Abstract
α,β-Unsaturated ketones are common feedstocks for the synthesis of fine chemicals, pharmaceuticals, and natural products. Transition metal-catalysed hydroacylation reactions of alkynes using aldehydes have been recognised as an atom-economical route to access α,β-unsaturated ketones through chemoselective aldehydic C–H activation. However, the previously reported hydroacylation reactions using rhodium, cobalt, or ruthenium catalysts require chelating moiety-bearing aldehydes to prevent decarbonylation of acyl-metal-hydride complexes. Herein, we report a nickel-catalysed anti-Markovnikov selective coupling process to afford non-tethered E-enones from terminal alkynes and S-2-pyridyl thioesters in the presence of zinc metal as a reducing agent. Utilization of a readily available thioester as an acylating agent and water as a proton donor enables the mechanistically distinctive and aldehyde-free hydroacylation of terminal alkynes. This non-chelation-controlled approach features mild reaction conditions, high step economy, and excellent regio- and stereoselectivity.
Similar content being viewed by others
Introduction
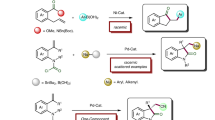
α,β-Unsaturated ketones have been extensively applied as versatile compounds in synthetic organic chemistry, for example, as key substrates for conjugate addition1,2,3,4, Morita–Baylis–Hillman5,6, Diels–Alder7,8, and epoxidation9,10,11 reactions. They have been conventionally prepared through aldol condensation12, Horner–Wadsworth–Emmons olefination13, the dehydrogenation of ketones14,15, or palladium-catalyzed carbonylation16,17 reactions. However, these methods often suffer from the use of strong bases, elevated reaction temperature, E/Z-selectivity control, and/or multi-step synthetic operations. Alkynes have been widely applied as readily available synthetic platforms for catalytic transformations to directly access functionalized cyclic or acyclic products18,19,20,21,22. The catalytic hydroacylation of alkynes using aldehydes inherently provides an atom-economical process leading to the formation of enones. Organocatalytic intramolecular hydroacylation reactions of alkynes using organophosphines or N-heterocyclic carbenes have been demonstrated (Fig. 1a)23,24,25. Metal-catalyzed intermolecular hydroacylation has emerged as a prominent method for rapidly accessing E-enones through alkyne–aldehyde coupling26,27,28,29,30,31. This atom-economical method involves the chemoselective activation of an aldehydic C(sp2)–H, and chelating moiety-bearing aldehydes have been applied to prevent decarbonylative side pathways (Fig. 1b).
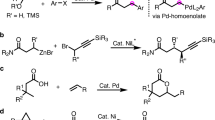
Stabilization of an acyl-metal complex assisted by heteroatom chelation is a powerful strategy for obtaining E-enones. However, the installation and removal of the coordinating moieties entailed extra synthetic steps while reducing the step economy of the hydrofunctionalization. Though nondirected hydroacylation methods have been developed for alkenes or dienes32,33,34,35,36, to our knowledge, there is no general hydroacylation method for unactivated terminal alkynes that lead to chelating moiety-free E-enones. Mukaiyama, Weix, and Kishi independently reported that S-2-pyridyl (SPy) thioesters act as potential acyl donors for (cross-electrophile) coupling reactions37,38,39,40. We surmised that the thioester may act as both a transient SPy ligand and an acyl component41,42,43,44 under nickel-catalyzed reductive coupling conditions to lead to acyl-Ni-SPy complex. A consecutive alkyne insertion and subsequent protodemetalation process may lead to hydroacylation product formation (Fig. 1c). However, the challenges of this anticipated reaction process to attain traceless alkyne hydroacylation are fourfold: (i) competition with non-conjunctive cross-electrophile (proton and thioester) coupling, (ii) reduction of substrates, (iii) iterative alkyne additions, and (iv) regio- and stereoselectivity issues. Therefore, precise reactivity and selectivity controls are crucial for a general approach to the hydroacylation of unactivated terminal alkynes. Herein, we report a nondirected and aldehyde-free approach to afford E-enones via a nickel-catalyzed reductive pathway.
