Abstract
The introduction of fish skin as a biological dressing for treating burns and wounds holds great promise, offering an alternative to existing management strategies. However, the risk of disease transmission is a significant concern. Therefore, this study aimed to examine how established sterilization and preservation procedures affected fish skin grafts' microbiological and histological properties for long-term usage. Lyophilization of the fish skin graft followed by rehydration in normal saline for 15 min did not change the collagen content. Furthermore, gamma irradiation of the lyophilized fish skin graft at different lengths 5, 10, and 25 KGy showed a significant reduction in microbial growth (aerobic bacteria, aerobic yeasts, and fungi) at 15- and 30 days after the irradiation. However, exposure to 10 KGy was found to be the most effective intensity among the different gamma irradiation lengths since it preserved the collagen fiber content and intensity in the lyophilized fish skin grafts at 15- and 30 days after the irradiation. These findings provide efficient preservation and sterilization methods for long-term usage of the fresh Tilapia skin grafts used for biological dressings.
Similar content being viewed by others
Introduction
Fish skin has been used as a biological dressing in the management of burns1,2,3,4,5,6,7,8,9 and wounds10. It has also been used as a biological graft in neovaginoplasty in cases of vaginal agenesis11,12. The histological characteristics of fish skin and human skin are similar13. The fish skin contains a high amount of collagen, making it a suitable biomaterial for tissue engineering9,13,14.
Fish skin perishability still poses the most enormous preservation difficulties15. It must be kept chilled or frozen, and even then, it has a very short shelf life16. Several diverse damage mechanisms, including microbiological spoilage, autolytic degradation, and lipid oxidation, are responsible for the depreciating of fresh fish skin during storage17. Therefore, most clinical practices have used fish skin grafts in fresh form for wound and burn management1,3,5,6,7,8,9,10.
Lyophilization, known also as freeze drying or cryodesiccation, is a low-temperature dehydration process involving freezing the product, lowering the pressure, then removing the ice by sublimation. This is in contrast to dehydration by most conventional methods use heat to evaporate water18.
Lyophilization can be a potential method for the long-term storage of fish skin grafts. In a previous study, lyophilization was addressed as a method for preserving fish skin grafts2. However, applying several detergents and sterilizing agents (e.g., chlorhexidine) to fish skin before lyophilization raises concerns about the integrity of collagen and the targeted material in the fish skin2,9,10,13. Moreover, the histopathology and microbiology of these lyophilized fish skin grafts were not evaluated before being used clinically.
The microbial colonization of the wound is an essential factor that affects the healing process of wounds19. Different sterilization methods have been described for biological dressings. These include physical methods such as irradiation and chemical techniques including treatment with chlorhexidine, povidone iodine, ethylene oxide gas, silver nanoparticles, and ozone10,13,20. Gamma irradiation represents an effective sterilization method as it has direct and indirect effects on microbial DNA21. Different lengths of Gamma irradiation have been used to sterilize fish skin (25, 30, and 50 kGy) without reference to its impact on collagen content1,2,13 or antimicrobial efficiency2,9.
The current study’s objectives are (1): to describe the optimized method of Tilapia skin lyophilization and (2): to define the standard length of gamma irradiation for sterilization of lyophilized Tilapia skin with special consideration to collagen integrity and microbial count of fish skin.
Materials and methods
Ethics statement
The Research Ethics Committee (REC) of the Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt, has approved all the procedures in this study in accordance with the Egyptian bylaws and OIE animal welfare standards for animal care and use in research and education. All methods were performed in accordance with relevant guidelines and regulations.
Fish skin sampling
The fish skin was collected from fresh Nile tilapia (Oreochromis niloticus) (weigh: 620 ± 35 gm; standard length: 20 ± 3 cm), obtained from The Aquatic Medicine Unit, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt. Fish were euthanized physically by decapitation. After removal the fish scales, the skin was dissected from the underlying tissue, cut into strips (6 × 2 cm), and then washed in sterile normal saline. Lyophilization was performed on skin strips.
Fish skin lyophilization
Lyophilization was carried out in a freeze dryer (LYOQUEST, SPAIN, Serial No.: 1812, manufacture company: Telstar). Fresh fish skin strips were placed in the flasks of the freeze dryer apparatus at − 80 °C for 24 h. Skin strips were then subjected to a cold vacuum for 5–6 h. Lyophilized skin strips were then vacuum-packed (Fig. 1A–C).
