44: 52–58.
Catz A, Bluvshtein V, Pinhas I, Akselrod S, Gelernter I, Nissel T et al. Hemodynamic effects of liquid food ingestion in mid-thoracic paraplegia: is postprandial hypotension related to thoracic spinal cord damage? Spinal Cord 2007; 45: 96–103.
Catz A, Bluvshtein V, Pinhas I, Akselrod S, Gelernter I, Nissel T et al. Cold pressor test in tetraplegia and paraplegia suggests an independent role of the thoracic spinal cord in the hemodynamic responses to cold. Spinal Cord 2008; 46: 33–38.
Catz A, Bluvshtein V, Korczyn AD, Pinhas I, Gelernter I, Nissel T et al. Modified cold pressor test by cold application to the foot after spinal cord injury suggests hemodynamic control by the spinal cord. Am J Phys Med Rehab 2007; 86: 875–882.
Bluvshtein V, Akselrod S, Korczyn AD, Pinhas I, Gelernter I, Catz A . Hemodynamic responses to head-up tilt after spinal cord injury support a role for the thoracic spinal cord in cardiovascular regulation. Spinal Cord 2010 (e-pub ahead of print).
Akselrod S . Spectral analysis of fluctuations in heart rate and other cardiovascular parameters as a tool for assessment of autonomic control. In: Korczyn AD (ed). Handbook of Autonomic Nervous System Dysfunction. Marcel Decker: New York, 1995, pp 469–493.
Maynard Jr FM, Bracken MB, Creasey G, Ditunno Jr JF, Donovan WH, Ducker TB et al. International standards for neurological and functional classification of spinal cord injury. Spinal Cord 1997; 35: 266–274.
Keselbrener L, Akselrod S . Selective discrete Fourier transform algorithm for time-frequency analysis: method and application on simulated and cardiovascular signals. IEEE Trans Biomedical Eng 1996; 43: 789–802.
Suzuki H, Fukushima M, Usami M, Ikeda M, Taniguchi A, Nakai Y et al. Factors responsible for development from normal glucose tolerance to isolated postchallenge hyperglycemia. Diabetes Care 2003; 26: 1211–1215.
Laszlo Z, Rosler A, Hinhhofer-Szalkay HG . Cardiovascular and hormonal changes with different angles of head-up tilt in man. Physiol Res 2001; 50: 71–82.
Raymond J, Harmer AR, Temesi J, van Kemenade C . Glucose tolerance and physical activity level in people with spinal cord injury. Spinal Cord 2010; 48: 591–597.
Acknowledgements
The study was supported by the Unit of Medical Services, Rehabilitation Department, Ministry of Defense and by the Tel Aviv University Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Bluvshtein, V., Korczyn, A., Pinhas, I. et al. Insulin resistance in tetraplegia but not in mid-thoracic paraplegia: is the mid-thoracic spinal cord involved in glucose regulation?. Spinal Cord 49, 648–652 (2011). https://doi.org/10.1038/sc.2010.152
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2010.152
Keywords
This article is cited by
-
The effect of level of injury on diabetes incidence and mortality after spinal cord injury – a longitudinal cohort study
Spinal Cord (2024)
-
Serum concentrations of ketones increase after hand-ergometer exercise in persons with cervical spinal cord injuries: a preliminary prospective study
Spinal Cord (2023)
-
Contributors to Metabolic Disease Risk Following Spinal Cord Injury
Current Physical Medicine and Rehabilitation Reports (2016)