Results and discussion
Optimization studies
To optimize the reaction conditions, S-(pyridin-2-yl) 4-methoxybenzothioate (1) and 3,3-dimethyl-1-butyne (2) were chosen as model substrates, and a thorough screening of catalysts, reducing agents, additives, and solvents was conducted (Table 1, see also the Supplementary Information (SI), Section III, Supplementary Tables 1–7). The standard conditions were established on the basis of inexpensive nickel(II) perchlorate hexahydrate, Zn, and ZnCl2 in 1,2-DME to exclusively afford E-enone 3 in 81% isolated yield at room temperature (entry 1). The use of THF resulted in a similar yield (entry 2). Interestingly, coordinated water molecules were also found to be a suitable proton source (entries 3–5). The use of 17 mol% of Ni catalyst was appropriate for providing a stoichiometric 1 equiv of protons to the reaction. No desired product formation was observed in the absence of the nickel catalyst, Zn, or ZnCl2 (entries 6–8). Mn as the reducing agent instead of Zn also appeared successful; however, this resulted in a diminished yield (entry 9). ZnCl2 was found to be superior to MgCl2 (entry 10). The complexation of thioester 1 by ZnCl2 was examined by 1H NMR spectroscopic studies (see the SI, Section VI). Using 1.5 equiv of terminal alkyne 2 was required for better conversion of the thioester (entry 11). The standard optimized reaction conditions were developed under an inert argon atmosphere; however, a significant amount of product formation was observed even under open atmosphere conditions (entry 12). Reduced reaction time or a decreased amount of Ni catalyst led to diminished yields (entries 13, 14). In addition, the employment of acyl chloride or aldehyde as an acyl donor instead of thioester appeared to be completely unproductive (entries 15, 16). Additional ligands in the hydroacylation resulted in slightly diminished yields for the substrates (entries 17, 18).
Scope of the reaction
With the optimized conditions in hand, we set out to explore the generality of this alkyne hydroacylation by determining the thioester scope (Fig. 2, see also Supplementary Figs. 1–5). Various alkyl substituents worked well to afford E-enone products (5–8) in moderate to good yields. Hydrogen and phenyl groups, however, led to slightly lower yields (4, 9). Generally, electron-donating groups gave the products in moderate to good yields (10–13). The thioester bearing -NMe2 gave enone 13′ in a low yield of 38%, suggesting competition between the Lewis-basic moiety and carbonyl group towards ZnCl2 (see the SI, Section VIII-2). Although yields were low due to the oxidative addition capability of Ni(0) species, halide groups were tolerated in the reaction (14–16). Electron-withdrawing groups such as CO2Me and CF3 led to products (17, 18) with low yields. Oxidative addition of C(sp2)–halides to Ni(0) or thioester homocoupling43 may result in reduced yields. Meta- and ortho-substitution patterns also provided the products in moderate yields (19–24). A 2-naphthyl substituent afforded the product a slightly higher yield (44%) than a 1-naphthyl substituent (32%) (25, 26). Upon 26 synthesis, naphthalene byproduct formation via decarbonylation was also observed in 15% yield with the consumption of substrates. An electron-rich indole substituent led to product 27 with an 82% yield. Both acyclic- and cyclic aliphatic substituents could access the products with moderate yields (28–32).
