Abstract
The purpose of this Perspective is to clarify for an interdisciplinary audience the fundamental concepts of human longevity and provide evidence for a limit to human lifespan. This observed limit is placed into a broader framework by showing how it has arisen through the process of evolution and by enumerating the molecular mechanisms that may enforce it. Finally, we look toward potential future developments and the prospects for possibly circumventing the current limit.
Similar content being viewed by others
Main
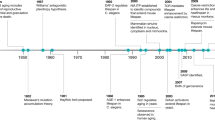
The duration of life has long been a topic of fascination. Ancient texts reflect this fascination in three ways. First, by contrasting humans’ limited lifespan with the permanence of immortal god(s). Second, by compiling lists of exceptionally long-lived individuals. Finally, by giving reasons for human mortality, generally explaining it as a process created by divine intervention. For example, in the mythology of Mesopotamia, death is created to relieve overpopulation1, while in the Bible it is God who declares that humans shall live no longer than 120 years2—although several figures, including Noah, were alleged to have lived longer.
In modern times, aging is still considered by some as an intentional process, albeit one driven not by supernatural agents but by the natural process of evolution3,4. This possibility was originally considered by August Weismann at the end of the 19th century. Aging was programmed, his reasoning went, so that older individuals could make room for fitter, younger ones5. However, this mechanism for aging was criticized as circular reasoning, on the basis that, if individuals did not decline in fitness as they got older, their removal would not be necessary. Indeed, Weismann himself seemed to have realized the flaws of considering aging as an adaptive process and moved to nonadaptive theories6. The key problem with theories of programmed aging is the need to identify an evolutionary advantage of such an evolved biological mechanism. While there is abundant evidence for an evolutionary advantage of adaptive processes promoting aging over the lifetime, most notably inflammation as a by-product of the immune response7, no basis for an evolutionary advantage of aging as a purposeful process has thus far been identified and the idea of programmed aging has essentially been abandoned. Instead, aging is now seen as a result of benign evolutionary neglect8,9.
The overwhelming majority of researchers on aging now prefer to see the process as multifactorial and consider treatments as merely extending healthspan rather than lifespan. Still, this is often not separated from an increase in lifespan10 and the idea of a limit to human lifespan is not generally accepted. Indeed, progress in geroscience on top of the dramatic improvements of the human condition since the 19th century is often interpreted as a new playing field in which each generation will gradually reach longer lifespans. New breakthrough interventions have been predicted to greatly accelerate this process and lead to new heights of longevity11.
The purpose of this Perspective is to clarify for an interdisciplinary audience the fundamental concepts of human longevity, and to show that the evidence for a limit to human lifespan—as understood by biologists—is now overwhelming. This observed limit is placed into a broader framework by showing how it has arisen through the process of evolution and by enumerating the molecular mechanisms that may enforce it. Finally, we look toward potential future developments and the prospects for possibly circumventing the current limit.
Is there a limit to human lifespan?
Without a programmed process leading to death, a hard limit—an age at which death is certain to occur—cannot exist. (Indeed, even if there were a genetic program, a hard limit would not exist, because occasionally individuals would be born with mutations deactivating that program.) However, although the probability of survival may never be exactly zero, there is an age beyond which survival is vanishingly unlikely. Although this is a soft limit, it is still a meaningful concept to denote the upper bound of a species’ lifespan.
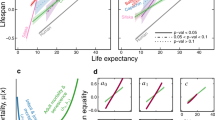
Some statisticians have modeled human lifespan using variations on the generalized extreme value (GEV) function. The GEV function gives the probability distribution of maxima of a random variable; when applied to lifespan, the GEV function is a model of the probability distribution of the oldest age a person can survive to. Statisticians have focused their attention on the shape parameter of the GEV function. In their interpretation, if the shape parameter of the function is less than zero, there is a hard limit to human lifespan, whereas if its value is zero or greater there is not a hard limit (Fig. 1). A few studies12,13 have estimated the shape parameter of the GEV function to be zero, while several others have come up with estimates that are negative14,15,16,17. In the latter case, the finding may be due to survival dropping so low as to be indistinguishable from zero. In the former case, the core finding may be true, even if it is trivial, but a lack of care in its interpretation can lead to misleading conclusions. Even if there is truly no hard limit to lifespan, declaring human life to be ‘unlimited’ is vacuous if no regard is given to the fact that the probability of survival becomes negligible after a certain point18,19,20. Scientists have now shifted their focus to a ‘probabilistic barrier’21, evaluating whether it is likely that an individual will be observed living past a certain age18,22,23,24.
The height of the curve represents the probability density of an individual surviving to a certain age. When the shape parameter is negative (the orange curve), the density function has support (nonzero probability) from –∞ to ω; there is no chance for anyone to live past age ω (a hard limit to lifespan). When the shape parameter is 0 (the blue curve), the density function has support over the interval (–∞,+∞); survival to any age is possible, although trending very close to zero for higher ages (a soft limit to lifespan). In a third possibility (not shown), the shape parameter could be positive; the density function would have support over the interval (ω,+∞) and would have similar lifespan predictions to a shape parameter of zero (as in the blue line). Graph created with code based on ref. 104.
