Abstract
This retrospective study investigated whether repetitive exposure to intravenous iodinated contrast media (ICM) affects long-term renal function in patients who undergo curative surgery for early gastric cancer (EGC) collected from the Korean Health Insurance and Review Assessment (HIRA) database. Patients diagnosed with gastric cancer between January 2010 and December 2013 underwent regular computed tomography (CT) scans to monitor for extragastric recurrence. Patients who already had chronic kidney disease (CKD) before cancer diagnosis or had undergone chemotherapy or repeated surgery were excluded. A nested case–control study design was chosen to analyze the effect of repetitive ICM exposure to long-term renal function by comparing patients who developed CKD 2 years after cancer diagnosis and patients who did not. Among 59,971 patients collected according to inclusion and exclusion criteria, 1021 were diagnosed with CKD 2 years after cancer diagnosis. Using 1:5 matching after adjusting for age, sex and date of cancer diagnosis, 5097 control patients were matched to 1021 CKD patients. Conditional logistic regression showed that the number of CTs taken using ICM slightly increased the odds of CKD (odds ratio, 1.080; 95% confidence interval (CI): 1.059, 1.100; P < 0.0001). Thus, the administration of ICM might contribute to chronic renal function impairment.
Similar content being viewed by others
Introduction
Computed tomography (CT) is widely used to diagnose cancer and intravenous iodinated contrast media (ICM) is essential for the detection and diagnosis of residual or recurrent gastric cancer or distant metastases1. Despite the consensus on its necessity for diagnosis, there is ongoing debate regarding the effects of contrast media exposure on renal function. Some studies have argued that the negative impact of contrast media on renal function may be overstated, even in cases of repetitive exposure2,3,4. However, in other studies, post-contrast acute kidney injury has been associated with negative short-term and long-term outcomes such as longer hospitalizations, higher chances of cardiac and neurologic incidents and higher mortality5,6,7. Although there are still questions regarding the actual risk of iodinated contrast media8, it is now evident in animal and human studies that contrast media can contribute to acute kidney injury7,9,10,11. The exact mechanism of nephrotoxicity from iodinated contrast media is not yet fully elucidated, but suggested causes are direct renal damage to the tubular epithelium, high osmolarity and viscosity which trigger increased oxidative stress, vasoconstriction, decreased urine flow and renal contrast media retention, and these factors can finally cumulate in medullary hypoxia and a decreased glomerular filtration rate12.
Stomach cancer was reported as the 5th most common cancer and the 4th most common cause of cancer-related deaths in 202013,14. With the development of treatment methods and implementation of a nationwide screening program in South Korea, the overall 5-year survival rates have increased to approximately 75%15. Furthermore, the 5-year overall survival rate of early gastric cancer (EGC) has actually been reported as being more than 97%16,17. Still, a protocol for CT surveillance after curative gastric cancer surgery has not yet been firmly established. In contrast to colorectal cancer for which intensive follow-up after curative surgery definitely improves overall survival18,19, the benefits of intensive follow-up are disputed in patients who undergo curative resection due to gastric cancer20,21. Hence, surveillance strategy after gastrectomy varies according to clinician and institution based on individual country guidelines22,23.
Recently, concerns have been raised about radiation exposure from CT follow-up scans in patients who undergo curative surgery due to EGC because the extragastric recurrence rate which is the main target of CT surveillance is only 1.4%24,25. Most CT scans are performed with contrast administration; hence, the nephrotoxicity of contrast media has to be considered. Therefore, the potential harm from radiation exposure as well as repeated administration of contrast media should be evaluated. There have been many studies regarding post-contrast acute kidney injury per one contrast-enhanced CT26,27,28,29, but only a few have focused on the harmful effect of repeated contrast administration on long-term renal function3,4,30.
Thus, the purpose of this study was to investigate whether repetitive exposure to iodinated CT contrast affects long-term renal function in patients who undergo curative surgery for EGC through an analysis of patients collected from a nationwide database.
Materials and methods
This retrospective study was approved by the Institutional Review Board of Severance Hospital (No. 4-2019-0252) with the requirement for informed consent waived because of its retrospective nature. This study abided by ethical guidelines stated in the Declaration of Helsinki.
Data source
Data were retrieved from the Korean Health Insurance and Review Assessment (HIRA) database (HIRA research data M20200206280). The HIRA database stores detailed information on drug prescriptions and treatment procedures performed based on a fee-for-service payment model and this medical information is available on all citizens who have registered for medical insurance in Korea31. Data are coded based on the Korean Classification of Diseases, 7th Revision (KCD-7) along with diagnosis, procedure and billing codes. KCD-7 was designed based on the International Classification of Diseases, 10th Revision (ICD-10) and has added a subdivision for frequent diseases that occur in South Korea.
Patients
Considering that not only chemotherapeutic agents, but also the cancer itself can contribute to kidney injury32,33,34,35, EGC patients who underwent curative treatment without recurrence during the follow-up period were included in this study. As these patients had minimal initial tumor burden that was removed after treatment and did not receive neoadjuvant or adjuvant chemotherapy36, we expected that any effect the cancer and chemotherapeutic agents would have on renal function would be minimized.
Initially, we searched the HIRA database for patients over 20 years old who were diagnosed with gastric cancer (KCD-7 C16) from 2010 to 2013. Among them, patients who underwent curative surgical resection (Procedure code Q0251, Q0252, Q0253, Q0254, Q0255, Q0256, