Abstract
The impact of bariatric surgery on metabolic and inflammatory status are reflected in the epigenetic profile and telomere length mediated by the changes in the metabolic status of the patients. This study compared the telomere length of children born before versus after maternal bariatric surgery as a surrogate to test the influence of the mother’s metabolic status on children’s telomere length. DNA methylation telomere length (DNAmTL) was estimated from Methylation-EPIC BeadChip array data from a total of 24 children born before and after maternal bariatric surgery in the greater Quebec City area. DNAmTL was inversely associated with chronological age in children (r = − 0.80, p < 0.001) and significant differences were observed on age-adjusted DNAmTL between children born before versus after the maternal bariatric surgery. The associations found between body mass index and body fat percentage with DNAmTL in children born after the surgery were influenced by maternal triglycerides, TG/HDL-C ratio and TyG index. This study reports the impact of maternal bariatric surgery on offspring telomere length. The influence of maternal metabolic status on the association between telomere length and markers of adiposity in children suggests a putative modulating effect of bariatric surgery on the cardiometabolic risk in offspring.
Similar content being viewed by others
Introduction
Obesity is a complex chronic disorder involving many different factors such as education, eating habits and physical activity, as well as genetic and epigenetic factors and their interactions1. Among these interactions, sedentary behaviour and the consumption of energy-dense foods promote the development of obesity through the modulation of epigenetic factors in genes related to metabolic homeostasis and aging2. The rising rates of obesity worldwide, together with the improvement and decreasing costs of surgical procedures, is driving the increase in bariatric surgery for body weight management3. To date, bariatric surgery is the most effective treatment for severe obesity in the long-term and is associated with nutritional changes having a beneficial impact on the metabolic and inflammatory status of patients4.
The recent development of clinical epigenetics has accelerated the analysis of the relationship between cardiometabolic risk and epigenetic factors5. In this regard, recent studies have explored the association between the cardiometabolic health improvement observed following bariatric surgery and DNA methylation profiles in blood and target metabolic tissues. These results suggested that the blood epigenetic profile reflects, at least partially, the metabolic changes induced after bariatric surgery in individuals with severe obesity6, 7. In this regard, recent meta-analyses suggested that epigenetic modifications could explain the association between body weight loss and cardiometabolic benefits8, 9. In addition to the link between the improvements of the cardiometabolic profile and the differential methylation marks related with regulatory cell pathways, a recent study also reported a reduction in epigenetic age acceleration after bariatric surgery in patients with severe obesity10.
Telomere length is one of the most studied factors associated with cellular lifespan, aging and the development of age-related diseases11. Despite evidence linking telomere length reduction to increased adiposity in adults12 and children13, results regarding the effects of bariatric surgery on telomere shortening are still inconsistent14. Moreover, it has been suggested that perinatal factors playing a key role in offspring telomere length are also influenced by maternal epigenetics15. The increase in bariatric procedures in women of childbearing age is encouraging the study of the effects of these surgeries on developmental markers16, 17, but the impact on epigenetic factors in children born after the surgery remain unknown or poorly studied18. Nevertheless, recent studies reported an association between maternal overweight, but not paternal, with shorter telomeres in offspring, highlighting the importance of maternal weight management for a healthy aging of children19, 20.
In the present study, we compared an epigenetic estimate of telomere length of children born before versus after a maternal bariatric surgery, and we analyzed the influence of the metabolic status of the mother at the time of delivery on telomere shortening.
Results
A total of 24 children, 12 born before and 12 born after their mother’s bariatric surgery, were eligible for the present study, after excluding individuals with missing information (Supplementary Fig. 1). Differences in anthropometric variables of mothers before and after the surgery are presented in Table 1. The age of the mothers was 27.1 years and 34.2 years at the delivery of the children before and after the bariatric surgery respectively. The time between the surgery and the birth of offspring (data not shown) varied from 0.6 to 11.0 years for the children born before and from 1.2 to 9.4 for those born after the surgery. Significant decreases in body weight (125.8 ± 4.5 to 80.6 ± 16.5 kg; p < 0.001) and body fatness indices (body mass index (BMI): 48.0 ± 7.7 to 31.0 ± 6.9 kg/m2, p < 0.001; waist circumference: 136.6 ± 13.5 to 100.9 ± 18.7 cm, p < 0.001; and waist-height ratio: 0.85 ± 0.09 to 0.63 ± 0.12, p = 0.001) were observed after the surgery. Body weight loss was accompanied by a significant improvement of the plasma lipid profile. Specifically, significant reductions in total-cholesterol (total-C; 4.6 ± 0.9 to 3.8 ± 0.6 mmol/l; p = 0.04), LDL-cholesterol (LDL-C; 2.6 ± 0.8 to 1.8 ± 0.5 mmol/l; p = 0.01), and triglyceride levels (TG; 1.8 ± 0.8 to 1.2 ± 0.5 mmol/l; p = 0.05) were observed, together with an increase in HDL-cholesterol levels (HDL-C; 1.1 ± 0.3 to 1.5 ± 0.5 mmol/l; p = 0.04). No significant reduction in plasma glucose levels was observed after the surgery (5.21 ± 1.61 to 4.60 ± 0.39 mmol/l; p = 0.25). Metabolic indices also exhibited a reduction of LDL-C/HDL-C ratio (2.51 ± 1.24 to 1.32 ± 0.68, p = 0.01), Non-HDL-C/HDL-C ratio (3.36 ± 1.72 to 1.74 ± 0.97 p = 0.011), TG/HDL-C ratio (1.78 ± 1.08 to 0.93 ± 0.67 p = 0.04), and triglyceride-glucose (TyG) index (8.82 ± 0.31 to 8.31 ± 0.39, p = 0.004).
Significant differences were only observed for age (19.8 ± 7.1 vs 12.8 ± 7.1 years old; p = 0.03) in children born before versus after the maternal surgery (Table 2). No significant differences of body fatness indices were found between the offspring before versus after the maternal bariatric surgery.
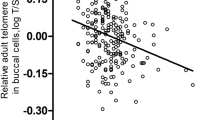
In children, an inverse association between chronological age and telomere length, estimated from DNA methylation (DNAmTL), was observed in the overall group (r = − 0.80, p < 0.001), independently of the maternal bariatric surgery (Fig. 1A). Residuals for the regression of DNAmTL by chronological age were estimated to test for differences in telomere length between birth groups. Significant differences were found for the age-adjusted DNAmTL between children born before versus after the maternal bariatric surgery (Δ = 0.1, p = 0.04) (Fig. 1B).
DNAmTL association with chronological age and differences in children born before versus after the maternal bariatric surgery. (A) Correlation between chronological age and DNAmTL by group. Pearson’s r and p-value for correlation between chronological age and DNAmTL are shown. (B) Differences of age-adjusted DNAmTL in blood samples of children born before versus after the maternal bariatric surgery.
Additionally, the interaction term between children’s characteristics and birth groups was added to the linear mixed model to further explore the effect of clinical parameters on DNAmTL differences (Fig. 2). Significant differences on DNAmTL were maintained after adjustment for birth height (p = 0.03), BMI z-score (p = 0.02) and waist-height ratio (p = 0.03). A trend was still observed for differences in birth weight (p = 0.06) and body fat percentage (p = 0.09) between birth groups.
Finally, a complete interaction model including the interaction between birth group (before versus after the surgery), children’s adiposity markers and maternal metabolic status indices was built to analyze the impact of maternal metabolic status on DNAmTL of children (Supplementary Table 1). Maternal metabolic status indices showed significant interactions with BMI z-score and body fat percentage (Supplementary Table 1). More specifically, BMI z-score and body fat percentage showed similar change with significant interactions for group being born before versus after bariatric surgery (Fig. 3 and Supplementary Fig. 2) for triglycerides (BMI z-score: Δ = 0.21, p = 0.02; body fat percentage: Δ = 0.02, p = 0.13), TG/HDL-C ratio (BMI z-score: Δ = 0.23, p = 0.03; body fat percentage: Δ = 0.03, p = 0.06) and TyG index (BMI z-score: Δ = 0.19, p = 0.02; body fat percentage: Δ = 0.03, p = 0.03).
