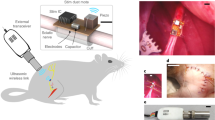
Abstract
Multichannel electrophysiological sensors and stimulators—particularly those used to study the nervous system—are usually based on monolithic microelectrode arrays. However, the architecture of such arrays limits flexibility in electrode placement and scaling to a large number of nodes, especially across non-contiguous locations. Here we report wirelessly networked and powered electronic microchips that can autonomously perform neural sensing and electrical microstimulation. The microchips, which we term neurograins, have an ~1 GHz electromagnetic transcutaneous link to an external telecom hub, providing bidirectional communication and control at the individual device level. To illustrate the potential of the approach, we show that 48 neurograins can be individually addressed on a rat cortical surface and used for the acute recording of neural activity. Theoretical calculations and experimental measurements show that the link configuration could potentially be scaled to 770 neurograins using a customized time-division multiple access protocol.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The dataset that supports the plots within this paper and other findings of this study are available in the Supplementary Information and from the corresponding author upon reasonable request.
Code availability
Custom-developed MATLAB codes for demodulation are available in the Supplementary Information and from the corresponding author upon reasonable request.
References
Hochberg, L. R. et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442, 164–171 (2006).
Afshar, A. et al. Single-trial neural correlates of arm movement preparation. Neuron 71, 555–564 (2011).
Truccolo, W., Friehs, G. M., Donoghue, J. P. & Hochberg, L. R. Primary motor cortex tuning to intended movement kinematics in humans with tetraplegia. J. Neurosci. 28, 1163–1178 (2008).
Nurmikko, A. V. et al. Listening to brain microcircuits for interfacing with external world—progress in wireless implantable microelectronic neuroengineering devices. Proc. IEEE 98, 375–388 (2010).
Rubehn, B., Bosman, C., Oostenveld, R., Fries, P. & Stieglitz, T. A MEMS-based flexible multichannel ECoG-electrode array. J. Neural Eng. 6, 036003 (2009).
Pistohl, T., Ball, T., Schulze-Bonhage, A., Aertsen, A. & Mehring, C. Prediction of arm movement trajectories from ECoG-recordings in humans. J. Neurosci. Methods 167, 105–114 (2008).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Musk, E. et al. An integrated brain-machine interface platform with thousands of channels. J. Med. Internet Res. 21, e16194 (2019).
Miranda, H., Gilja, V., Chestek, C. A., Shenoy, K. V. & Meng, T. H. HermesD: a high-rate long-range wireless transmission system for simultaneous multichannel neural recording applications. IEEE Trans. Biomed. Circuits Syst. 4, 181–191 (2010).
Borton, D. A., Yin, M., Aceros, J. & Nurmikko, A. An implantable wireless neural interface for recording cortical circuit dynamics in moving primates. J. Neural Eng. 10, 026010 (2013).
Yin, M., Borton, D. A., Aceros, J., Patterson, W. R. & Nurmikko, A. V. A 100-channel hermetically sealed implantable device for chronic wireless neurosensing applications. IEEE Trans. Biomed. Circuits Syst. 7, 115–128 (2013).
Simeral, J. D. et al. Home use of a wireless intracortical brain-computer interface by individuals with tetraplegia. IEEE Trans. Biomed. Eng. 68, 2313–2325 (2021).
Song, Y.-K. et al. Active microelectronic neurosensor arrays for implantable brain communication interfaces. IEEE Trans. Neural Syst. Rehabil. Eng. 17, 339–345 (2009).
Zanos, S., Richardson, A. G., Shupe, L., Miles, F. P. & Fetz, E. E. The Neurochip-2: an autonomous head-fixed computer for recording and stimulating in freely behaving monkeys. IEEE Trans. Neural Syst. Rehabil. Eng. 19, 427–435 (2011).
Liu, X. et al. The PennBMBI: design of a general purpose wireless brain-machine-brain interface system. IEEE Trans. Biomed. Circuits Syst. 9, 248–258 (2015).
Zhou, A. et al. A wireless and artefact-free 128-channel neuromodulation device for closed-loop stimulation and recording in non-human primates. Nat. Biomed. Eng. 3, 15–26 (2019).
Seo, D., Carmena, J. M., Rabaey, J. M., Maharbiz, M. M. & Alon, E. Model validation of untethered, ultrasonic neural dust motes for cortical recording. J. Neurosci. Methods 244, 114–122 (2015).
Seo, D. et al. Wireless recording in the peripheral nervous system with ultrasonic neural dust. Neuron 91, 529–539 (2016).
Piech, D. K. et al. A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nat. Biomed. Eng. 4, 207–222 (2020).
Jia, Y. et al. A mm-sized free-floating wirelessly powered implantable optical stimulation device. IEEE Trans. Biomed. Circuits Syst. 13, 608–618 (2019).