We then examined the scope of a wide range of alkynes. It is noteworthy that the reaction conditions b in Fig. 2 employ 2,2′-dipyridyl disulfide (Py2S2) to improve the reaction efficacy. The reduced SPy anion from Py2S2 may provide an additional ligand source to stabilize nickel complexes (see also Fig. 1c). Acyclic- as well as cyclic aliphatic terminal alkynes, underwent the reaction to afford the corresponding vinyl ketones (33–38) in moderate yields, although cyclopropyl- and cyclopentyl-derived alkynes gave the diminished yields. Aromatic alkynes bearing alkyl, phenyl, and phenoxy substituents worked well to give the desired products (39–45) with moderate to good yields. Ortho-, meta-, and para-substituted methoxy groups were also tolerated to obtain the products (46–48). Product 49 was isolated in 55% yield by using a disubstituted arylalkyne. Fluoro- and chloro-groups were examined and gave 50 and 51 in 63% and 56% yields, respectively. The strongly electron-withdrawing trifluoromethyl group led to product formation (52, 53) with diminished yields. 2-Ethylnyl-6-methoxy-naphthalene efficiently produced 54 in 76% yield. 2-Ethynylthiophene and 3-ethynylthiophene underwent the reaction smoothly to obtain the corresponding products (55, 56) in 39% and 58% yields, respectively. A free hydroxyl group was compatible affording 57 in 59% yield. We were delighted to find that the reaction was feasible with ethisterone, an agent for gynecological disease treatment, to afford 58 in 56% yield. The chemistry was also operative on a 1 mmol scale to give a similar yield. Symmetrical as well as unsymmetrical internal alkynes gave hydroacylation products (59–61) in low yields. The iterative reactivity yielding double-alkyne-insertion byproducts was observed (see also Fig. 3b). Activated alkenes also underwent the reaction smoothly to afford 62–64 in good yields.
Mechanistic investigations and deuterium labeling experiments
Control experiments were conducted to gain the insight into the reaction pathway. When the reaction was performed using Ni(COD)2 as a catalyst, the desired hydroacylation product 3 was obtained in a 35% isolated yield. This study corroborates the oxidative addition of thioester 1 to zero-valent nickel species over the reaction course (Fig. 3a). The formation of hydroacylation product 60 suggests the protodemetalation of nucleophilic vinyl nickel complex I45. Interestingly, 65 and 66 were also isolated, verifying an iterative double-alkyne-insertion (Fig. 3b) and the presence of vinyl nickel species II46,47,48.
To identify the proton source of the hydroacylation, deuterium labeling tests were performed (Fig. 3c, see also Supplementary Figs. 6–11). First, a reaction conducted using deuterium oxide (3.0 equiv) resulted in the formation of the product with 64 and 33% [D] incorporation at the β- and α-positions, respectively, of unsaturated ketone [D]-3. π-Complexation of terminal alkynes to transition metal species gives its increased acidity49,50. Reversible H–D exchange between alkyne complex C (shown in Fig. 4) and D2O and subsequent migratory insertion lead to the incorporation of deuterium in the α-position of enone framework (see also the SI, Supplementary Fig. 12). Employing deuterated nickel(II) perchlorate hexahydrate also resulted in the deuterated enone [D]-3 (see also the SI, Section V). When we also performed the hydroacylation reaction in THF-d8, enone 3 was obtained with no deuterium labeling. The employment of terminally deuterated alkyne [D]-2 for the Ni-catalyzed reductive coupling reaction also reveals H/D scrambling behavior.
Proposed mechanism
Based on the above mechanistic studies and findings from previous reports45,46,47,48,51,52, we proposed a reaction pathway for this reductive hydroacylation as illustrated in Fig. 4. The reaction is initiated by the generation of Ni(0) species A through the reduction of Ni(II) catalyst with the help of zinc. With the assistance of zinc chloride as a Lewis-acidic additive (see also the SI, Section VI, Supplementary Figs. 13 and 14), oxidative addition of electrophilic thioester to Ni(0) can be promoted53,54, forming acyl-Ni(II)-X complex B. Terminal alkynes approached complex B to give Ni(II)-alkyne complex C. Migratory insertion of terminal alkynes gives rise to vinyl nickel complex D. Then, protodemetalation of nucleophilic vinyl Ni(II) species D with the help of water affords the desired E-enone45. The reduction of Ni(II)(OH)X species E by zinc regenerates the active Ni(0) species A. Overall, readily available electrophiles (i.e., “H+” and “Ac+”) are incorporated into terminal alkynes in a reductive fashion55 with excellent regio- and E-selectivity under mild conditions. This method can avoid the use of strong bases, organometallic reagents, and pre-installed chelating moieties.