Modulatory effect of maternal metabolic status on the association between BMI z-score and age-adjusted DNAmTL in offspring. (A) Interaction between children’s BMI z-score and maternal triglycerides p = 0.008. Triglycerides percentiles estimated for birth group: Before: p25 = 1.30, p75 = 1.94; After: p25 = 0.85, p75 = 1.35. (B) Interaction between children’s BMI z-score and maternal TG/HDL-C ratio (Maternal TG:HDL-C) p = 0.026. TG/HDL-C percentiles estimated for birth group: Before: p25 = 0.92, p75 = 1.76; After: p25 = 0.49, p75 = 1.13. (C) Interaction between children’s BMI z-score and maternal TyG index (Maternal TyG) p = 0.009. TyG percentiles estimated for birth group: Before: p25 = 8.6, p75 = 8.9; After: p25 = 8.1, p75 = 8.5.
Discussion
The present study reports the effect of maternal bariatric surgery on telomere length in the offspring. In addition, this study hypothesized about the potential role of the maternal metabolic improvements after the bariatric surgery on the relationship between telomere shortening and adiposity in the offspring. Present findings support the impact of the maternal plasma lipid profile and insulin resistance status (assessed by TyG index) on the association between age-adjusted telomere length and adiposity markers of children.
Bariatric surgery has been associated with beneficial effects on insulin sensitivity and on the plasma lipid profile21. More specifically, an improvement of the atherogenic-related lipoprotein profile, by reducing plasma LDL-C levels and LDL particle size, together with increasing HDL-C levels, has been observed following bariatric surgery22. These benefits seem to be associated with the degree of body weight reduction achieved23 and to its long-term maintaining after surgery24, therefore reducing the cardiometabolic risk of these patients. Accordingly, in the present study, bariatric surgery was associated in mothers with an improved cardiometabolic risk profile. A recent longitudinal study carried out in twins, reported on the association between changes in plasma lipid profile and methylation levels of ABCG1, AKAP1 and SREBF1, three genes directly related with lipid metabolism25.
The development of new tools in the assessment of biological aging allowed to investigate the synchronisation between the chronological and the biological age26. Telomere shortening has been widely associated with aging and plays an important role in cellular function and senescence27. Herein, we observed that chronological age and telomere length was inversely correlated in both groups of children born before and after the bariatric surgery. In this respect, some authors have also suggested the significant impact of paternal age on TL28, which has been partially evaluated in the present study by including family features as random effects in the mixed model.
In addition to the inherent individual characteristics of age, sex and ethnicity affecting telomere length29, different modifiable factors, such as smoking habit, physical activity or drinking behaviour, has been proposed to accelerate telomere shortening30. These modifiable factors and their interactions with the genetic background are among those classically associated with an increased risk of developing cardiometabolic diseases31. Similarly, telomere length has been associated with a higher prevalence of cardiometabolic disorders, such as diabetes32, high blood pressure33 or cardiovascular events34. These findings may suggest that telomere length would play a major role on the reported association between modifiable risk factors and the development of cardiometabolic complications35. Indeed, the association between the increase of adiposity markers and telomere shortening found herein in children has been previously reported by several authors32, 36. All of this has led to the hypothesis that bariatric surgery would have a significant influence on telomere length. However, there are still some discrepancies about its actual impact37, 38. Thus, a prospective study with a 10-year follow-up after bariatric surgery suggested that there is an increase in telomere length induced by body weight loss38. Results of the present study support this hypothesis and suggest that the difference in telomere length found between children born before versus after the surgery may be also attributable to an imprinting effect of the metabolic improvements observed in mothers.
