Abstract
Heart failure (HF) is a leading cause of morbidity and mortality. Studies in animal models and individuals with HF revealed a prominent role for CD4+ T cell immune responses in the pathogenesis of HF and highlighted an active cross-talk between cardiac fibroblasts and interferon (IFN)-γ-producing CD4+ T cells that results in profibrotic myofibroblast transformation. Whether cardiac fibroblasts concomitantly modulate pathogenic cardiac CD4+ T cell immune responses is unknown. Here we report that mouse cardiac fibroblasts express major histocompatibility complex type II (MHCII) in two different experimental models of cardiac inflammation. We demonstrate that cardiac fibroblasts take up and process antigens for presentation to CD4+ T cells via MHCII induced by IFN-γ. Conditional deletion of MhcII in cardiac fibroblasts ameliorates cardiac remodeling and dysfunction induced by cardiac pressure overload. Collectively, we demonstrate that cardiac fibroblasts function as antigen-presenting cells and contribute to cardiac fibrosis and dysfunction through MHCII induced by IFN-γ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data used to generate figures are included in the accompanying files.
Change history
09 November 2022
A Correction to this paper has been published: https://doi.org/10.1038/s44161-022-00179-6
References
Pinto, A. R. et al. Revisiting cardiac cellular composition. Circ. Res. 118, 400–409 (2016).
Prabhu, S. D. & Frangogiannis, N. G. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ. Res. 119, 91–112 (2016).
Kaur, H. et al. Targeted ablation of periostin-expressing activated fibroblasts prevents adverse cardiac remodeling in mice. Circ. Res. 118, 1906–1917 (2016).
Aghajanian, H. et al. Targeting cardiac fibrosis with engineered T cells. Nature 573, 430–433 (2019).
Nevers, T. et al. Left ventricular T cell recruitment contributes to the pathogenesis of heart failure. Circ. Heart. Fail. 8, 776–787 (2015).
Nevers, T. et al. TH1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J. Exp. Med. 214, 3311–3329 (2017).
Laroumanie, F. et al. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation 129, 2111–2124 (2014).
Epelman, S., Liu, P. P. & Mann, D. L. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat. Rev. Immunol. 15, 117–129 (2015).
Kambayashi, T. & Laufer, T. M. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell. Nat. Rev. Immunol. 14, 719–730 (2014).
Umetsu, D. T., Katzen, D., Jabara, H. H. & Geha, R. S. Antigen presentation by human dermal fibroblasts: activation of resting T lymphocytes. J. Immunol. 136, 440–445 (1986).
Kanisicak, O. et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 7, 12260 (2016).
Burgdorf, S., Kautz, A., Bohnert, V., Knolle, P. A. & Kurts, C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 316, 612–616 (2007).
Ramachandra, L., Noss, E., Boom, W. H. & Harding, C. V. Phagocytic processing of antigens for presentation by class II major histocompatibility complex molecules. Cell Microbiol. 1, 205–214 (1999).
Ngwenyama, N. et al. CXCR3 regulates CD4+ T cell cardiotropism in pressure overload-induced cardiac dysfunction. JCI Insight https://doi.org/10.1172/jci.insight.125527 (2019).
Michailowsky, V. et al. Intercellular adhesion molecule 1 deficiency leads to impaired recruitment of T lymphocytes and enhanced host susceptibility to infection with Trypanosoma cruzi. J. Immunol. 173, 463–470 (2004).
Cardillo, F., Voltarelli, J. C., Reed, S. G. & Silva, J. S. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect. Immun. 64, 128–134 (1996).
Haverson, K., Singha, S., Stokes, C. R. & Bailey, M. Professional and nonprofessional antigen-presenting cells in the porcine small intestine. Immunology 101, 492–500 (2000).
Geppert, T. D. & Lipsky, P. E. Antigen presentation by interferon-gamma-treated endothelial cells and fibroblasts: differential ability to function as antigen-presenting cells despite comparable Ia expression. J. Immunol. 135, 3750–3762 (1985).