Conclusions
In summary, we have demonstrated a nickel-catalyzed hydroacylation method of terminal alkynes to exclusively afford non-tethered E-enones. S-(2-Pyridyl) thioesters not only serve as the acyl donor but also give the acyl-Ni(II)-SPy species as a key intermediate over the course of non-chelation-controlled catalytic events. It requires neither additional steps for removal of the coordinating group nor the use of a nucleophilic hydride source, further enhancing the efficacy of the method. We anticipate that these Ni-catalyzed reductive hydroacylation reactions will have an impact on synthesizing important synthetic intermediates, functional materials, and pharmaceuticals.
Methods
General experimental procedure for E-enones
A 1-dram screw-cap vial equipped with a magnetic stir bar was charged with thioester (0.2 mmol, 1.0 equiv), Zn (33 mg, 0.5 mmol, 2.5 equiv), ZnCl2 (27 mg, 0.2 mmol, 1.0 equiv), and Ni(ClO4)2·6H2O (12 mg, 0.034 mmol, 17 mol%) inside a glove box. The mixture was dissolved in 1,2-DME (1 mL). Then, alkyne (0.3 mmol, 1.5 equiv) was added. The reaction mixture was stirred for 24 h at room temperature. After completion of the reaction, the mixture was purified by flash column chromatography to afford the desired product.
Data availability
Detailed experimental procedures, HRMS-ESI data and NMR spectra (PDF) for all compounds were provided in the Supplementary Information. Single-crystal X-ray data for 27 and 66 (CIF) are available free of charge from the Cambridge Crystallographic Database Centre (CCDC) under reference numbers 2039192 and 2036543.
References
Wu, H. et al. Mechanism of NHC-catalyzed conjugate additions of diboron and borosilane reagents to α,β-unsaturated carbonyl compounds. J. Am. Chem. Soc. 137, 10585–10602 (2015).
Wu, F., Li, H., Hong, R. & Deng, L. Construction of quaternary stereocenters by efficient and practical conjugate additions to α,β‐unsaturated ketones with a chiral organic catalyst. Angew. Chem. Int. Ed. 45, 947–950 (2006).
Lee, S. et al. Enantioselective conjugate radical addition to α′-hydroxy enones. Org. Lett. 8, 4311–4313 (2006).
Chi, Y. & Gellman, S. H. Diphenylprolinol methyl ether: a highly enantioselective catalyst for Michael addition of aldehydes to simple enones. Org. Lett. 7, 4253–4256 (2005).
Li, Y.-Q., Wang, H.-J. & Huang, Z.-Z. Morita–Baylis–Hillman reaction of α,β-unsaturated ketones with allylic acetates by the combination of transition-metal catalysis and organomediation. J. Org. Chem. 81, 4429–4433 (2016).
Satpathi, B. & Ramasastry, S. S. V. Morita–Baylis–Hillman reaction of β,β‐disubstituted enones: an enantioselective organocatalytic approach for the synthesis of cyclopenta[b]annulated arenes and heteroarenes. Angew. Chem. Int. Ed. 55, 1777–1781 (2016).
Singh, R. P. et al. Enantioselective Diels−Alder reaction of simple α,β-unsaturated ketones with a cinchona alkaloid catalyst. J. Am. Chem. Soc. 130, 2422–2423 (2008).
Northrup, A. B. & MacMillan, D. W. C. The first general enantioselective catalytic Diels−Alder reaction with simple α,β-unsaturated ketones. J. Am. Chem. Soc. 124, 2458–2460 (2002).
Lifchits, O. et al. The cinchona primary amine-catalyzed asymmetric epoxidation and hydroperoxidation of α,β-unsaturated carbonyl compounds with hydrogen peroxide. J. Am. Chem. Soc. 135, 6677–6693 (2013).
