Abstract
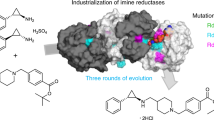
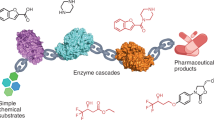
Enzymatic reductive amination, being a direct, selective and green methodology, has attracted significant interest in a short period of time and is emerging as a powerful tool for the synthesis of chiral alkylated amines. The discovery of an increasing number of imine reductases with reductive aminase (RedAm) activity has enabled mechanistic and substrate profiling studies. However, their potential for commercial applications has not been realized. Here, we report the discovery of RedAm activity in an imine reductase enzyme for the direct reductive amination of a cyclic ketone with methylamine. We also investigate engineering the enzyme to access a cis-cyclobutyl-N-methylamine for the manufacturing of a late-stage drug candidate, Janus kinase 1 (JAK1) inhibitor abrocitinib. The engineered enzyme, SpRedAm-R3-V6, showed >200-fold improvement in performance over the wild-type enzyme and was successfully used to develop a commercial manufacturing process with 73% isolated yield at 99.5% purity and high selectivity (>99:1 cis:trans). This process has been successfully used to manufacture multi-metric tons of the amine, demonstrating the potential of RedAm technology for commercial manufacturing.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Additional data supporting the findings reported in this paper are available as Supplementary Information. All other data are available from the authors upon reasonable request.
References
Nugent, T. C. & El‐Shazly, M. Chiral amine synthesis–recent developments and trends for enamide reduction, reductive amination, and imine reduction. Adv. Synth. Catal. 352, 753–819 (2010).
Afanasyev, O. I., Kuchuk, E., Usanov, D. L. & Chusov, D. Reductive amination in the synthesis of pharmaceuticals. Chem. Rev. 119, 11857–11911 (2019).
Topczewski, J. J., Cabrera, P. J., Saper, N. I. & Sanford, M. S. Palladium-catalysed transannular C–H functionalization of alicyclic amines. Nature 531, 220–224 (2016).
Verzijl, G. K. et al. Asymmetric synthesis of a key intermediate for tofacitinib via a dynamic kinetic resolution-reductive amination protocol. Org. Process Res. Dev. 22, 1817–1822 (2018).
Koszelewski, D., Tauber, K., Faber, K. & Kroutil, W. ω-Transaminases for the synthesis of non-racemic α-chiral primary amines. Trends Biotechnol. 28, 324–332 (2010).
Gomm, A. & O’Reilly, E. Transaminases for chiral amine synthesis. Curr. Opin. Chem. Biol. 43, 106–112 (2018).
Hughes, D. L. Biocatalysis in drug development—highlights of the recent patent literature. Org. Process Res. Dev. 22, 1063–1080 (2018).
Abrahamson, M. J., Wong, J. W. & Bommarius, A. S. The evolution of an amine dehydrogenase biocatalyst for the asymmetric production of chiral amines. Adv. Synth. Catal. 355, 1780–1786 (2013).
Abrahamson, M. J., Vázquez‐Figueroa, E., Woodall, N. B., Moore, J. C. & Bommarius, A. S. Development of an amine dehydrogenase for synthesis of chiral amines. Angew. Chem. Int. Ed. 51, 3969–3972 (2012).
Bornscheuer, U. et al. Engineering the third wave of biocatalysis. Nature 485, 185–194 (2012).
Wu, S. et al. Biocatalysis: enzymatic synthesis for industrial applications. Angew. Chem. Int. Ed. 60, 88–119 (2021).
Bornscheuer, U. T. The fourth wave of biocatalysis is approaching. Philos. Trans. R. Soc. A 376, 20170063 (2018).
Devine, P. N. et al. Extending the application of biocatalysis to meet the challenges of drug development. Nat. Rev. 2, 409–421 (2018).
Midelfort, K. S. et al. Redesigning and characterizing the substrate specificity and activity of Vibrio fluvialis aminotransferase for the synthesis of imagabalin. Protein Eng. Des. Sel. 26, 25–33 (2013).
Savile, C. K. et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science 329, 305–309 (2010).
Chen, H. et al. Engineered imine reductases and methods for the reductive amination of ketone and amine compounds. US patent 9803224B2 (2017)..
Scheller, P. N. et al. Enzyme toolbox: novel enantiocomplementary imine reductases. Chembiochem. 15, 2201–2204 (2014).
Li, H., Luan, Z. J., Zheng, G. W. & Xu, J. H. J. A. S. Efficient synthesis of chiral indolines using an imine reductase from Paenibacillus lactis. Adv. Synth. Catal. 357, 1692–1696 (2015).
Wetzl, D. et al. Expanding the imine reductase toolbox by exploring the bacterial protein‐sequence space. Chembiochem. 16, 1749–1756 (2015).
France, S. P. et al. Biocatalytic routes to enantiomerically enriched dibenz[c,e]azepines. Angew. Chem. Int. Ed. 56, 15589–15593 (2017).