Maternal health risk factors and metabolic status during pregnancy have been proposed as determinants of epigenetic aging in the offspring, affecting both fetal growth and maturation, body size at birth and, ultimately, the future metabolic health of children39. This emphasizes the fact that changes in the environment during pregnancy would have an impact on the epigenetic age of the offspring40. Maternal circulating fatty acids, B-group vitamins, and homocysteine concentrations have been reported to be associated with an accelerated epigenetic gestational age of newborns18, 41. Furthermore, other authors have evidenced the key role of high n-3 polyunsaturated fatty acids in the maternal lipid profile during gestation with lower body fat mass and plasma TG levels, and higher HDL-C during childhood42. Consistent with these findings, our results suggest that maternal lipid markers may have an impact on telomere shortening in offspring, making children more sensitive to metabolic alterations during development. All these results pave the way for considering the use of epigenetic markers of biological age, more specifically epigenetic estimates of telomere length, as potential tool for assessing the risk of developing metabolic diseases. However, additional prospective analyses are still needed to strengthen the evidence and establish causality, in order to allow the design of decision aid tools for applications in precision medicine and precision nutrition43.
Study strengths and limitations
Although the homogeneity of ethnicity in the present study sample is an advantage that allowed us to conduct a more robust analysis of the data, future studies in this area should include a greater diversity in order to generalize these results to other ethnicities. In addition, conducting studies in children was a challenge that limited the depth of the profiling on health indicators. In this regard, future studies should also include more accurate methods for estimating adiposity in children. The close collaboration with the medical team minimized these problems and reduced potential bias in recruitment and sampling for this study. Moreover, the assessment of nutritional status in children and young adults is an area where there is still considerable room of improvement44.
Conclusion
These results suggest that there is an inherited effect of maternal bariatric surgery on offspring telomere length that may influence adiposity markers of children. In addition, this inherited effect of bariatric surgery on children’s metabolic health seems to be mediated by the maternal metabolic status.
Methods
Study population
A total of 24 children born to 11 French-Canadian women who had given birth before and after undergoing bariatric surgery in the Quebec City region were selected for the present study. The mothers had a biliopancreatic diversion with duodenal switch (BPD-DS) at the Institut universitaire de cardiologie et de pneumologie de Québec (IUCPQ) for the treatment of severe obesity (BMI > 30). Anthropometric measurements and blood sampling of mothers and children were performed at IUCPQ between August and October 2019. Adult women and the legal representative of minor children provided written informed consent for their participation. The study has been approved by the Ethics Committee of IUCPQ (2020-326421791).
Sample collection and measurements
For mothers, data were collected during the post-surgery clinical follow-up using standardized procedures. For children, a visit was scheduled to the IUCPQ during which body weight, height, waist and hip circumference were measured. Body fat percentage was estimated using bioelectrical impedance analysis (TANITA Corp, Tokyo, Japan), and blood samples were taken from children for biochemical analysis.
Body mass index (BMI) for mothers was calculated dividing weight in kilograms by the square of height in meters. BMI z-score was estimated for children using charts for individuals between 2 and 20 years old, adapted for Canada from the World Health Organization45. In order to have a consistent metric of relative weight across offspring that could be analyzed as a continuous variable, reference values for BMI z-score at 20 years old were used for children older than 20 years old (n = 6), as suggested by Must and Anderson46. An additional waist-height ratio was estimated by dividing waist circumference by the height in centimeters for both mothers and children. Lipoprotein particle atherogenic ratios were estimated for mothers as follows: total-C to HDL-C (total-C/HDL-C ratio); non-HDL-C to HDL-C (non-HDL-C/HDL-C); TG to HDL-C (TG/HDL-C ratio). The TyG index was calculated using the following formula: TyG = Ln[TG (mg/dl) × fasting glucose (mg/dl)/2]47 to assess the maternal insulin resistance status48.