Gama (γ) irradiation sterilization of lyophilized fish skin
Gamma irradiation was performed at a gamma station (60Co gamma cell (2000 Ci), 30 ± 5 °C, 1.5 Gy/s, 150 rad/s), the National Center for Radiation Research and Technology (NCRRT) of the Egyptian Atomic Energy Authority, Cairo, Egypt. The vacuum-packed lyophilized Tilapia skin was subjected to Cobalt 60 γ sterilization at 5, 10, and 25 kGy2. Sterilized skin strips subjected to microbiological and histopathological evaluations after each treatment 15- and 30-days post-sterilization (3 skin strips each).
Microbiological evaluation
Following Morton et al.22, the dilution plate method was employed to count the lyophilized fish skin microbiology under different lengths of gamma irradiation (0, 5, 10, and 25 KGy) after 15 and 30 days of irradiation. Fish skin strips were swabbed with a sterilized swab needle and suspended in 10 mL of sterile saline solution. For microbiological counting and identification, various media types were employed; nutrient agar and MacConkey agar media to count all aerobic bacteria, yeast malt extract medium (YME) to count the aerobic yeasts, and potato dextrose agar medium (PDA) to count the fungi23,24,25. One milliliter (1 mL) of the saline-swabbed solution was added to sterilized Petri dishes before the dishes were filled with sterilized isolation medium, three replicates were performed for each medium, and left to solidify. For bacteria, plates were incubated for 24 h at 35 °C, for yeasts, for 72 h at 28 °C, and for fungi, 7 days at 28 °C. The developed colony count was estimated as CFU/cm2. Using the same media, developed colonies with variations in their morphological characteristics, such as size, color, colony edge, and pigmentation, were sub-cultured and purified for identification using Bergey’s Manual of Systematic Bacteriology26, Yeasts: characteristics and identification24 and fungal identification27.
Histological evaluation
Fish skin strips (0.5 × 0.5 cm) were fixed in 10% neutral buffered formalin, routinely processed, and subsequently embedded in paraffin. Afterwards, they were sectioned into 5 μm thick sections and stained with Mayer’s hematoxylin (Merck, Darmstadt, Germany) and eosin (Sigma, Missouri, USA). After microscopically examining the slides, histological evaluations were performed blindly on coded samples, with a comparison to control group. Collagen fibers integrity and organization were assessed histologically based on 0–3 scale10,28,29. Collagen fibers integrity scores: 0 = continue, long fiber, 1 = slightly fragmented, 2 = moderately fragmented, 3 = severely fragmented. Collagen fibers organization scores: 0 = compact and parallel, 1 = slightly loose and wave, 2 = moderately loose, wavy and cross to each other, 3 = no identifiable pattern.
Histochemical evaluation
The collagen content evaluation was carried out by the Gomori’s trichrome stain30. After deparaffinization in xylene, paraffin-embedded sections were and rehydrated in a graded series of ethanol solutions (into 0.1 M phosphate-buffered saline (PBS), pH 7.2) to distilled water. Sections were stained with Gomori’s trichrome according to the manufacturer’s protocol, dehydrated in graded alcohol, made transparent with xylene, and mounted. Slides were then microscopically examined to verify collagen staining with green. The collagen content was evaluated based on the depth of the green staining.
The percentage of collagen-positive area was calculated by ImageJ (1.48v) using threshold area fraction determination. The amount of collagen was expressed as a percentage from the total number of pixels in the optical view as a percentage and expressed as mean ± SEM.
Statistical analysis
One-way ANOVA followed by Tukey's post hoc test was performed using GraphPad Prism software version 8.0.1 (GraphPad Software Inc., La Jolla, CA, USA). P values < 0.05 were considered statistically significant.
Results
Microbiological evaluation of the lyophilized fish skin after gamma irradiation
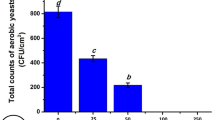
Gamma irradiation exhibited an efficient sterilizing effect on fish skin surface microbiota. Different lengths of gamma irradiation (5, 10, and 25 KGy) were applied to the lyophilized fish skin. The microbial counts of aerobic bacteria, aerobic yeasts, and fungi were detected 15- and 30- days after the irradiation as cleared in Fig. 2A–C. It was clear that gamma irradiation is a great microbial sterilizer, especially with the high length of 25 KGy that inhibited the aerobic bacterial counts significantly by 98.53% and 98.96%, aerobic yeast counts by 99.2% and 99.8%, and fungal counts by 98.48% and 99.25% 15- and 30- days after irradiation, respectively, (P < 0.05). Gamma irradiation also maintained the low microbial skin counts for a longer period (30 days) and remarkably at the low length of 5 KGy, gamma irradiation was effective against aerobic bacteria.