Jia, Y. et al. A dual-band wireless power transmission system for evaluating mm-sized implants. IEEE Trans. Biomed. Circuits Syst. 13, 595–607 (2019).
Gutruf, P. et al. Fully implantable optoelectronic systems for battery-free, multimodal operation in neuroscience research. Nat. Electron. 1, 652–660 (2018).
Ho, J. S. et al. Wireless power transfer to deep-tissue microimplants. Proc. Natl Acad. Sci. USA 111, 7974–7979 (2014).
Khalifa, A. et al. The microbead: a 0.009 mm3 implantable wireless neural stimulator. IEEE Trans. Biomed. Circuits Syst. 13, 971–985 (2019).
Yeon, P., Bakir, M. S. & Ghovanloo, M. Towards a 1.1 mm2 free-floating wireless implantable neural recording SoC. In 2018 IEEE Custom Integrated Circuits Conference (CICC) 1–4 (IEEE, 2018).
Agrawal, D. R. et al. Conformal phased surfaces for wireless powering of bioelectronic microdevices. Nat. Biomed. Eng. 1, 0043 (2017).
Lee, J. et al. An implantable wireless network of distributed microscale sensors for neural applications. In 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER) 871–874 (IEEE, 2019).
Lee, J. et al. Wireless power and data link for ensembles of sub-mm scale implantable sensors near 1Ghz. In 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS) 1–4 (IEEE, 2018).
Leung, V. W. et al. Distributed microscale brain implants with wireless power transfer and Mbps bi-directional networked communications. In 2019 IEEE Custom Integrated Circuits Conference (CICC) 1–4 (IEEE, 2019).
Laiwalla, F. et al. A distributed wireless network of implantable sub-mm cortical microstimulators for brain-computer interfaces. In 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 6876–6879 (IEEE, 2019).
Ha, S. et al. Silicon-integrated high-density electrocortical interfaces. Proc. IEEE 105, 11–33 (2016).
Ahmadi, N. et al. Towards a distributed, chronically-implantable neural interface. In 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER) 719–724 (IEEE, 2019).
Spilker, J. J. Jr. Digital Communications by Satellite (Prentice-Hall, 1977).
Ahn, D. & Ghovanloo, M. Optimal design of wireless power transmission links for millimeter-sized biomedical implants. IEEE Trans. Biomed. Circuits Syst. 10, 125–137 (2015).
Feng, P., Maslik, M. & Constandinou, T. G. EM-lens enhanced power transfer and multi-node data transmission for implantable medical devices. In 2019 IEEE Biomedical Circuits and Systems Conference (BioCAS) 1–4 (IEEE, 2019).
Theilmann, P. T., Presti, C. D., Kelly, D. J. & Asbeck, P. M. A µW complementary bridge rectifier with near zero turn-on voltage in SOS CMOS for wireless power supplies. IEEE Trans. Circuits Syst. I, Reg. Papers 59, 2111–2124 (2012).
Leung, V. W. et al. A CMOS distributed sensor system for high-density wireless neural implants for brain-machine interfaces. In ESSCIRC 2018—IEEE 44th European Solid State Circuits Conference (ESSCIRC) 230–233 (IEEE, 2018).
Lee, A.-H. et al. A scalable and low stress post-CMOS processing technique for implantable microsensors. Micromachines 11, 925 (2020).
Yang, K., Dong, Q., Blaauw, D. & Sylvester, D. 8.3 A 553F2 2-transistor amplifier-based physically unclonable function (PUF) with 1.67% native instability. In 2017 IEEE International Solid-State Circuits Conference (ISSCC) 146–147 (IEEE, 2017).
Suh, G. E. & Devadas, S. Physical unclonable functions for device authentication and secret key generation. In 2007 44th ACM/IEEE Design Automation Conference 9–14 (IEEE, 2007).
Huang, J. et al. A 0.01-mm2 mostly digital capacitor-less AFE for distributed autonomous neural sensor nodes. IEEE Solid-State Circuits Lett. 1, 162–165 (2018).
Jeong, J. et al. Conformal hermetic sealing of wireless microelectronic implantable chiplets by multilayered atomic layer deposition (ALD). Adv. Funct. Mater. 29, 1806440 (2019).
Steriade, M., Contreras, D., Amzica, F. & Timofeev, I. Synchronization of fast (30–40 Hz) spontaneous oscillations in intrathalamic and thalamocortical networks. J. Neurosci. 16, 2788–2808 (1996).
Brindley, G. S. & Lewin, W. S. The sensations produced by electrical stimulation of the visual cortex. J. Physiol. 196, 479–493 (1968).