Some of the discussion of a human lifespan limit has been based on the question of what pattern late-life mortality takes as a function of age (Fig. 2). Does it follow the Gompertz model of increasing exponentially25, or does the chance of dying level off at a certain age? Originally, Gompertz had only intended his model to explain mortality between the ages of 20 and 60, after which survivors were—at the time—rare26. Some studies have claimed that, after decades of exponential increase, mortality suddenly plateaus at a certain age: 105 or 108 or 110 years12,13,27. However, the existence of a mortality plateau is heavily disputed, with criticisms questioning both the reliability of the data and the suitability of the models used to analyze it28,29,30,31. Other studies have not found a mortality plateau32 or have found that mortality temporarily decelerates and then accelerates again later in life33,34.
a, In the Gompertz model, mortality increases exponentially with age. b, Some have proposed a model in which mortality is constant (at around 50%) after a certain age, usually in the range of 105–110 years. Graph based on ref. 18.
What unites all of these competing models is their convergence when evaluating them on the basis of an empirical endpoint, such as the probability of living past 130 years. An extreme value model might find a zero chance of living past 130 years, an increasing-mortality model would find a nonzero yet tiny probability of living past 130 years, and the most optimistic model—a constant mortality of 50% after age 110—would give each supercentenarian a chance to survive past 130 years of just under 1 in a million, which dwarfs the current population of 17 living, verified supercentenarians35.
Another, and arguably more important, way of defining a limit to human lifespan is in terms of a temporal limit: in other words, a limit—hard or soft—that changes over time. If that limit can be increased without bound, there is no temporal limit; if the limit is stationary over time, a temporal limit exists. In general, life expectancy at birth has been going up, and continues to go up, although its rate of increase has been slowing for decades36,37. Life expectancy has even experienced stagnation or acute decline, caused in the United States by the opioid epidemic and more globally by the coronavirus pandemic38. At higher ages, there is almost no evidence of a continued increase in longevity. Past age 100, improvements in survival have plateaued23, and statistical and demographic models are almost unanimous—even when arguing against a limit to lifespan—that late-life mortality has seen no improvement12,13,14,15,16,17,32,39.
Hence, there is overwhelming evidence for a limit to human lifespan. Such a limit is soft, that is, probabilistic in nature and may be occasionally exceeded depending on its stringency; but for sufficiently stringent milestones the chance of observing an individual living past the limit is—given a reasonable time frame and the size of the human population—vanishingly small. Furthermore, human lifespan has reached a temporal limit: the age of the soft limit has not gone up since the 1990s.
Why is there a limit to human lifespan?
The process that caps human lifespan, even under the best of conditions, is known as aging. Discarding both theological explanations and models based on a theory of programmed death, aging arises as a phenomenon of unplanned functional decline and increased risk of disease40,41. To understand the mechanisms underlying aging, it is vital to understand the difference between life expectancy or mean lifespan and maximum lifespan. The former reflects all individuals in the species and is amenable to environmental interventions; the latter is more genetically driven, reflects only the species’ highest achievers and may prove resistant to changes that primarily benefit below-average individuals. Let’s first discuss why the human lifespan is now much higher than it has ever been and why further progress is currently stalled.
With some ups and downs, life expectancy at birth throughout human history has never been much higher than 30 years, mostly due to the horrific rate of childhood mortality42,43,44. Before the 19th century technological explosion known as the Industrial Revolution, famine and infectious disease were the most common causes of death. Since then, there has been a massive inversion of conditions contributing to mortality. Improvements in agriculture, food storage and food transportation soon made famines a thing of the past, at least in Europe and the United States. Food security and alleviation of crowded living conditions greatly reduced the prevalence of infectious diseases. Even as late as 1900, influenza was the leading cause of death in the United States, followed by tuberculosis; by 2010, influenza had sunk to ninth place, and tuberculosis did not even break into the top ten. Instead, heart disease, cancer, Alzheimer’s, diabetes and other noncommunicable conditions dominate the leaderboard45. Although there was certainly an age-related aspect to mortality in historical periods (for example, risk of death due to infection is exacerbated by the reduced effectiveness of an aged immune system and, indeed, Gompertz derived his law of mortality from observing what was essentially a preindustrial population), death was more common among younger individuals than it is now, as evidenced by infant mortality that claimed more than 1 in every 10 babies46. Influenza is liable to strike at any age; with rare exceptions, cancer and heart disease are confined to individuals well past middle age.
In the United States and Europe, the 19th century saw the beginning of several public health efforts to extend life. In addition to food security and improved living conditions, public sanitation and mass vaccination were major factors in reducing the incidence of infection. Awareness, further public health measures and the systematic application of disinfection and, ultimately, the use of antibiotics eliminated diseases, such as typhus and cholera, and greatly reduced mortality of women during childbirth and their babies in the early phases of life. Indeed, childhood mortality made an enormous dip, from about 200 per 1,000 live births halfway through the 19th century to less than 10 per 1,000 live births now47. The current life expectancy in the developed world of about 80 years, driven to its height by a century of steady gains, is owed to these 19th century innovations, although emerging trends of vaccine hesitancy and antibiotic resistance threaten to undermine some of its foundations.
In addition to the aforementioned reductions in early-life and mid-life causes of mortality, progress has also been made in reducing the prominent causes of late-life mortality48. Taking cancer as an example, anti-smoking campaigns have focused on prevention, while advances in surgery, chemotherapy and immunotherapy have improved treatment. Also, drugs against high blood pressure, high cholesterol and late-onset diabetes, preventing heart disease, stroke, and kidney disease, and many other medical accomplishments, such as efficient replacement of hips and knees by prostheses, have now made age 70 the new age 60. Gains in healthy lifespan among the aged were most dramatic from the 1940s to the 1980s, but began to show diminishing returns as early as the 1990s. As the number of survivors to old age has now grown so much, the human species for the first time began to get a glimpse of its maximum lifespan. For example, in the 1950s, the oldest verified person was 113 years at the time of death; by 1997, Jeanne Calment had shattered longevity records by surviving past age 122. Since then, however, in spite of the enormously increased number of healthy older adults, progress has ceased. No other person has even lived past age 120 and, while the interpretation of this finding is widely debated49,50,51,52,53,54,55,56,57,58,59,60,61,62,63, this stagnation cannot be explained as a fluke due to Calment’s outlier status64,65,66. It is certainly possible that someone will eventually surpass Jeanne Calment’s record, but the data suggest that they will surpass it only slightly, and the chance of observing any individual living past a higher milestone—such as 125 or 130 years—is so small as to be negligible. As discussed earlier, this signifies a soft limit to human lifespan.
Furthermore, the odds, for a single supercentenarian, of living past Jeanne Calment’s age at death are essentially unchanged since she set her record. The emergence of a new record holder would be driven by an increase not in survival but in the number of supercentenarians, due to either larger cohort sizes or the accumulation of data on more cohorts over time. The fact that old-age survival has ceased to improve signifies, as discussed earlier, a temporal limit to human lifespan.
The reason for this stagnation and for a limit to the human lifespan is given by the mechanism of aging itself. Aging in animals is a consequence of the logic of evolution, which is based on genetic variation and natural selection. Genetic variants will be selected when their corresponding traits positively affect development and reproduction in its given niche, even when these same variants have adverse effects at old age67,68,69. But the efficacy of selection against variants with adverse effects decreases with advancing age, based on the steep decline in the probability of being alive in most natural environments. Lifespan of a species, therefore, depends on its niche, with animals subject to high extrinsic mortality generally having a shorter maximum lifespan than those living in a more protective environment70. Humans now live in a highly protective environment, which could lead to an increase in maximum lifespan if late-fecund females would become the main contributors to future generations. But this would require many generations to have a noticeable effect.
Hence, the gains in longevity over the past century were driven by improvements in the human condition as a consequence of the Industrial Revolution. They affected the main causes of death, such as food shortages and acute, nonsystemic causes of mortality (primarily infectious diseases). More recent gains were mediated by improvements in treating specific conditions. But the fundamental biology of aging has remained unchanged, leading to the exhaustion of options for further improvement and the current stagnation of human lifespan.
Can we succeed where Gilgamesh failed?
In the nearly 4,000-year-old Epic of Gilgamesh, the titular hero is king of the Mesopotamian city of Uruk. He befriends the wild warrior Enkidu. When Enkidu dies, Gilgamesh sets out to conquer death and consults the wise Utnapishtim who offers him two routes to immortality. First, he is told he will escape death if he stays awake for a week straight; he fails and falls asleep. His second chance is to consume an herb found underwater. Gilgamesh successfully obtains the herb and plans to first test it by feeding it to the elders of Uruk. But before he can get the chance, a snake slithers by and steals the herb away71.
After the breakthrough inventions of the 19th century provided the solutions that now allow most humans in developed countries to live fairly healthy lives, often deep into old age, new findings promising to extend maximum lifespan of our species were lacking for much of the 20th century. Not until the 1990s was it shown that in the nematode worm, Caenorhabditis elegans, a single genetic mutation could increase natural lifespan at least twofold72,73. Such mutations increase lifespan of the worm because they dampen the insulin/insulin growth factor 1 (IGF1)/FOXO (IIF) signal transduction pathway. Since then, mutations in other pathways often linked to IIF, such as the mechanistic target of rapamycin (mTOR) pathway, have been found to increase lifespan and most of these mutations were found to have similar effects in flies and mice with evidence for health benefits in humans too74. The same pathways have now been targeted pharmacologically with very similar effects on longevity. Importantly, in flies and mice the effects of such mutations on longevity are much less than in worms and there is very little evidence that such interventions can affect species-specific maximum lifespan. Nevertheless, the conclusion from these results that animal lifespan is fluid and not necessarily limited was the starting point of a new development in research on aging, called geroscience.