Chu, Y. et al. Asymmetric catalytic epoxidation of α,β-unsaturated carbonyl compounds with hydrogen peroxide: additive-free and wide substrate scope. Chem. Sci. 3, 1996–2000 (2012).
Ooi, T., Ohara, D., Tamura, M. & Maruoka, K. Design of new chiral phase-transfer catalysts with dual functions for highly enantioselective epoxidation of α,β-unsaturated ketones. J. Am. Chem. Soc. 126, 6844–6845 (2004).
Wang, W., Mei, Y., Li, H. & Wang, J. A novel pyrrolidine imide catalyzed direct formation of α,β-unsaturated ketones from unmodified ketones and aldehydes. Org. Lett. 7, 601–604 (2005).
Umezawa, T., Seino, T. & Matsuda, F. Novel one-pot three-component coupling reaction with trimethylsilylmethyl-phosphonate, acyl fluoride, and aldehyde through the Horner–Wadsworth–Emmons reaction. Org. Lett. 14, 4206–4209 (2012).
Hirao, T. Synthetic strategy: palladium-catalyzed dehydrogenation of carbonyl compounds. J. Org. Chem. 84, 1687–1692 (2019).
Diao, T. & Stahl, S. S. Synthesis of cyclic enones via direct palladium-catalyzed aerobic dehydrogenation of ketones. J. Am. Chem. Soc. 133, 14566–14569 (2011).
Zhang, S., Neumann, H. & Beller, M. Synthesis of α,β-unsaturated carbonyl compounds by carbonylation reactions. Chem. Soc. Rev. 49, 3187–3210 (2020).
Zhang, S., Neumann, H. & Beller, M. Pd-catalyzed synthesis of α,β-unsaturated ketones by carbonylation of vinyl triflates and nonaflates. Chem. Commun. 55, 5938–5941 (2019).
Gao, D.-W. et al. Cascade CuH-catalysed conversion of alkynes into enantioenriched 1,1-disubstituted products. Nat. Catal. 3, 23–29 (2020).
Derosa, J., Tran, V. T., Boulous, M. N., Chen, J. S. & Engle, K. M. Nickel-catalyzed β,γ-dicarbofunctionalization of alkenyl carbonyl compounds via conjunctive cross-coupling. J. Am. Chem. Soc. 139, 10657–10660 (2017).
Kim, W. G. et al. Nickel-catalyzed azide–alkyne cycloaddition to access 1,5-disubstituted 1,2,3-triazoles in air and water. J. Am. Chem. Soc. 139, 12121–12124 (2017).
Zhang, L. et al. Catalytic conjunctive cross-coupling enabled by metal-induced metallate rearrangement. Science 351, 70–74 (2016).
Li, L. & Herzon, S. B. Temporal separation of catalytic activities allows anti-Markovnikov reductive functionalization of terminal alkynes. Nat. Chem. 6, 22–27 (2014).
Mondal, A., Hazra, R., Grover, J., Raghu, M. & Ramasastry, S. S. V. Organophosphine-catalyzed intramolecular hydroacylation of activated alkynes. ACS Catal. 8, 2748–2753 (2018).
Vedachalam, S. et al. N‐Heterocyclic carbene catalyzed intramolecular hydroacylation of activated alkynes: synthesis of chromones. Adv. Synth. Catal. 353, 219–225 (2011).
Biju, A. T., Wurz, N. E. & Glorius, F. N-Heterocyclic carbene-catalyzed cascade reaction involving the hydroacylation of unactivated alkynes. J. Am. Chem. Soc. 132, 5970–5971 (2010).
Coxon, T. J. et al. Exploiting carbonyl groups to control intermolecular rhodium-catalyzed alkene and alkyne hydroacylation. J. Am. Chem. Soc. 139, 10142–10149 (2017).
Ghosh, A., Johnson, K. F., Vickerman, K. L., Walker, J. A. & Standley, L. M. Recent advances in transition metal-catalysed hydroacylation of alkenes and alkynes. Org. Chem. Front. 3, 639–644 (2016).