By increasing the gamma irradiation, the total counts of aerobic bacteria decrease dramatically giving 92.67 ± 2.62 (88.36% inhibition), 39.33 ± 4.92 (95.06% inhibition), and 11.67 ± 1.7 (98.53% inhibition) at 5, 10, and 25 KGy 15 days post-irradiation, respectively, (P < 0.05). At 30 days post-irradiation, the bacterial counts were 74.33 ± 2.49 (88.93% inhibition), 32 ± 5.7 (95.24% inhibition), and 7 ± 1.6 (98.96% inhibition) at 5, 10, and 25 KGy, respectively, compared to the untreated sample (796 ± 9.93 and 671.7 ± 7.4) 15- and 30-days after irradiation, respectively, (P < 0.05).
For yeasts, it was clear that by increasing the length of gamma irradiation, the total counts of yeasts decreased significantly after 15- and 30-days irradiation giving 208.67 ± 6.13 (55.25% inhibition), 19 ± 1.63 (95.93% inhibition), and 3.67 ± 0.47 (99.2% inhibition) at 5, 10, and 25 KGy, respectively, at 15 days (P < 0.05). At 30 days, the yeast counts were 104.67 ± 4.9 (76.21% inhibition), 8.7 ± 1.2 (98.03% inhibition), and 1 ± 0 (99.77% inhibition) at 5, 10, and 25 KGy, respectively, compared to the untreated sample (466.3 ± 2.87 and 440 ± 5.72) 15- and 30-days after irradiation, respectively, (P < 0.05).
Filamentous fungi had the same criteria as bacteria and yeasts, the total counts of fungi decreased significantly (P < 0.05) with increasing the length of gamma irradiation giving 95.7 ± 4.9 (45.54% inhibition), 34.7 ± 3.7 (80.27% inhibition), and 2.67 ± 0.4, (98.48% inhibition) at 5, 10, and 25 KGy, respectively, after 15 days. After 30 days, the yeast counts were 55.67 ± 4.9 (68.49% inhibition), 15.7 ± 3.3 (91.13% inhibition), and 1.3 ± 0.1 (99.24% inhibition) at 5, 10, and 25 KGy, respectively, compared to the untreated sample (175.7 ± 3.7 and 176.67 ± 3.8) 15- and 30-days after irradiation, respectively.
By investigating the microbial species present on the fish skin, Bacillus sp., Escherichia coli, Micrococcus luteus, and Serratia marcescens were the dominant aerobic bacteria, Candida sp., Saccharomyces sp. and Rhodotorula sp. were the dominant aerobic yeasts, whereas Aspergillus niger, A. flavus, A. fumigatus, and Rhizopus stolonifer were the dominant aerobic fungi.
Impact of lyophilization on fish skin
In the control group, fresh fish skin was examined histologically, and the collagen fibers were tightly packed, well-organized, parallel-distributed, and no evidence of disaggregation (Fig. 3A,B). In addition, the lyophilized fish skin rehydrated in normal saline for 15 min showed the preservation of the collagen fibers in a well-organized pattern with no signs of disaggregation (Fig. 3C,D).
Histological evaluation of the lyophilized fish skin after gamma irradiation
In the lyophilized fish skin subjected to gamma irradiation at 5, 10, and 25 kGy, histological examination of the fish skin 15-day post-sterilization revealed slightly to moderately disorganized and disaggregated in irradiated skin at 5 and 25 kGy (Figs. 4A,E, 6A,B) (P < 0.05). However, the collagen fibers were well organized in a parallel pattern at 10 kGy (Fig. 4C). At 30-days post-sterilization, the collagen fiber structure did not change in the irradiated skin at 5 and 10 kGy (Figs. 5A,C, 6A,B) (P < 0.05), with disorganization and disaggregation were more prominent at 25 kGy (Figs. 5E, 6A,B) (P < 0.05).