Kim, S. et al. Behavioral assessment of sensitivity to intracortical microstimulation of primate somatosensory cortex. Proc. Natl Acad. Sci. USA 112, 15202–15207 (2015).
Fi´ath, R. et al. Large-scale recording of thalamocortical circuits: in vivo electrophysiology with the two-dimensional electronic depth control silicon probe. J. Neurophysiol. 116, 2312–2330 (2016).
Determining the peak spatial-average specific absorption rate (SAR) in the human body from wireless communications devices, 30 MHz to 6 GHz—part 3: specific requirements for using the finite difference time domain (FDTD) method for SAR calculations of mobile phones. IEC/IEEE 62704-3:2017 1–76 (2017).
IEEE standard for safety levels with respect to human exposure to radio frequency electromagnetic fields, 3 kHz to 300 GHz. IEEE Std C95.1-2005 (Revision of IEEE Std C95.1-1991) 1–238 (2006).
Shilimkar, V. S., Gaskill, S. G. & Weisshaar, A. Experimental characterization of metal fill placement and size impact on spiral inductors. In 2009 IEEE 18th Conference on Electrical Performance of Electronic Packaging and Systems 101–104 (IEEE, 2009).
Sigurdsson, S. A., Yu, Z., Lee, J. & Nurmikko, A. A method for large-scale implantation of 3D microdevice ensembles into brain and soft tissue. Microsyst. Nanoeng. 6, 97 (2020).
Kiani, M., Jow, U.-M. & Ghovanloo, M. Design and optimization of a 3-coil inductive link for efficient wireless power transmission. IEEE Trans. Biomed. Circuits Syst. 5, 579–591 (2011).
Finkenzeller, K. RFID Handbook: Fundamentals and Applications in Contactless Smart Cards, Radio Frequency Identification and Near-Field Communication (John Wiley & Sons, 2010).
Jeong, J. et al. A miniaturized, eye-conformable, and long-term reliable retinal prosthesis using monolithic fabrication of liquid crystal polymer (LCP). IEEE Trans. Biomed. Eng. 62, 982–989 (2014).
Cai, H. et al. A software-defined radio for wireless brain implants network. In Proc. 24th Annual International Conference on Mobile Computing and Networking 852–854 (Association for Computing Machinery, 2018).
Acknowledgements
We thank Y.-K. Song for insights into microfabrication and ASIC design, J. Jeong for his expertise with neurograin hermetic sealing and materials processing, and R. Rao for guidance on wireless networking design. We also thank C. Kilfoyle, E. Mok, S. Sigurdsson, and the Animal Care facility at Brown University for their contributions. We also acknowledge S. Li, S. Yu, L. Cui, S. Alluri and M. Lokhandwala at UCSD for their work on ASIC design and benchtop test. We have greatly benefited from the insight of our colleagues across multiple fields from microelectronics to brain sciences and clinical neurology: J. Donoghue, B. Dutta, J. Groe, L. Hochberg, T. Sejnowsky, K. Shenoy and S. Cash, among others. This research was initially supported by Defense Advanced Research Projects Agency N66001-17-C-4013, with subsequent support from private gifts. The post-processing work benefited from the equipment funded by MRI award no. DMR-1827453.
Author information
Authors and Affiliations
Contributions
A.N. conceived the project with P.A., P.P.M. and L.L. J.L. and A.N. designed the neural experiment concept. J.L. designed the RF wireless powering and data communication method, performed system characterization and in vivo experiments, and analysed the results. V.L., P.A., S.S. and L.L. designed the RF data communication protocol. V.L., J.H., F.L. and P.P.M. designed the neurograin ASICs. A.-H.L. performed microfabrication and packaging. J.L., V.L., F.L. and A.N. wrote the paper. All the authors commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Electronics thanks Nitish Thakor, Samantha Santacruz and Jonathan Viventi for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–16 and Tables 1 and 2.
Supplementary Data 1
MATLAB codes for the neurograin BPSK dataset and demodulation.
Rights and permissions
About this article
Cite this article
Lee, J., Leung, V., Lee, AH. et al. Neural recording and stimulation using wireless networks of microimplants. Nat Electron 4, 604–614 (2021). https://doi.org/10.1038/s41928-021-00631-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41928-021-00631-8
This article is cited by
-
Machine learning-based high-frequency neuronal spike reconstruction from low-frequency and low-sampling-rate recordings
Nature Communications (2024)
-
An asynchronous wireless network for capturing event-driven data from large populations of autonomous sensors
Nature Electronics (2024)
-
Wireless agents for brain recording and stimulation modalities
Bioelectronic Medicine (2023)
-
An interface connects
Nature Electronics (2023)
-
Nanomaterial-based microelectrode arrays for in vitro bidirectional brain–computer interfaces: a review
Microsystems & Nanoengineering (2023)