Spear, T. T., Evavold, B. D., Baker, B. M. & Nishimura, M. I. Understanding TCR affinity, antigen specificity, and cross-reactivity to improve TCR gene-modified T cells for cancer immunotherapy. Cancer Immunol. Immunother. 68, 1881–1889 (2019).
Ngwenyama, N. et al. Isolevuglandin-modified cardiac proteins drive CD4+ T cell activation in the heart and promote cardiac dysfunction. Circulation https://doi.org/10.1161/CIRCULATIONAHA.120.051889 (2021).
Rieckmann, M. et al. Myocardial infarction triggers cardioprotective antigen-specific T helper cell responses. J. Clin. Invest. 129, 4922–4936 (2019).
Tallquist, M. D. & Molkentin, J. D. Redefining the identity of cardiac fibroblasts. Nat. Rev. Cardiol. 14, 484–491 (2017).
Kong, P., Christia, P. & Frangogiannis, N. G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 71, 549–574 (2014).
Fields, P. E. et al. B7.1 is a quantitatively stronger costimulus than B7.2 in the activation of naive CD8+ TCR-transgenic T cells. J. Immunol. 161, 5268–5275 (1998).
Pechhold, K. et al. Inflammatory cytokines IFN-gamma plus TNF-alpha induce regulated expression of CD80 (B7-1) but not CD86 (B7-2) on murine fibroblasts. J. Immunol. 158, 4921–4929 (1997).
Smythe, J. A. et al. Human fibroblasts transduced with CD80 or CD86 efficiently trans-costimulate CD4+ and CD8+ T lymphocytes in HLA-restricted reactions: implications for immune augmentation cancer therapy and autoimmunity. J. Immunol. 163, 3239–3249 (1999).
Sansom, D. M. CD28, CTLA-4 and their ligands: who does what and to whom? Immunology 101, 169–177 (2000).
Hulsmans, M. et al. Cardiac macrophages promote diastolic dysfunction. J. Exp. Med. 215, 423–440 (2018).
Seeberg, J. C. et al. Nonprofessional phagocytosis: a general feature of normal tissue cells. Sci Rep. 9, 11875 (2019).
Nakaya, M. et al. Cardiac myofibroblast engulfment of dead cells facilitates recovery after myocardial infarction. J. Clin. Invest. 127, 383–401 (2017).
Salvador, A. M. et al. Intercellular adhesion molecule 1 regulates left ventricular leukocyte infiltration, cardiac remodeling, and function in pressure overload-induced heart failure. J. Am. Heart Assoc. 5, e003126 (2016).
Wang, H. et al. Role of bone marrow-derived CD11c+ dendritic cells in systolic overload-induced left ventricular inflammation, fibrosis and hypertrophy. Basic Res. Cardiol. 112, 25 (2017).
Virani, S. S. et al. Heart Disease and Stroke Statistics—2020 update: a report from the American Heart Association. Circulation 141, e139–e596 (2020).
Ren, Z. et al. Single-cell reconstruction of progression trajectory reveals intervention principles in pathological cardiac hypertrophy. Circulation 141, 1704–1719 (2020).
Wang, L. et al. Single-cell reconstruction of the adult human heart during heart failure and recovery reveals the cellular landscape underlying cardiac function. Nat. Cell Biol. 22, 108–119 (2020).
Dubrot, J. et al. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4+ T cell tolerance. J. Exp. Med. 211, 1153–1166 (2014).
Perez-Shibayama, C. et al. Type I interferon signaling in fibroblastic reticular cells prevents exhaustive activation of antiviral CD8+ T cells. Sci. Immunol. https://doi.org/10.1126/sciimmunol.abb7066 (2020).
Richards, D. A. et al. Distinct phenotypes induced by three degrees of transverse aortic constriction in mice. Sci Rep. 9, 5844 (2019).