Geroscience aims at understanding the basic mechanisms driving aging and using that knowledge to develop clinical interventions against multimorbidity at old age75,76,77. Because this multimorbidity is mechanistically anchored in basic mechanisms of aging, such interventions would then prevent, delay or cure multiple chronic age-related diseases simultaneously. Thus far, geroscience has been remarkably successful in increasing our insight into aging and convincingly demonstrating that lifespan, at least mean lifespan, as well as healthspan, can be modulated, based on interventions targeting the molecular pathways first discovered in the worm. What it has not done, however, is demonstrate that the maximum lifespan of a vertebrate can be radically extended.
The possibility of doing just that, however, is suggested by the large diversity of mortality curves across species78. Indeed, the extreme longevity of certain species as compared to humans, such as the tortoise, bowhead whale, Greenland shark and rockfish suggests that human maximum lifespan at least in theory can be greatly extended. However, this neglects the immense physiological differences between species and the close relationship of these differences with the mechanisms that determine lifespan20. If radical life extension is to be pursued by emulating the strategies used by these species, it may also require a radical alteration of fundamental aspects of human biology.
Mortality is perhaps the most fundamental aspect of aging, but it also has the disadvantage of taking a long time to measure. A desire to more rapidly determine an individual’s biological age (as opposed to their chronological age), and to gain deeper insight into the biological mechanisms of aging, has led to the development of dozens of biomarkers of aging79. These biomarkers, or aging clocks, run the gamut from phenotypic observations of traits such as grip strength and conventional medical blood tests to advanced (epi)genetic tests. It has even been suggested that an accurate assessment of a person’s biological age can be obtained merely by examining the appearance of their face80. Even a single area, such as DNA methylation, can be broken out into multiple clocks that vary in both their timing and amenability to intervention81. Which biomarker—or combination of biomarkers—is best suited to use when studying aging is still an open question. Indeed, the best biomarker may even be one that has yet to be discovered. The lack of an experimentally tractable metric that is universally agreed upon further complicates assessment of the efficacy of antiaging treatments.
In spite of these considerations, confidence in technological progress has now become so high that it has been argued that new medical interventions will soon emerge and radically increase human longevity. Such optimism is the driving force behind the very large sums of money recently donated by billionaires to new organizations active in geroscience. These include: the Methuselah Foundation82, which has set up a series of prizes to demonstrate longevity extension in mice83,84,85,86,87; the SENS Foundation, which has funded research into aging and rejuvenation88,89; Calico, launched by Google, has engaged in multiple collaborations with academic and commercial researchers90,91,92; Human Longevity, founded by Craig Venter of human genome fame, and largely focused on a concierge longevity service93,94,95; and Altos Labs, a newcomer with $3 billion of funding96.
Despite their impressive rosters and large cash flows, these organizations face great difficulty in achieving their lofty goals. Given that, thus far, no interventions have been demonstrated to increase maximum species lifespan, there are other considerations that make such efforts dubious to say the least. To expand species-specific limits to life would require the discovery of master regulatory pathways, the downstream effects of which would improve most or even all phenotypes of aging to such an extent that its maximum lifespan is increased while retaining all other characteristics of the species. While the pathways mentioned above, such as IIF or TOR, do affect lifespan in model organisms in a conserved manner, there is no evidence that intervening in such pathways extends the species-specific limits to lifespan. The same is true for dietary restriction, which is known to increase active and healthy lifespan in a variety of species97. Indeed, they are promising targets for improving healthspan but not maximum lifespan, perhaps with the exception of the nematode worm. To identify pathways that can be targeted to increase maximum lifespan we have to consider the causes of aging, which is the process that limits it.
Currently, there is little consensus as to the cause, or causes, of aging98. Most would agree that aging is the result of damage, that is, deleterious changes, that are ultimately molecular in nature99. In this respect, damage to DNA is the most likely candidate because this has been shown to affect most, if not all, aspects of the aging phenotype100. Antiaging interventions can then be categorized as either preventative (geroprotectors) or reparative (gerotherapeutics). Assuming that a causal molecular change has been identified, then lifespan extension will require a reparative and/or regenerative—not merely preventative—approach. Preventative measures reduce the rate at which damage accumulates. However, they cannot bring it to zero, and, as damage tends to beget more damage in a positive feedback loop that leads to an exponential increase, even dramatic reductions in the rate of damage accumulation yield only incremental improvements to lifespan. Although preventative measures can be useful, a damage-repair approach, like the one advocated by the SENS Foundation and others, will be necessary101,102.