Neuhaus, J. D., Morrow, S. M., Brunavs, M. & Willis, M. C. Diversely substituted quinolines via rhodium-catalyzed alkyne hydroacylation. Org. Lett. 18, 1562–1565 (2016).
Castaing, M., Wason, S. L., Estepa, B., Hooper, J. F. & Willis, M. C. 2-Aminobenzaldehydes as versatile substrates for rhodium-catalyzed alkyne hydroacylation: application to dihydroquinolone synthesis. Angew. Chem. Int. Ed. 52, 13280–13283 (2013).
Willis, M. C. Transition metal catalyzed alkene and alkyne hydroacylation. Chem. Rev. 110, 725–748 (2010).
Jun, C.-H., Lee, H., Hong, J.-B. & Kwon, B.-I. Efficient and selective hydroacylation of 1-alkynes with aldehydes by a chelation-assisted catalytic system. Angew. Chem. Int. Ed. 41, 2146–2147 (2002).
Zhou, Y., Bandar, J. S. & Buchwald, S. L. Enantioselective CuH-catalyzed hydroacylation employing unsaturated carboxylic acids as aldehyde surrogates. J. Am. Chem. Soc. 139, 8126–8129 (2017).
Ociepa, M., Baka, O., Narodowiec, J. & Gryko, D. Light-driven vitamin B12-catalyzed generation of acyl radicals from 2-S-pyridyl thioesters. Adv. Synth. Catal. 359, 3560–3565 (2017).
Chen, Q.-A., Kim, D. K. & Dong, V. M. Regioselective hydroacylation of 1,3-dienes by cobalt catalysis. J. Am. Chem. Soc. 136, 3772–3775 (2014).
Leung, J. C. & Krische, M. J. Catalytic intermolecular hydroacylation of C–C π-bonds in the absence of chelation assistance. Chem. Sci. 3, 2202–2209 (2012).
Omura, S., Fukuyama, T., Horiguchi, J., Murakami, Y. & Ryu, I. Ruthenium hydride-catalyzed addition of aldehydes to dienes leading to β,γ-unsaturated ketones. J. Am. Chem. Soc. 130, 14094–14095 (2008).
Wang, J., Cary, B. P., Beyer, P. D., Gellman, S. H. & Weix, D. J. Ketones from nickel-catalyzed decarboxylative, non-symmetric cross-electrophile coupling of carboxylic acid esters. Angew. Chem. Int. Ed. 58, 12081–12085 (2019).
Ai, Y., Ye, N., Wang, O., Yahata, K. & Kishi, Y. Zirconium/nickel‐mediated one‐pot ketone synthesis. Angew. Chem. Int. Ed. 56, 10791–10795 (2017).
Wotal, A. C. & Weix, D. J. Synthesis of functionalized dialkyl ketones from carboxylic acid derivatives and alkyl halides. Org. Lett. 14, 1476–1479 (2012).
Onaka, M., Matsuoka, Y. & Mukaiyama, T. A convenient method for the direct preparation of ketones from 2-(6-(2-methoxyethyl)pyridyl)carboxylates and alkyl iodides by use of zinc dust and a catalytic amount of nickel dichloride. Chem. Lett. 10, 531–534 (1981).
Kumar, V. P., Babu, V. S., Yahata, K. & Kishi, Y. Fe/Cu-mediated one-pot ketone synthesis. Org. Lett. 19, 2766–2769 (2017).
Zhang, Y. & Rovis, T. A unique catalyst effects the rapid room-temperature cross-coupling of organozinc reagents with carboxylic acid fluorides, chlorides, anhydrides, and thioesters. J. Am. Chem. Soc. 126, 15964–15965 (1980).
Goto, T., Onaka, M. & Mukaiyama, T. A new method for the preparation of symmetrical aromatic ketones from aromatic carboxylic acids. Chem. Lett. 9, 51–52 (1980).