Acknowledgements
These studies were supported by NIH grants R01 HL123658 and R01 HL144477 (to P.A.), NIH T32 HL 69770, NIH T32AI007077-34, NIH F31HL140883 and the American Heart Association grant 18PRE34020084 (to N.N.), NIH R01 HL141187 and HL142624 (to J.D.) and NIH T32AG066574 (to D.B.).
Author information
Authors and Affiliations
Contributions
N.N. designed and conducted experiments, analyzed data and wrote the manuscript. K.K., R.B., S.P. and B.T. planned and conducted experiments. C.G. and M.A.P. contributed to experiment design and intellectually contributed to data analysis. M.A. performed the TAC surgeries and contributed to experiment design. J.D. and D.B. performed TAC and immunofluorescence in Tcf21iCre/+; R26eGFP mice, sorted GFP+ cardiac fibroblasts and intellectually contributed to the manuscript. P.A. overviewed the design and interpretation of all studies and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
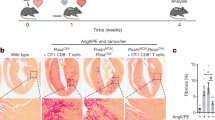
Extended Data Fig. 1 Cardiac fibroblasts express MHCII in vivo in response to acute T. cruzi infection.
(A) Wt mice were inoculated with 20,000 T. cruzi parasites by intraperitoneal injection and the hearts were harvested at 19 days post-infection. (B-E) Ventricular CD31-CD45-MEFSK4+ cardiac fibroblasts were analysed by flow cytometry (≥ 5,000 target cells acquired) to determine surface expression of (B-C) MHCII and (D-E) CD80. n= 4 (mock) and n=3 (Chagas) mice. Error bars represent mean ± SD. (* p≤0.05; *** p<0.001; Mann-Whitney test, two tailed).
Extended Data Fig. 2 MhcII recombination in cardiac fibroblasts occurred in a cell-specific manner.
Detection of Intact allele (310bp), recombined alleles (475 bp) and GAPDH(156 bp) in sorted CD31-CD45-MEFSK4+ Fibroblasts and leukocytes from Tcf21iCre/+MhcIIfl/fl mice treated with vehicle or Tamoxifen. GAPDH was used as loading control.
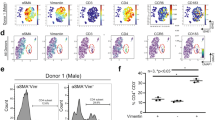
Extended Data Fig. 3 Tamoxifen treatment of Tcf21iCre/+/ MhcIIflox/flox mice reduces MHCII surface protein expression specifically in cardiac fibroblasts.
Transverse aortic constriction (TAC) surgery was performed on vehicle and Tamoxifen treated Tcf21iCre/+MhcIIfl/fl mice and the left ventricle was harvested after 4 weeks, digested, stained and analysed by flow cytometry. (A) Representative FACS plot showing gating of CD31+CD45-MESFK4- endothelial cells, CD31-CD45+MESFK4- leukocytes and CD31-CD45-MESFK4+ cardiac fibroblasts. (B-C) Representative FACS plots (B) and quantification of mean fluorescence intensity (MFI) (C) of MHC-II expression in endothelial cells (top), leukocytes (middle) and cardiac fibroblasts (bottom) in the hearts of vehicle and tamoxifen treated mice. n=4 mice/group. Error bars represent mean ± SE. (* p<0.05; Mann-Whitney test, two tailed. C: p value=0.0286).
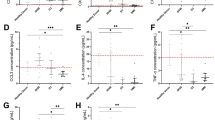
Extended Data Fig. 4 Cardiac fibroblast MHCII expression does not affect CD4+ T cell activation in the mLNs in response to TAC.