While in theory targeting cellular defense systems, including systems for DNA repair, detoxification, immune response and programmed cell death, to boost the quick removal of damage to biological macromolecules, protein aggregates and senescent cells, should be feasible in the long term, singular causes of aging are conflicting with evolutionary theory. Indeed, if there would be one highly conserved central cause of aging, possibly going back in evolutionary time to the early replicators, multicellular organisms would fall prey to the late-life adverse effects of mutations that accumulate in the germline due to the age-related decline in efficacy of natural selection. This would mean that, independent of any hypothetical central cause of aging, a host of additional adverse late-life effects have to be taken into account. This would essentially mean that any fix of the limits to lifespan would require interventions at many choke points. Such multipoint targeting would also need to be fine-balanced so as to avoid side effects. Indeed, there are few if any gene regulatory pathways exclusively involved in somatic maintenance and it is this complexity that essentially rules out successful interventions aiming to exclusively extend maximum lifespan of a species. In essence, what needs to be done is to mimic evolution as to how this gave rise to extremely long-lived species, such as those mentioned above, but in real time. As this would involve possibly millions of genetic variants, this seems an impossible quest.
Based on the above, geroscientists should clearly distinguish between mean and maximum lifespan and not give the impression that their research can substantially increase the current limits to human lifespan. Their focus should be on improving life expectancy and healthspan, that is, bringing more people closer to the maximum lifespan possible for members of Homo sapiens and improving the quality of those years. A global extension in the median number of healthy years would benefit billions of people. Moreover, healthspan is much more amenable to study than maximum lifespan, where hypotheses may take generations to test (Box 1), Meanwhile, basic research into the mechanisms that underlie species-specific limits to lifespan should be an important focus as well. This not only will satisfy our curiosity about the nature of lifespan limits and why these are sometimes absent in organisms, such as the small freshwater cnidarian polyp Hydra103, but also may well open up a new frontier of untapped, unrealized potential for improving healthspan.
Can modern science succeed where Gilgamesh, and so many others, failed? Only time will tell, but the obstacles faced are certainly formidable. Indeed, all current scientific evidence tells us that breaking through the biological limits of human lifespan is impossible. However, past centuries have learned that in science no possibility can ever be excluded and new insights and more advanced technologies may emerge to radically extend the maximum lifespan of our species above and beyond the current limit established from demographic analysis. Aging is the ultimate challenge of humankind. Defeating it will require groundbreaking research that utilizes a wide range of knowledge and techniques across many areas of science and clinical practice. To accomplish this would certainly warrant the name we have given to our species: Homo sapiens.
References
Dalley, D. Myths from Mesopotamia (Oxford Univ. Press, 2009).
Bible Gateway. Genesis 6:3 NRSV. https://www.biblegateway.com/passage/?search=Genesis+6%3A3&version=NRSV
Goldsmith, T. C. Evolvability, population benefit, and the evolution of programmed aging in mammals. Biochemistry 82, 1423–1429 (2017).
Longo, V. D., Mitteldorf, J. & Skulachev, V. P. Programmed and altruistic ageing. Nat. Rev. Genet. 6, 866–872 (2005).
Weismann, A. Essays Upon Heredity and Kindred Biological Problems. Available at https://www.gutenberg.org/ebooks/48132/pg48132-images.html.utf8 (1889).
Kirkwood, T. B. L. & Cremer, T. Cytogerontology since 1881: a reappraisal of August Weismann and a review of modern progress. Hum. Genet. 60, 101–121 (1982).
Finch, C. E. Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc. Natl Acad. Sci. USA 107, 1718–1724 (2010).
Vijg, J. & Kennedy, B. K. The essence of aging. Gerontology 62, 381–385 (2016).
Kowald, A. & Kirkwood, T. B. L. Can aging be programmed? A critical literature review. Aging Cell 15, 986–998 (2016).
Ismail, K. et al. Compression of morbidity is observed across cohorts with exceptional longevity. J. Am. Geriatr. Soc. 64, 1583–1591 (2016).
Fleur, N. S., Williams, C. & Wood, C. Can we live to 200? Here’s a roadmap. The New York Times (27 April 2021).
Rootzén, H. & Zholud, D. Human life is unlimited—but short. Extremes 20, 713–728 (2017).
Belzile, L. R., Davison, A. C., Rootz‚n, H. & Zholud, D. Human mortality at extreme age. R. Soc. Open Sci. 8, 202097 (2021).
Gbari, S., Poulain, M., Dal, L. & Denuit, M. Extreme value analysis of mortality at the oldest ages: a case study based on individual ages at death. N. Am. Actuar. J. 21, 397–416 (2017).
Einmahl, J. J., Einmahl, J. H. J. & de Haan, L. Limits to human life span through extreme value theory. J. Am. Stat. Assoc. 114, 1075–1080 (2019).
Feifel, J., Genz, M. & Pauly, M. The myth of immortality: an analysis of the maximum lifespan of US females. https://www.ifa-ulm.de/fileadmin/user_upload/download/forschung/2018_ifa_Feifel-etal_The-Myth-of-Immortality-An_Analysis-of-the-Maximum-Lifespan-of-US-Females.pdf (2018).