Methylation DNA analysis and telomere length estimation
Blood samples from children were used to extract genomic DNA from isolated buffy coat using GenElute™ Blood Genomic DNA kit (Sigma, St Louis, MO, USA). Quality control and quantification of DNA were carried out by using NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and PicoGreen DNA methods. Isolated DNA samples were bisulfite converted to perform the quantitative genome-wide methylation analysis conducted by using the Infinium Methylation-EPIC BeadChip array (Illumina, San Diego, CA, USA) according to manufacturer’s instructions at the Centre d’expertise et de services Génome Québec (Montreal, QC, Canada). Methylation reads were preprocessed and normalized using standard protocols with the minfi R package49. Probes mapped on sex chromosomes, overlapping with known single nucleotide polymorphisms or with cross reactive regions were removed. Data quality was assessed using multi-dimensional scaling to identify technical confounding bias and association with cell type composition. Beta values were estimated as the ratio of signal intensity of the methylated alleles to the sum of methylated and unmethylated intensity signals of the alleles. Telomere length was estimated from methylation data using the methylclock R package26, that includes the algorithm proposed by Lu et al. based on the methylation levels of 140 cytosine-phosphate-guanine dinucleotides for blood samples50.
Statistical analysis
Differences in anthropometric and biochemical measurements before and after the bariatric surgery were analysed in mothers and children, using t-test and chi-square test, respectively for continuous and categorical variables. Pearson correlation coefficient between chronological age and DNAmTL was computed for children by birth group (before versus after the bariatric surgery). Due to the direct association showed between both variables, age-adjusted DNAmTL was estimated as residuals values from DNAmTL regressed by chronological age of children, this adjusted measurement was used to determine the effects of maternal surgery on the offspring removing the differences caused by aging. Linear mixed models with random effects by family (including mother id, same father and parity to avoid the effects of familiar relatedness between subjects) were performed using lme4 and emmeans R packages51. An initial model adjusted for children’s sex was used to test the differences on age-adjusted DNAmTL between children born before and after the maternal surgery. An additional interaction term was included to estimate the effect of children’s adiposity markers on the variations of the age-adjusted DNAmTL. A subsequent model was constructed to evaluate the effects of the interaction between children’s adiposity markers and the metabolic status of mothers on age-adjusted DNAmTL of children. This model was adjusted for children’s sex, age of the mother at delivery and time in years from the surgery. A p-value ≤ 0.05 was considered as significant.
Ethics approval and consent to participate
The study was approved according to the ethical standards of the Declaration of Helsinki by the Institut universitaire de cardiologie et de pneumologie de Québec ethic committee with the aprobal number: 2020-326421791. All participants provided written, informed consent.
Data availability
Datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
References
San-Cristobal, R., Navas-Carretero, S., Martinez-Gonzalez, M. A., Ordovas, J. M. & Martinez, J. A. Contribution of macronutrients to obesity: Implications for precision nutrition. Nat. Rev. Endocrinol. 16, 305–320. https://doi.org/10.1038/s41574-020-0346-8 (2020).
Welendorf, C. et al. Obesity, weight loss, and influence on telomere length: New insights for personalized nutrition. Nutrition 66, 115–121. https://doi.org/10.1016/j.nut.2019.05.002 (2019).
Alalwan, A. A. et al. US national trends in bariatric surgery: A decade of study. Surgery 170, 13–17. https://doi.org/10.1016/j.surg.2021.02.002 (2021).
Santos, J. et al. Effect of bariatric surgery on weight loss, inflammation, iron metabolism, and lipid profile. Scand. J. Surg. 103, 21–25. https://doi.org/10.1177/1457496913490467 (2014).
Izquierdo, A. G. & Crujeiras, A. B. Obesity-related epigenetic changes after bariatric surgery. Front. Endocrinol. (Lausanne) 10, 232. https://doi.org/10.3389/fendo.2019.00232 (2019).
Nicoletti, C. F. et al. DNA methylation screening after roux-en Y gastric bypass reveals the epigenetic signature stems from genes related to the surgery per se. BMC Med. Genom. 12, 72. https://doi.org/10.1186/s12920-019-0522-7 (2019).
Faenza, M., Benincasa, G., Docimo, L., Nicoletti, G. F. & Napoli, C. Clinical epigenetics and restoring of metabolic health in severely obese patients undergoing batriatric and metabolic surgery. Updates Surg. 74, 431–438. https://doi.org/10.1007/s13304-021-01162-9 (2022).