Transverse aortic constriction (TAC) surgery was performed on vehicle and Tamoxifen treated Tcf21iCre/+MhcIIfl/fl mice and the mediastinal lymph nodes were harvested after 4 weeks, stained and analysed by flow cytometry for the indicated T cell activation markers. (A) Representative FACS plot showing CD62L and CD44 staining. (B-C) quantification of CD62LlowCD44hi effector T CD4+ T cell numbers (B) and frequency (C) in vehicle and tamoxifen treated TAC mice. n=5 mice (vehicle) and n=6 mice (tamoxifen). Error bars represent mean ± SD. (Mann-Whitney test, two tailed. C: p value=0.0286).
Extended Data Fig. 5 Cardiac fibroblast MHCII expression contributes to cardiomyocyte hypertrophy and to cardiac fibroblast abundance in response to pressure overload.
Transverse aortic constriction (TAC) was performed on vehicle and Tamoxifen treated Tcf21iCre/+MhcIIfl/fl mice and harvested after 4 weeks. Conditional deletion of MhcII on cardiac fibroblasts was induced by administering Tamoxifen via intraperitoneal injections prior to TAC surgery, followed by Tamoxifen citrate chow for 4 weeks. (A) Wheat germ agglutinin (WGA) staining of frozen LV tissue sections was performed and used to calculate (B) mean cardiomyocyte area. Representative images of n=3 hearts (Sham), n=4 (TAC vehicle) and n=7 (TAC TMX). (C) Gross LV mass was acquired and is shown normalized to tibia length. (D-E) LV tissue was digested, stained for CD31 and MESFK4 and analysed by flow cytometry. Representative FACS plot (D) and quantification (E) of vehicle and tamoxifen treated mice hearts (n=4 mice/group). Error bars represent mean ± SE. (* p<0.01; Mann-Whitney test, two tailed: E: p=0286).
Extended Data Fig. 6 Schematic Diagram showing bidirectionality of cardiac fibroblast and Th1 cell interactions.
Cardiac fibroblasts express the costimulatory molecule CD80 and efficiently capture and process extracellular antigens into small peptides that are uploaded into MHC-II molecules. In response to IFNγ stimulation, cardiac fibroblasts express MHCII and present peptide antigens that induce IFNγ + Th1 cell immune responses and promote cardiac remodelling and dysfunction.
Supplementary information
Source data
Source Data Fig. 1
Statistics and data for graphs in Fig. 1.
Source Data Fig. 2
Statistics and data for graphs in Fig. 2.
Source Data Fig. 3
Statistics and data for graphs in Fig. 3.
Source Data Fig. 4
Statistics and data for graphs in Fig. 4.
Source Data Fig. 5
Statistics and data for graphs in Fig. 5.
Source Data Fig. 6
Statistics and data for graphs in Fig. 6.
Source Data Fig. 7
Statistics and data for graphs in Fig. 7.
Source Data Extended Data Fig. 1
Statistics and data for graphs in Extended data Fig. 1.
Source Data Extended Data Fig. 3
Statistics and data for graphs in Extended data Fig. 3.
Source Data Extended Data Fig. 4
Statistics and data for graphs in Extended data Fig. 4.
Source Data Extended Data Fig. 5
Statistics and data for graphs in Extended data Fig. 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ngwenyama, N., Kaur, K., Bugg, D. et al. Antigen presentation by cardiac fibroblasts promotes cardiac dysfunction. Nat Cardiovasc Res 1, 761–774 (2022). https://doi.org/10.1038/s44161-022-00116-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-022-00116-7
This article is cited by
-
Immunomodulation and immunopharmacology in heart failure
Nature Reviews Cardiology (2024)
-
Immune and inflammatory mechanisms in hypertension
Nature Reviews Cardiology (2024)
-
AMPK signaling inhibits the differentiation of myofibroblasts: impact on age-related tissue fibrosis and degeneration
Biogerontology (2024)
-
Bone morphogenic protein-4 availability in the cardiac microenvironment controls inflammation and fibrosis in autoimmune myocarditis
Nature Cardiovascular Research (2024)
-
Immunology of human fibrosis
Nature Immunology (2023)