Ferreira, A. & Huang, F. Is human life limited or unlimited? (A discussion of the paper by Holger Rootzén and Dmitrii Zholud). Extremes 21, 373–382 (2018).
Milholland, B., Dong, X. & Vijg, J. The shortness of human life constitutes its limit. Preprint at https://doi.org/10.48550/arXiv.1803.04024 (2018).
Beltrán-Sánchez, H., Austad, S. N. & Finch, C. E. Comment on ‘The plateau of human mortality: demography of longevity pioneers’. Science 361, eaav1200 (2018).
Olshansky, S. J. & Carnes, B. A. Inconvenient truths about human longevity. J. Gerontol. A. Biol. Sci. Med. Sci. 74, S7–S12 (2019).
Antero-Jacquemin, J. et al. Learning from leaders: lifespan trends in olympians and supercentenarians. J. Gerontol. A. Biol. Sci. Med. Sci. 70, 944–949 (2015).
Fleming, N. Scientists up stakes in bet on whether humans will live to 150. Nature https://doi.org/10.1038/nature.2016.20818 (2016).
Dong, X., Milholland, B. & Vijg, J. Evidence for a limit to human lifespan. Nature 538, 257–259 (2016).
Holden, C. A long-lived bet. Science https://doi.org/10.1126/article.36961 (2001).
Gompertz, B. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. In a letter to Francis Baily, Esq. F. R. S. &c. By Benjamin Gompertz, Esq. F. R. S. Abstract paper. Philos. Trans. R. Soc. Lond. 2, 252–253 (1833).
Olshansky, S. J. & Carnes, B. A. Ever since Gompertz. Demography 34, 1–15 (1997).
Barbi, E., Lagona, F., Marsili, M., Vaupel, J. W. & Wachter, K. W. The plateau of human mortality: demography of longevity pioneers. Science 360, 1459–1461 (2018).
Cabreros, I. A new study argues you might be able to live forever. You still can’t. slate.com, https://slate.com/technology/2018/07/a-new-study-argues-you-might-be-able-to-live-forever-you-still-cant.html (2018).
Newman, S. Errors as a primary cause of late-life mortality deceleration and plateaus. PLoS Biol. https://doi.org/10.1371/journal.pbio.2006776 (2018).
Newman, S. J. Plane inclinations: a critique of hypothesis and model choice in Barbi et al. PLoS Biol. 16, e3000048 (2018).
Gavrilov, L. A. & Gavrilova, N. S. Late-life mortality is underestimated because of data errors. PLoS Biol. 17, e3000148 (2019).
Gavrilov, L. A. & Gavrilova, N. S. New trend in old-age mortality: Gompertzialization of mortality trajectory. Gerontology 65, 451–457 (2019).
Huang, F., Maller, R. & Ning, X. Modelling life tables with advanced ages: an extreme value theory approach—ScienceDirect. Insur. Math. Econ. 93, 95–115 (2020).
Huang, F., Maller, R., Milholland, B. & Ning, X. A mixture model incorporating individual heterogeneity in human lifetimes. Preprint at https://doi.org/10.1101/2021.01.29.428902v1 (2021).
Gerontology Research Group. Table C—World’s Oldest Person (WOP) Titleholders Since 1955 https://gerontology.fandom.com/wiki/World%27s_Oldest_Person_titleholders (2019).
Cardona, C. & Bishai, D. The slowing pace of life expectancy gains since 1950. BMC Public Health 18, 151 (2018).
Raleigh, V. S. Trends in Life Expectancy in EU and other OECD Countries: Why are Improvements Slowing? https://doi.org/10.1787/223159ab-en (2019).
Harper, S., Riddell, C. A. & King, N. B. Declining life expectancy in the United States: missing the trees for the forest. Annu. Rev. Public Health 42, 381–403 (2021).
Modig, K., Andersson, T., Vaupel, J., Rau, R. & Ahlbom, A. How long do centenarians survive? Life expectancy and maximum lifespan. J. Intern. Med. 282, 156–163 (2017).
Blagosklonny, M. V. Aging is not programmed. Cell Cycle 12, 3736–3742 (2013).
Gladyshev, V. N. The origin of aging: imperfectness-driven non-random damage defines the aging process and control of lifespan. Trends Genet. 29, 506–512 (2013).
Galor, O. & Moav, O. Natural selection and the evolution of life expectancy. SSRN Electron. J. https://doi.org/10.2139/ssrn.563741 (2005).
Morris, I. Economic growth in Ancient Greece. J. Institutional Theor. Econ. 160, 709–742 (2004).
Roser, M., Ortiz-Ospina, E. & Ritchie, H. Life expectancy. https://ourworldindata.org/life-expectancy (2013).
Jones, D. S., Podolsky, S. H. & Greene, J. A. The burden of disease and the changing task of medicine. N. Engl. J. Med. 366, 2333–2338 (2012).
Brosco, J. P. The early history of the infant mortality rate in America. Pediatrics 103, 478–485 (1999).