Histological and histochemical evaluations of the lyophilized Tilapia fish skin submitted to different irradiation dosages 15-days post-sterilization. Hematoxylin and eosin stained sections were irradiated at 5 (A), 10 (C), and 25 (E) kGy. (B), (D), and (F) Gomori’s trichrome stained sections for collagen. The scale bars = 100 μm.
Histological and histochemical evaluations of the lyophilized Tilapia fish skin submitted to different irradiation dosages 30-days post-sterilization. Hematoxylin and eosin-stained sections were irradiated at 5 (A), 10 (C), and 25 (E) kGy. (B), (D), and (F) Gomori’s trichrome stained sections for collagen. The scale bars = 100 μm.
Evaluation of the collagen integrity, organization, and intensity in the lyophilized Tilapia fish skin submitted to different irradiation dosages. Collagen integrity and organization were evaluated based on a 0–3 scale as described in the materials and methods. The collagen intensity was quantified using ImageJ. The results are expressed as a percentage of the total number of pixels and are normalized to the control-treated group. Differences were evaluated using one-way ANOVA. *p < 0.05; **p < 0.001; ***p < 0.0001.
Histochemical evaluation of the fish skin
The collagen fibers were also stained with Gomori’s trichrome stain and the collagen intensity in the skin was measured using ImageJ (1.48v). At 15- and 30-days post-sterilization, the collagen disposition and intensity in skin samples irradiated at 5 and 10 kGy did not show significant differences compared with the control (Figs. 4B,D, 5B,D, 6C). However, collagen deposition and intensity were significantly decreased in skin samples exposed to 25 kGy after 15 and 30 days of irradiation (Figs. 4F, 5F, 6C) (P < 0.0001).
Discussion
Recently, biological dressings have become indispensable in modern strategies of burn and wound management. The current study investigated the use of lyophilization as a method for the long-term preservation of fish skin grafts in a manner that maintains the integrity of fish skin collagen. Moreover, the study revealed that gamma irradiation at a length of 10 KGy is optimal for sterilizing the lyophilized fish skin grafts without disrupting the collagen content.
Autologous skin grafts have a limited availability and add an additional scaring31. Allografts have many limitations such as the finding of suitable donors and the risk of infection transmission, especially, viral infections20. Therefore, various xenografts have been described as biological wound dressings in humans. This includes the bovine embryo skin32, bovine amnion33, canine skin34, frog skin35, and porcine skin36,37,38. However, there are concerns about their clinical application due to immunological causes, zoonotic risk, and religious beliefs20,39,40.
The fish skin was found to be consistent with human skin8,13 and has a higher collagen content than other skins9,13,14, which is a potential wound healing promotor41. Moreover, fish skin has advantages such as low antigenicity, a high potential adherent to the skin, a lower risk of transmitting diseases, and a degree of moisture symbolizing the human skin8,42. Fish skin graft is highly porous. Since the pore size is within the range of typical cell size, fish skin is well suited to support cell ingrowth43. Therefore, the fish skin has recently been suggested as a potential xenograft1,2,3,4,5,6,7,9,10,11,12.
Although the tilapia skin contains a normal non-infectious microbiota, it may be exposed to microbial contaminants from a contaminated water environment7,44. Bacteria, yeasts, fungi, and viruses represent serious fish contaminants45. Enterobacteriaceae, yeast, and aerobic spoilers are the main spoilage microorganisms of fish during the storage46. Due to the high-water activity, and low acidity in the fish skin, bacteria proliferate quickly causing its spoilage47. Therefore, in most studies, Tilapia skin was used as a biological dressing graft for burn or wound treatment shortly after its processing1,3,4,5,6,7,9,10. This creates an urgent need to find a way for long-term preservation and storage of the fish skin grafts.
Various methods have been used for the preservation of different biological dressings, such as cryopreservation48,49 and glycerolization4,5,7,9,50,51,52. Although these methods could prolong the shelf life of biological dressings, they may also affect their effectiveness53.
Lyophilization or freeze-drying is a specific dehydration process, which provides benefits to the final product through improved composition stability and reduced water content to low levels, which allow the cell to survive during long storage18,54. Microbial cells need high stabilization processes to survive on the lyophilized parts. Otherwise, they will be damaged and/or die55.