Onaka, M., Matsuoka, Y. & Mukaiyama, T. Reductive coupling of S-(2-pyridyl) aliphatic thioates by use of bis(1,5-cyclooctadiene)nickel. Chem. Lett. 9, 905–906 (1980).
Iqbal, N., Iqbal, N., Maiti, D. & Cho, E. J. Access to multifunctionalized benzofurans by aryl nickelation of alkynes: efficient synthesis of the anti‐arrhythmic drug amiodarone. Angew. Chem. Int. Ed. 58, 15808–15812 (2019).
Barber, E. R. et al. Nickel-catalyzed hydroarylation of alkynes under reductive conditions with aryl bromides and water. J. Org. Chem. 84, 11612–11622 (2019).
Zhang, X., Xie, X. & Liu, Y. Nickel-catalyzed highly regioselective hydrocyanation of terminal alkynes with Zn(CN)2 using water as the hydrogen source. J. Am. Chem. Soc. 140, 7385–7389 (2018).
Clarke, C., Incerti-Pradillos, C. A. & Lam, H. W. Enantioselective nickel-catalyzed anti-carbometallative cyclizations of alkynyl electrophiles enabled by reversible alkenylnickel E/Z isomerization. J. Am. Chem. Soc. 138, 8068–8071 (2016).
Chatterjee, B. & Gunanathan, C. The ruthenium-catalysed selective synthesis of mono-deuterated terminal alkynes. Chem. Commun. 52, 4509–4512 (2016).
Arndt, M. et al. Mechanistic investigation of the Ru-catalyzed hydroamidation of terminal alkynes. J. Am. Chem. Soc. 133, 7428–7449 (2011).
Richmond, E. & Moran, S. Recent advances in nickel catalysis enabled by stoichiometric metallic reducing agents. Synthesis 50, 499–513 (2018).
Huihui, K. M. M. et al. Decarboxylative cross-electrophile coupling of N-hydroxyphthalimide esters with aryl iodides. J. Am. Chem. Soc. 138, 5016–5019 (2016).
Erdelmeier, I. et al. Cross-coupling reaction of alkenyl sulfoximines and alkenyl aminosulfoxonium salts with organozincs by dual nickel catalysis and Lewis acid promotion. Chem. Eur. J. 25, 8371–8386 (2019).
Jia, X. G., Guo, P., Duan, J. & Shu, X.-Z. Dual nickel and Lewis acid catalysis for cross-electrophile coupling: the allylation of aryl halides with allylic alcohols. Chem. Sci. 9, 640–645 (2018).
Everson, D. A. & Weix, D. J. Cross-electrophile coupling: principles of reactivity and selectivity. J. Org. Chem. 79, 4793–4798 (2014).
Acknowledgements
The authors are grateful for the financial support provided by the Electronics and Telecommunications Research Institute (ETRI) grant funded by the Korean government (20ZB1200, development of ICT materials, components and equipment technologies), and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2020R1A5A1019631). W.C. acknowledges Pohang Accelerator Laboratory for 2D beamline use (2020-3rd-2D-012). S.M. is grateful for the financial support from the Institute of Basic Science (IBS-R022-D1) Korea.
Author information
Authors and Affiliations
Contributions
J.H.R., S.M., K.M., and S.Y.H. conceived the project. J.H.R., S.M., J.W.L., I.A.B., and H.S.L. prepared substrates and examined the catalytic reactions. S.L. and W.C. solved single-crystal X-ray structures of compounds 27 and 66. J.H.R., J.P., S.J.K., Y.S.K., and J.K.S. worked on analytical sections for the mechanistic and deuterium labeling experiments. J.H.R., S.M., J.W.L., and S.Y.H. wrote the manuscript. All authors discussed results and provided input on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rhlee, J.H., Maiti, S., Lee, J.W. et al. Synthesis of α,β-unsaturated ketones through nickel-catalysed aldehyde-free hydroacylation of alkynes. Commun Chem 5, 13 (2022). https://doi.org/10.1038/s42004-022-00633-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-022-00633-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.