Lenz, M., Borlinghaus, N., Weinmann, L. & Nestl, B. M. Recent advances in imine reductase-catalyzed reactions. World J. Microbiol. Biotechnol. 33, 199 (2017).
Mangas-Sanchez, J. et al. Imine reductases (IREDs). Curr. Opin. Chem. Biol. 37, 19–25 (2017).
Wetzl, D. et al. Asymmetric reductive amination of ketones catalyzed by imine reductases. ChemCatChem 8, 2023–2026 (2016).
Aleku, G. A. et al. A reductive aminase from Aspergillus oryzae. Nat. Chem. 9, 961–969 (2017).
France, S. P. et al. Identification of novel bacterial members of the imine reductase enzyme family that perform reductive amination. ChemCatChem 10, 510–514 (2018).
Sharma, M. et al. A mechanism for reductive amination catalyzed by fungal reductive aminases. ACS Catal. 8, 11534–11541 (2018).
Roiban, G. D. et al. Efficient biocatalytic reductive aminations by extending the imine reductase toolbox. ChemCatChem 9, 4475–4479 (2017).
Schober, M. et al. Chiral synthesis of LSD1 inhibitor GSK2879552 enabled by directed evolution of an imine reductase. Nat. Catal. 2, 909–915 (2019).
Vazquez, M. L. et al. Identification of N-{cis-3-[methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclobutyl} propane-1-sulfonamide (PF-04965842): a selective JAK1 clinical candidate for the treatment of autoimmune diseases. J. Med. Chem. 61, 1130–1152 (2018).
Marshall, J. R. et al. Screening and characterization of a diverse panel of metagenomic imine reductases for biocatalystic reductive amination. Nat. Chem. 13, 140–140 (2021).
Matzel, P. et al. Synthesis of β-chiral amines by dynamic kinetic resolution of α-branched aldehydes appling imine reductases. ChemCatChem 11, 4281–4285 (2019).
Matzel, P. et al. Photometric characterization of the reductive amination scope of the imine reductases from Streptomyces tsukubensis and Streptomyces ipomoease. Chembiochem. 18, 2022–2027 (2017).
Xia, G. et al. Novel process for preparing Pramipexole and its optical isomeric mixture by reduction with sodium triacetoxyborohydride. WO patent WO2006070349 (2006).
Patil, P., Pansare, P., Jagtap, A. & Krishnamurthy, D. An improved process for the preparation of pramipexole dihydrochloride monohydrate. WO patent WO 2015155704A1 (2015).
Reetz, M. T. & Carballeira, J. D. Iterative saturation mutagenesis (ISM) for rapid directed evolution of functional enzymes. Nat. Protoc. 2, 891 (2007).
Bommarius, A. S., Blum, J. K. & Abrahamson, M. J. Status of protein engineering for biocatalysts: how to design an industrially useful biocatalyst. Curr. Opin. Chem. Biol. 15, 194–200 (2011).
Lu, X., Hora, B., Cai, F. & Gao, F. Generation of random mutant libraries with multiple primers in a single reaction. J. Virol. Methods 167, 146–151 (2010).
Acknowledgements
We would like to thank Pfizer leadership L. Handanyan, S. Caron, N. Thomson, J. Nelson and C. McWilliams for their support for the work and publication. We would also like to thank R. Lewis and S. France for proof-reading the manuscript.
Author information
Authors and Affiliations
Contributions
R.K., M.J.K., J.S., M.P.B. and C.A.M. contributed to enzyme engineering and enzyme process development work. R.K., E.L.M., N.M.D., C.G.C. and E.C. performed process engineering and process development. D.B., L.A.C., K.M., D.M. and S.H. ran the manufacturing scale reactions. C.A.L., K.M.D., R.P. and J.W. provided analysis, sourcing support and participated in discussion. R.K., M.J.K. and J.S. wrote the paper and the other co-authors provided information and feedback.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks John Woodley and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Notes 1 and 2, Figs. 1–4 and Tables 1–4.
Rights and permissions
About this article
Cite this article
Kumar, R., Karmilowicz, M.J., Burke, D. et al. Biocatalytic reductive amination from discovery to commercial manufacturing applied to abrocitinib JAK1 inhibitor. Nat Catal 4, 775–782 (2021). https://doi.org/10.1038/s41929-021-00671-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-021-00671-5
This article is cited by
-
Transaminase-catalysis to produce trans-4-substituted cyclohexane-1-amines including a key intermediate towards cariprazine
Communications Chemistry (2024)
-
Engineered enzymes for the synthesis of pharmaceuticals and other high-value products
Nature Synthesis (2024)
-
Synthesis and clinical application of new drugs approved by FDA in 2022
Molecular Biomedicine (2023)
-
Catalytic 4-exo-dig carbocyclization for the construction of furan-fused cyclobutanones and synthetic applications
Nature Communications (2023)
-
Actinomycetes-derived imine reductases with a preference towards bulky amine substrates
Communications Chemistry (2022)