Lyophilization has been addressed in previous studies for fish skin preservation2,3. However, using a rigorous process of chemical sterilization of the fish skin before the lyophilization raises concerns about collagen integrity and other biological components potential for healing43, especially, since these studies were not followed by histological evaluation before clinical application1,2,7. Moreover, using several sterilizing steps with several chemicals make such procedures cost-effective and impractical for industrial application. Chlorhexidine is commonly used to sterilize and disinfect grafting procedures. However, many bacterial spores or mycobacteria are chlorhexidine-resistant56,57. It also has a low activity against viruses. A potential limitation of chlorhexidine is its cytotoxicity and alteration in the biochemical properties of collagen10,58.
Here, this study describes the lyophilization of fish skin without using any disinfectants during the processing of fish skin grafts to ensure the collagen safety and subsequently its effectiveness. This was confirmed by histological and histochemical evaluations of the lyophilized fish skin after rehydration in normal saline for 15 min that there was no change in the arrangement and content of the collagen fibers of the fish skin.
Sterilization of fish skin with gamma irradiation represents an efficient sterilizing method for the fish skin microbiota. High-energy gamma irradiations are released by several radioisotopes, including the comparatively cheap byproducts of nuclear fission Caesium-137 (137Cs) and Cobalt-60 (60Co). By subjecting the plentiful, non-radioactive Co-59 isotope to neutron irradiation inside a nuclear reactor, radioactive Co is created. Then, after emitting one electron and two gamma rays with energies of 1.17 MeV and 1.33 MeV, Co-60 atoms decompose into nonradioactive Ni-60 atoms. Gamma rays don't have enough energy to cause radioactivity in other materials because they are released isotropically. Similar to x-rays, gamma rays have a shorter wavelength and higher energy. Thus, Gamma rays are appealing for industrial sterilization of materials with a significant thickness or volume, such as packaged food, medical equipment, or medical supplies59.
The combination of lyophilization and gamma irradiation inhibited the microbial growth by 99.8%. Gram-negative and Gram-positive bacteria, as proteobacteria and the Lactobacillales, are more vulnerable to Gamma irradiation than spore-forming bacteria, such as Bacilli and Clostridia60. This is in accordance with our findings that Bacillus sp., Escherichia coli, Micrococcus luteus, and Serratia marcescens were the dominant aerobic bacteria. Similar findings were recorded by Dharmarha et al.61, who found that Gamma irradiation decreased Pseudomonas sp., Escherichia coli, and Yersinia sp. total counts highly than spore-forming bacteria. Gamma irradiation was found to have an effect on the microbial DNA by altering its composition21. It reacts with water molecules forming free radicals damage DNA and breakage the DNA double strand with non-repaired effects causing microbial cell death62.
An infection is deemed to exist when there are more than 105 CFU of bacteria per gram of skin graft63. On wound beds with more than 105 bacteria/g of tissue, the absorption of a skin graft is decreased64. In our results, the total bacterial counts were less than 35 CFU/cm2 at 10 and 25 kGy after 30 days of sterilization, indicating the high sterilizing efficiency of these irradiations; however, 10 kGy is preferred as it maintains the collagen integrity and content. Also, the total counts of yeasts and fungi were less than 10 and 16 CFU/cm2, respectively, at 10 and 25 kGy after 30 days. In accordance with our results, clinical investigations found that skin transplant failure occurred in wounds that were significantly infected with 107 pathogens65,66. Also, Nsaful et al.67 found that wound graft failure mainly occurs by the microbial contamination, especially bacterial contamination with the bacteria counts ranged from 3.7 × 105 to 9 × 105 CFU/cm2.
In accordance, histological evaluation of the fish skin grafts revealed that the exposure to gamma irradiation at 25 kGy caused the collagen fibers to dissociate and disintegrate with a reduction in the collagen intensity, whereas exposure at 5 kGy altered collagen fiber arrangement and integrity. However, the exposure to gamma irradiation at 10 kGy showed the preferred intensity since it preserved the collagen fiber content and intensity in the skin graft.
The limitation of this study is the lack of histological and microbiological assessment of the sterilized vacuum-packed lyophilized fish skin grafts on further long period (6 and 12 months). Therefore, future studies should be conducted to address this concern.
Conclusion
This study established an optimized method for tilapia skin lyophilization. It defined the standard length of gamma irradiation for sterilizing lyophilized tilapia skin, considering the collagen integrity and microbial count of fish skin. These processed, sterilized, vacuum-packed, lyophilized fish skin patches could be suitable for long-term storage for burn and wound management in hospitals and medical centers. Further clinical studies are still being conducted on this product.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Costa, B. A. et al. Use of Tilapia skin as a Xenograft for pediatric burn treatment: A case report. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 40(5), 714–717 (2019).