Metere, A. & Graves, C. E. Factors influencing epigenetic mechanisms: Is there a role for bariatric surgery?. High Throughput 9, 141. https://doi.org/10.3390/ht9010006 (2020).
ElGendy, K., Malcomson, F. C., Bradburn, D. M. & Mathers, J. C. Effects of bariatric surgery on DNA methylation in adults: A systematic review and meta-analysis. Surg. Obes. Relat. Dis. 16, 128–136. https://doi.org/10.1016/j.soard.2019.09.075 (2020).
Fraszczyk, E. et al. The effects of bariatric surgery on clinical profile, DNA methylation, and ageing in severely obese patients. Clin. Epigenet. 12, 14. https://doi.org/10.1186/s13148-019-0790-2 (2020).
Li, C. et al. Genome-wide association analysis in humans links nucleotide metabolism to leukocyte telomere length. Am. J. Hum. Genet. 106, 389–404. https://doi.org/10.1016/j.ajhg.2020.02.006 (2020).
Gielen, M. et al. Body mass index is negatively associated with telomere length: A collaborative cross-sectional meta-analysis of 87 observational studies. Am. J. Clin. Nutr. 108, 453–475. https://doi.org/10.1093/ajcn/nqy107 (2018).
Kahrizi, M. S. et al. Leukocyte telomere length and obesity in children and adolescents: A systematic review and meta-analysis. Front. Genet. 13, 861101. https://doi.org/10.3389/fgene.2022.861101 (2022).
Pena, E. et al. Telomere length in patients with obesity submitted to bariatric surgery: A systematic review. Eur. Eat Disord. Rev. 29, 842–853. https://doi.org/10.1002/erv.2865 (2021).
Andreu-Sanchez, S. et al. Genetic, parental and lifestyle factors influence telomere length. Commun. Biol. 5, 565. https://doi.org/10.1038/s42003-022-03521-7 (2022).
Dell’Agnolo, C. M., Carvalho, M. D. & Pelloso, S. M. Pregnancy after bariatric surgery: Implications for mother and newborn. Obes. Surg. 21, 699–706. https://doi.org/10.1007/s11695-011-0363-8 (2011).
Kwong, W., Tomlinson, G. & Feig, D. S. Maternal and neonatal outcomes after bariatric surgery; a systematic review and meta-analysis: Do the benefits outweigh the risks?. Am. J. Obstet. Gynecol. 218, 573–580. https://doi.org/10.1016/j.ajog.2018.02.003 (2018).
Monasso, G. S., Voortman, T. & Felix, J. F. Maternal plasma fatty acid patterns in mid-pregnancy and offspring epigenetic gestational age at birth. Epigenetics 1–11, 2022. https://doi.org/10.1080/15592294.2022.2076051 (2022).
Martens, D. S., Plusquin, M., Gyselaers, W., De Vivo, I. & Nawrot, T. S. Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med. 14, 148. https://doi.org/10.1186/s12916-016-0689-0 (2016).
Wei, B. et al. Maternal overweight but not paternal overweight before pregnancy is associated with shorter newborn telomere length: Evidence from Guangxi Zhuang birth cohort in China. BMC Pregn. Childbirth 21, 283. https://doi.org/10.1186/s12884-021-03757-x (2021).
Piche, M. E., Tardif, I., Auclair, A. & Poirier, P. Effects of bariatric surgery on lipid-lipoprotein profile. Metabolism 115, 154441. https://doi.org/10.1016/j.metabol.2020.154441 (2021).
Genua, I. et al. Changes in the composition and function of lipoproteins after bariatric surgery in patients with severe obesity. J. Clin. Med. 10, 8. https://doi.org/10.3390/jcm10081716 (2021).
Ooi, G. J. et al. Detailed description of change in serum cholesterol profile with incremental weight loss after restrictive bariatric surgery. Obes. Surg. 28, 1351–1362. https://doi.org/10.1007/s11695-017-3015-9 (2018).