Roser, M. Mortality in the past—around half died as children https://ourworldindata.org/child-mortality-in-the-past (2019).
Beltrán-Sánchez, H., Soneji, S. & Crimmins, E. M. Past, present and future of healthy life expectancy. Cold Spring Harb. Perspect. Med. 5, a025957 (2015).
Olshansky, S. J. Measuring our narrow strip of life. Nature 538, 175–176 (2016).
Brown, N. J. L., Albers, C. J. & Ritchie, S. J. Contesting the evidence for limited human lifespan. Nature 546, E6–E7 (2017).
Dong, X., Milholland, B. & Vijg, J. Dong et al. reply. Nature 546, E7 (2017).
Hughes, B. G. & Hekimi, S. Many possible maximum lifespan trajectories. Nature 546, E8–E9 (2017).
Dong, X., Milholland, B. & Vijg, J. Dong et al. reply. Nature 546, E9–E10 (2017).
Lenart, A. & Vaupel, J. W. Questionable evidence for a limit to human lifespan. Nature 546, E13–E14 (2017).
Dong, X., Milholland, B. & Vijg, J. Dong et al. reply. Nature 546, E12 (2017).
Rozing, M. P., Kirkwood, T. B. L. & Westendorp, R. G. J. Is there evidence for a limit to human lifespan? Nature 546, E11–E12 (2017).
Dong, X., Milholland, B. & Vijg, J. Dong et al. reply. Nature 546, E14–E15 (2017).
de Beer, J., Bardoutsos, A. & Janssen, F. Maximum human lifespan may increase to 125 years. Nature 546, E16–E17 (2017).
Dong, X., Milholland, B. & Vijg, J. Dong et al. reply. Nature 546, E21 (2017).
Dolgin, E. There’s no limit to longevity, says study that revives human lifespan debate. Nature 559, 14–15 (2018).
The limits to human lifespan must be respected. Nature 538, 6 (2016).
Geddes, L. Human age limit claim sparks debate. Nature https://doi.org/10.1038/nature.2016.20750 (2016).
Eisenstein, M. Does the human lifespan have a limit? Nature 601, S2–S4 (2022).
Dong, X., Milholland, B. & Vijg, J. Reply to Kashnitsky. https://doi.org/10.2139/ssrn.2890500 (2016).
Milholland, B., Dong, X. & Vijg, J. ‘Best-guess’ MRAD provides robust evidence for a limit to human lifespan: reply to de Grey (Rejuvenation Res. 20, 261–262 (2017)). Rejuvenation Res. 20, 437–440 (2017).
Milholland, B. Jeanne Calment, actuarial paradoxography and the limit to human lifespan. Rejuvenation Res. 23, 17–18 (2020).
Williams, G. C. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 (1957).
Le Bourg, E. Evolutionary theories of aging can explain why we age. Interdiscip. Top. Gerontol. 39, 8–23 (2014).
Kowald, A. & Kirkwood, T. B. L. Evolutionary significance of ageing in the wild. Exp. Gerontol. 71, 89–94 (2015).
Austad, S. N. Retarded senescence in an insular population of Virginia opossums (Didelphis virginiana). J. Zool. 229, 695–708 (1993).
Sandars, N. The Epic of Gilgamesh (Penguin, 1973).
Kenyon, C., Chang, J., Gensch, E., Rudner, A. & Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464 (1993).
Kimura, K. D., Tissenbaum, H. A., Liu, Y. & Ruvkun, G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277, 942–946 (1997).
Fontana, L., Partridge, L. & Longo, V. D. Extending healthy life span—from yeast to humans. Science 328, 321–326 (2010).
Kennedy, B. K. et al. Geroscience: linking aging to chronic disease. Cell 159, 709–713 (2014).
Olshansky, S. J., Perry, D., Miller, R. A. & Butler, R. N. In pursuit of the longevity dividend: what should we be doing to prepare for the unprecedented aging of humanity? Scientist 20, 28–37 (2006).
DeVito, L. M. et al. Extending human healthspan and longevity: a symposium report. https://doi.org/10.1111/nyas.14681 (2022).
Jones, O. R. et al. Diversity of ageing across the tree of life. Nature 505, 169–173 (2014).
Hartmann, A. et al. Ranking biomarkers of aging by citation profiling and effort scoring. Front. Genet. 12, 686320 (2021).
Chen, W. et al. Three-dimensional human facial morphologies as robust aging markers. Cell Res. 25, 574–587 (2015).
Levine, M. E., Higgins-Chen, A., Thrush, K., Minteer, C. & Niimi, P. Clock work: deconstructing the epigenetic clock signals in aging, disease and reprogramming. Preprint at bioRxiv https://doi.org/10.1101/2022.02.13.480245 (2022).
Mary Ann Liebert, Inc. Methuselah Foundation. Rejuvenation Res. 7, 154–159 (2004).
Sprague, V. Battle for ‘old mouse’ prize. BBC News (2003).