Júnior, E. M. L. et al. Lyophilised tilapia skin as a xenograft for superficial partial thickness burns: A novel preparation and storage technique. J. Wound Care 29(10), 598–602 (2020).
Lima Júnior, E. M. et al. A randomized comparison study of lyophilized Nile tilapia skin and silver-impregnated sodium carboxymethylcellulose for the treatment of superficial partial-thickness burns. J. Burn Care Res. 42(1), 41–48 (2021).
Lima Junior, E. M. et al. Nile tilapia fish skin-based wound dressing improves pain and treatment-related costs of superficial partial-thickness burns: A phase III randomized controlled trial. Plast. Reconstr. Surg. 147(5), 1189–1198 (2021).
Lima Júnior, E. M. et al. Innovative burn treatment using tilapia skin as a xenograft: A phase II randomized controlled trial. J. Burn Care Res. 41(3), 585–592 (2020).
Lima Júnior, E. M. et al. Pediatric burn treatment using tilapia skin as a xenograft for superficial partial-thickness wounds: A pilot study. J. Burn Care Res. 41(2), 241–247 (2020).
Lima-Junior, E. M. et al. Innovative treatment using tilapia skin as a xenograft for partial thickness burns after a gunpowder explosion. J. Surg. Rep. 6, 181 (2019).
Lordello, A., Bianco, A. & Ribas, A. Leather of the tilapia, how healing in the treatment of burns. Nurse Care Open Access J. 6(4), 147–153 (2019).
Meq, L. V. et al. Nile tilapia skin (Oreochromis niloticus) for burn treatment: Ultrastructural analysis and quantitative assessment of collagen. Acta Histochem. 123(6), 151762–151762 (2021).
Ibrahim, A., Hassan, D., Kelany, N., Kotb, S. & Soliman, M. Validation of three different sterilization methods of Tilapia skin dressing: Impact on microbiological enumeration and collagen content. Front. Vet. Sci. 7, 597751 (2020).
Dias, M. T. P. M. et al. Neovaginoplasty using Nile Tilapia fish skin as a new biologic graft in patients with Mayer–Rokitansky–Küster–Hauser syndrome. J. Minim. Invasive Gynecol. 27(4), 966–972 (2020).
Dias, M. T. P. M. et al. Tilapia fish skin as a new biologic graft for neovaginoplasty in Mayer–Rokitansky–Kuster–Hauser syndrome: A video case report. Fertil. Steril. 112(1), 174–176 (2019).
Alves, A. P. N. N. et al. Study of tensiometric properties, microbiological and collagen content in Nile tilapia skin submitted to different sterilization methods. Cell Tissue Bank. 19(3), 373–382 (2018).
Tang, J. & Saito, T. Biocompatibility of novel type I collagen purified from tilapia fish scale: an in vitro comparative study. BioMed Res. Int. https://doi.org/10.1155/2015/139476 (2015).
Prabhakar, P. K., Vatsa, S., Srivastav, P. P. & Pathak, S. S. A comprehensive review on freshness of fish and assessment: Analytical methods and recent innovations. Food Res. Int. 133, 109157 (2020).
Ježek, F. & Buchtová, H. Physical and chemical changes in fresh chilled muscle tissue of common carp (Cyprinus carpio L.) packed in a modified atmosphere. Acta Vet. Brno 76(8), 83–92 (2007).
Ghaly, A. E., Dave, D., Budge, S. & Brooks, M. Fish spoilage mechanisms and preservation techniques. Am. J. Appl. Sci. 7(7), 859 (2010).
Haseley, P. & Oetjen, G.-W. Freeze-Drying (Wiley, 2017).
Mihai, M. M. et al. Nanocoatings for chronic wound repair—Modulation of microbial colonization and biofilm formation. Int. J. Mol. Sci. 19(4), 1179 (2018).
Chiu, T. & Burd, A. “Xenograft” dressing in the treatment of burns. Clin. Dermatol. 23(4), 419–423 (2005).
Manas, P. & Pagán, R. Microbial inactivation by new technologies of food preservation. J. Appl. Microbiol. 98(6), 1387–1399 (2005).