Arnaiz, E. G. et al. Evaluation of lipoprotein profile and residual risk three years after bariatric surgery. Obes. Surg. 31, 4033–4044. https://doi.org/10.1007/s11695-021-05543-2 (2021).
Wu, Z. et al. Temporal associations between leukocytes DNA methylation and blood lipids: A longitudinal study. Clin. Epigenet. 14, 132. https://doi.org/10.1186/s13148-022-01356-x (2022).
Pelegi-Siso, D., de Prado, P., Ronkainen, J., Bustamante, M. & Gonzalez, J. R. methylclock: A Bioconductor package to estimate DNA methylation age. Bioinformatics 37, 1759–1760. https://doi.org/10.1093/bioinformatics/btaa825 (2021).
Lulkiewicz, M., Bajsert, J., Kopczynski, P., Barczak, W. & Rubis, B. Telomere length: How the length makes a difference. Mol. Biol. Rep. 47, 7181–7188. https://doi.org/10.1007/s11033-020-05551-y (2020).
De Meyer, T. et al. Paternal age at birth is an important determinant of offspring telomere length. Hum. Mol. Genet. 16, 3097–3102. https://doi.org/10.1093/hmg/ddm271 (2007).
Vyas, C. M. et al. Telomere length and its relationships with lifestyle and behavioural factors: Variations by sex and race/ethnicity. Age Ageing 50, 838–846. https://doi.org/10.1093/ageing/afaa186 (2021).
Davis, S. K., Xu, R., Khan, R. J. & Gaye, A. Modifiable mediators associated with the relationship between adiposity and leukocyte telomere length in US adults: The National Health and Nutrition Examination Survey. Prev. Med. 138, 106133. https://doi.org/10.1016/j.ypmed.2020.106133 (2020).
San-Cristobal, R., de Toro-Martin, J. & Vohl, M. C. Appraisal of gene-environment interactions in GWAS for evidence-based precision nutrition implementation. Curr. Nutr. Rep. https://doi.org/10.1007/s13668-022-00430-3 (2022).
Cheng, F. et al. Diabetes, metabolic disease, and telomere length. Lancet Diabetes Endocrinol. 9, 117–126. https://doi.org/10.1016/S2213-8587(20)30365-X (2021).
Martens, D. S. et al. Association of newborn telomere length with blood pressure in childhood. JAMA Netw. Open 5, e2225521. https://doi.org/10.1001/jamanetworkopen.2022.25521 (2022).
Zhan, Y. & Hagg, S. Telomere length and cardiovascular disease risk. Curr. Opin. Cardiol. 34, 270–274. https://doi.org/10.1097/HCO.0000000000000613 (2019).
Mazidi, M., Kengne, A. P., Sahebkar, A. & Banach, M. Telomere length is associated with cardiometabolic factors in US adults. Angiology 69, 164–169. https://doi.org/10.1177/0003319717712860 (2018).
Azcona-Sanjulian, M. C. Telomere length and pediatric obesity: A review. Genes Basel 12, 946. https://doi.org/10.3390/genes12060946 (2021).
Formichi, C. et al. Weight loss associated with bariatric surgery does not restore short telomere length of severe obese patients after 1 year. Obes. Surg. 24, 2089–2093. https://doi.org/10.1007/s11695-014-1300-4 (2014).
Laimer, M. et al. Telomere length increase after weight loss induced by bariatric surgery: Results from a 10 year prospective study. Int. J. Obes. (Lond.) 40, 773–778. https://doi.org/10.1038/ijo.2015.238 (2016).
Girchenko, P. et al. Associations between maternal risk factors of adverse pregnancy and birth outcomes and the offspring epigenetic clock of gestational age at birth. Clin. Epigenet. 9, 49. https://doi.org/10.1186/s13148-017-0349-z (2017).
Simpkin, A. J. et al. Prenatal and early life influences on epigenetic age in children: A study of mother-offspring pairs from two cohort studies. Hum. Mol. Genet. 25, 191–201. https://doi.org/10.1093/hmg/ddv456 (2016).