Pilcher, H. R. Money for old mice. Nature https://doi.org/10.1038/news030915-13 (2003).
Christensen, B. First Methuselah Mouse Rejuvenation ‘M Prize’ Awarded. Live Science https://www.livescience.com/3725-methuselah-mouse-rejuvenation-prize-awarded.html (2004).
A Special Mprize Award. Fight Aging! https://www.fightaging.org/archives/2009/10/a-special-mprize-award/ (2009).
Methuselah Foundation Announces Award to Dr. Huber Warner. Fight Aging! https://www.fightaging.org/archives/2014/06/methuselah-foundation-announces-award-to-dr-huber-warner/ (2014).
SENS Research Foundation. SENS Research Foundation Annual Report (2019).
SENS Research Foundation. SENS Research Foundation Annual Report (2020).
Google. Google announces Calico, a new company focused on health and well-being—news announcements. http://googlepress.blogspot.com/2013/09/calico-announcement.html (2013).
Calico. Calico announces collaboration with the University of Pennsylvania for Translational Medicine efforts in aging and age-related diseases; Garret FitzGerald to become senior advisor to Calico. https://calicolabs.com/press/calico-announces-collaboration-with-the-university-of-pennsylvania-for-translational-medicine-efforts-in-aging-and-age-related-diseases-garret-fitzgerald-to-become-senior-advisor-to-calico (2018).
Calico. AbbVie and Calico announce second extension of collaboration focused on aging and age-related diseases. https://calicolabs.com/press/abbvie-and-calico-announce-second-extension-of-collaboration-focused-on-aging-and-age-related-diseases (2021).
Nast, C. ‘Supercharged’ genomics: 100 years of breakthroughs possible in 10 years. Wired UK https://www.wired.co.uk/article/brad-perkins-human-longevity-wired-health-2015 (2015).
Winkler, R. Genomics startup Human Longevity’s valuation falls 80%. Wall Street Journal https://www.wsj.com/articles/genomics-startup-human-longevitys-valuation-falls-80-1544187724 (2018).
Human Longevity Inc. Changing healthcare ‘one patient at a time’. Pubs—Diagnostics World News https://www.diagnosticsworldnews.com/news/2020/03/06/human-longevity-inc.-changing-healthcare-one-patient-at-a-time (2020).
The Economist. A $3bn bet on finding the fountain of youth. https://www.economist.com/science-and-technology/a-3bn-bet-on-finding-the-fountain-of-youth/21807244 (2022).
Katewa, S. D. & Kapahi, P. Dietary restriction and aging, 2009. Aging Cell 9, 105–112 (2010).
Gems, D. & de Magalhães, J. P. The hoverfly and the wasp: a critique of the hallmarks of aging as a paradigm. Ageing Res. Rev. 70, 101407 (2021).
da Silva, P. F. L. & Schumacher, B. Principles of the molecular and cellular mechanisms of aging. J. Invest. Dermatol. 141, 951–960 (2021).
Schumacher, B., Pothof, J., Vijg, J. & Hoeijmakers, J. H. J. The central role of DNA damage in the ageing process. Nature 592, 695–703 (2021).
Grey, A. de & Rae, M. Ending Aging: The Rejuvenation Breakthroughs That Could Reverse Human Aging in Our Lifetime (St. Martin’s Griffin, 2008).
Hébert, J. Replacing Aging (Science Unbound, 2020).
Tomczyk, S., Fischer, K., Austad, S. & Galliot, B. Hydra, a powerful model for aging studies. Invertebr. Reprod. Dev. 59, 11–16 (2015).
Gill, R. File:GevDensity 2.svg—Wikipedia. Generalized Extreme Value Densities https://commons.wikimedia.org/wiki/File:GevDensity_2.svg (2020).
Finch, C. E. & Pike, M. C. Maximum life span predictions from the Gompertz mortality model. J. Gerontol. Ser. A 51A, B183–B194 (1996).
Harrison, D. E. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009).
Dhahbi, J. M., Kim, H.-J., Mote, P. L., Beaver, R. J. & Spindler, S. R. Temporal linkage between the phenotypic and genomic responses to caloric restriction. Proc. Natl Acad. Sci. USA 101, 5524–5529 (2004).
Acknowledgements
This work was supported by National Institutes of Health grants to J.V. We thank N. Barzilai and Y. Suh for critically reading the manuscript.
Author information
Authors and Affiliations
Contributions
B.M. analyzed data and B.M. and J.V. jointly wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.V. is cofounder of SingulOmics. B.M. declares no competing interests.
Peer review
Peer review information
Nature Aging thanks S. Jay Olshansky, Joop De Beer and Elsa Logarinho for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Milholland, B., Vijg, J. Why Gilgamesh failed: the mechanistic basis of the limits to human lifespan. Nat Aging 2, 878–884 (2022). https://doi.org/10.1038/s43587-022-00291-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-022-00291-z
This article is cited by
-
Brain age Prediction and the Challenge of Biological Concepts of Aging
Neuroethics (2023)