Morton, R. D. Aerobic plate count, compendium of methods for the microbiological examination of foods 4, 63–67 (2001)
Atlas, R. Handbook of Microbiology Media (CRC Press, 1993).
Barnett, J. A., Payne, R. W. & Yarrow, D. Yeasts: Characteristics and Identification (Cambridge University Press, 1990).
Leslie, J. F. & Summerell, B. A. The Fusarium Laboratory Manual (Wiley, 2008).
Bergey, D. H. Bergey’s Manual of Determinative Bacteriology (Lippincott Williams & Wilkins, 1994).
Moubasher, A. Soil Fungi in Qatar and Other Arab Countries (University of Qatar, 1993).
Chen, J. et al. Autologous tenocyte therapy for experimental Achilles tendinopathy in a rabbit model. Tissue Eng. Part A 17(15–16), 2037–2048 (2011).
Farfaras, S. et al. More histologic and ultrastructural degenerative signs in the subscapularis tendon and the joint capsule in male patients with shoulder impingement. Knee Surg. Sports Traumatol. Arthrosc. 26(1), 79–87 (2018).
GoMori, G. A rapid one-step trichrome stain. Am. J. Clin. Pathol. 20(7_ts), 661–664 (1950).
Gajiwala, K. & Gajiwala, A. L. Evaluation of lyophilised, gamma-irradiated amnion as a biological dressing. Cell Tissue Bank. 5(2), 73–80 (2004).
Rogers, B. O. & Converse, J. M. Bovine embryo skin zoografts as temporary biologic dressings for burns and other skin defects. Plast. Reconstr. Surg. 22(5), 471–485 (1958).
Zhu, Z., Xu, G. & Zhao, J. Clinical study on application of bovine amnion on burn wounds. Chin. J. Repar. Reconstr. Surg. 20(7), 735–738 (2006).
Switzek, W. E., Moncrief, J. A., Mills, W. Jr. & Lindberg, R. B. The use of canine heterografts in the therapy of thermal injury. J. Trauma Acute Care Surg. 6(3), 391–398 (1966).
Sarto Piccolo, N., Sarto Piccolo, M. & Sarto Piccolo, M. The Use of Frogskin as a Biological Dressing for Temporary Cover of Burn Wounds. Innovations in Plastic and Aesthetic Surgery 129–137 (Springer, 2008).
Chiu, T. & Shah, M. Porcin xenograft dressing for facial burns: Beware of the mesh imprint. Burns 28(3), 279–282 (2002).
Davis, D. A. & Arpey, C. J. Porcine heterografts in dermatologic surgery and reconstruction. Dermatol. Surg. 26(1), 76–80 (2000).
Haller, H. L. et al. Porcine xenograft and epidermal fully synthetic skin substitutes in the treatment of partial-thickness burns: A literature review. Medicina 57(5), 432 (2021).
Denner, J. Porcine endogenous retroviruses and xenotransplantation, 2021. Viruses 13(11), 2156 (2021).
Weiss, R. A. Xenografts and retroviruses. Science 285(5431), 1221–1222 (1999).
Rangaraj, A., Harding, K. & Leaper, D. Role of collagen in wound management. Wounds uk 7(2), 54–63 (2011).
Yamamoto, K. et al. Biological safety of fish (tilapia) collagen. BioMed Res. Int. 1, 1. https://doi.org/10.1155/2014/630757 (2014).
Magnusson, S., Baldursson, B. T., Kjartansson, H., Rolfsson, O. & Sigurjonsson, G. F. Regenerative and antibacterial properties of acellular fish skin grafts and human amnion/chorion membrane: Implications for tissue preservation in combat casualty care. Mil. Med. 182(suppl_1), 383–388 (2017).
Seo, S. B. et al. Silver nanoparticles enhance wound healing in zebrafish (Danio rerio). Fish Shellfish Immunol. 68, 536–545 (2017).
Mahmoud, G.A.-E., Osman, Y. A. & Abdel-Hakeem, S. S. Hydrolytic bacteria associated with natural helminth infection in the midgut of Red Sea marbled spinefoot rabbit fish Siganus rivulatus. Microb. Pathog. 147, 104404 (2020).
Gram, L. & Huss, H. H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 33(1), 121–137 (1996).
Tavares, J. et al. Fresh fish degradation and advances in preservation using physical emerging technologies. Foods 10(4), 780 (2021).