Monasso, G. S., Kupers, L. K., Jaddoe, V. W. V., Heil, S. G. & Felix, J. F. Associations of circulating folate, vitamin B12 and homocysteine concentrations in early pregnancy and cord blood with epigenetic gestational age: The Generation R Study. Clin. Epigenet. 13, 95. https://doi.org/10.1186/s13148-021-01065-x (2021).
Voortman, T. et al. Plasma fatty acid patterns during pregnancy and child’s growth, body composition, and cardiometabolic health: The Generation R Study. Clin. Nutr. 37, 984–992. https://doi.org/10.1016/j.clnu.2017.04.006 (2018).
Gorenjak, V., Akbar, S., Stathopoulou, M. G. & Visvikis-Siest, S. The future of telomere length in personalized medicine. Front. Biosci. (Landm. Ed.) 23, 1628–1654. https://doi.org/10.2741/4664 (2018).
Kyle, U. G., Earthman, C. P., Pichard, C. & Coss-Bu, J. A. Body composition during growth in children: Limitations and perspectives of bioelectrical impedance analysis. Eur. J. Clin. Nutr. 69, 1298–1305. https://doi.org/10.1038/ejcn.2015.86 (2015).
Group W. H. O. M. G. R. S. WHO Child Growth Standards based on length/height, weight and age. Acta. Paediatr. Suppl. 450, 76–85. https://doi.org/10.1111/j.1651-2227.2006.tb02378.x (2006).
Must, A. & Anderson, S. E. Body mass index in children and adolescents: Considerations for population-based applications. Int. J. Obes. (Lond.) 30, 590–594. https://doi.org/10.1038/sj.ijo.0803300 (2006).
Simental-Mendia, L. E., Rodriguez-Moran, M. & Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 6, 299–304. https://doi.org/10.1089/met.2008.0034 (2008).
Zheng, S. et al. Triglyceride glucose-waist circumference, a novel and effective predictor of diabetes in first-degree relatives of type 2 diabetes patients: Cross-sectional and prospective cohort study. J. Transl. Med. 14, 260. https://doi.org/10.1186/s12967-016-1020-8 (2016).
Aryee, M. J. et al. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 30, 1363–1369. https://doi.org/10.1093/bioinformatics/btu049 (2014).
Lu, A. T. et al. DNA methylation-based estimator of telomere length. Aging (Albany N.Y.) 11, 5895–5923. https://doi.org/10.18632/aging.102173 (2019).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. https://doi.org/10.18637/jss.v067.i01 (2015).
Acknowledgements
We thank all participants for their collaboration, the surgeons and the nurse Suzy Laroche of the Institut universitaire de cardiologie et de pneumologie de Québec (IUCPQ) for their invaluable collaboration in clinical care and patient recruitment. R.S.-C. is supported through a postdoctoral fellowship from the Centre Nutrition, Santé et Société (NUTRISS) at Université Laval. NUTRISS is financed by the Fonds de recherche du Québec—Santé (FRQS). M.-C. Vohl is Tier 1 Canada Research Chair in Genomics Applied to Nutrition and Metabolic Health.
Funding
Fondation de l’Université Laval—Fonds Dr Simon-Biron pour l’intervention clinique en obésité.
Author information
Authors and Affiliations
Contributions
RS-C performed statistical analysis, interpreted the data and wrote the manuscript; MCV and FG conceived and designed the research; MCV, JT-M and LP revised analytical methods, findings, and manuscript. FG, LP, SB, SM, ALP and MCV participated in the elaboration of the study design and critically reviewed the manuscript. FG, SB, SM and ALP participated in patient recruitment, and tissue sampling. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
San-Cristobal, R., de Toro-Martín, J., Guénard, F. et al. Impact of maternal cardiometabolic status after bariatric surgery on the association between telomere length and adiposity in offspring. Sci Rep 13, 20771 (2023). https://doi.org/10.1038/s41598-023-47813-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47813-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.