Cleland, H. et al. Clinical application and viability of cryopreserved cadaveric skin allografts in severe burn: A retrospective analysis. Burns 40(1), 61–66 (2014).
Eldad, A. et al. Cryopreserved cadaveric allografts for treatment of unexcised partial thickness flame burns: Clinical experience with 12 patients. Burns 23(7–8), 608–614 (1997).
Guerrero, L. & Camacho, B. Comparison of different skin preservation methods with gamma irradiation. Burns 43(4), 804–811 (2017).
Iyun, A. O. et al. Glycerolised skin allografts for extensive burns in low-and middle-income countries. J. West Afr. Coll. Surg. 11(3), 35 (2021).
Zidan, S. M. & Eleowa, S. A. Banking and use of glycerol preserved full-thickness skin allograft harvested from body contouring procedures. Burns 40(4), 641–647 (2014).
Pruitt, B. A. Jr. The evolutionary development of biologic dressings and skin substitutes. J. Burn Care Rehabil. 18(suppl_1_pt_2), S2–S5 (1997).
Fissore, D. & Pisano, R. Computer-aided framework for the design of freeze-drying cycles: Optimization of the operating conditions of the primary drying stage. Processes 3(2), 406–421 (2015).
Quintana, G., Gerbino, E. & Gómez-Zavaglia, A. Okara: A nutritionally valuable by-product able to stabilize Lactobacillus plantarum during freeze-drying, spray-drying, and storage. Front. Microbiol. 8, 641 (2017).
Gomes, B. et al. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int. Endod. J. 34(6), 424–428 (2001).
Weber, D. J., Rutala, W. A. & Sickbert-Bennett, E. E. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob. Agents Chemother. 51(12), 4217–4224 (2007).
Alomar, A. Z., Gawri, R., Roughley, P. J., Haglund, L. & Burman, M. The effects of chlorhexidine graft decontamination on tendon graft collagen and cell viability. Am. J. Sports Med. 40(7), 1646–1653 (2012).
Willey, J. M., Sherwood, L. & Woolverton, C. J. Prescott’s Microbiology (McGraw-Hill, 2011).
Monk, J. D., Beuchat, L. R. & Doyle, M. P. Irradiation inactivation of food-borne microorganisms. J. Food Prot. 58(2), 197–208 (1995).
Dharmarha, V. et al. Gamma irradiation influences the survival and regrowth of antibiotic-resistant bacteria and antibiotic-resistance genes on romaine lettuce. Front. Microbiol. 10, 710 (2019).
Hall, E. J. & Giaccia, A. J. Radiobiology for the Radiologist (Lippincott Williams and Wilkins, 2006).
Bowler, P., Duerden, B. & Armstrong, D. G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 14(2), 244–269 (2001).
Rubin, J. P. & Neligan, P. C. Plastic Surgery. Aesthetic Surgery Vol. 2 (Elsevier, 2017).
Bacchetta, C. A., Magee, W., Rodeheaver, G., Edgerton, M. T. & Edlich, R. F. Biology of infections of split thickness skin grafts. Am. J. Surg. 130(1), 63–67 (1975).
Geethabanu, S. & Vanaja, R. A study to analyse the influence of bacterial bio-burden on the success rate of split thickness skin grafting. J. Clin. Diagn. Res. 12(6), 23 (2018).
Nsaful, K. O., Paintsil, A. B., Dakubo, J. C., Nsaful, J., Appiah-Labi, K., Nartey, E. An evaluation of bacterial infection of split thickness skin grafts: At the Korle Bu Teaching Hospital. LAP LAMBERT Academic Publishing (2020).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A.I.: Conceptualization, Methodology, Validation, Data curation, Visualization, Investigation, Supervision, Writing- Original draft preparation, Writing- Reviewing and Editing. H.F.: Conceptualization, Methodology, Data curation, Investigation, Writing- Original draft preparation. G.A.-E.M.: Methodology, Visualization, Investigation, Writing- Original draft preparation. A.E.: Methodology, Validation, Data curation, Investigation, Writing- Original draft preparation. M.S.: Methodology, Validation, Data curation, Software, Visualization, Investigation, Supervision, Writing- Original draft preparation, Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, A., Fahmy, H.M., Mahmoud, G.AE. et al. New strategies for sterilization and preservation of fresh fish skin grafts. Sci Rep 14, 1253 (2024). https://doi.org/10.1038/s41598-024-51608-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51608-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.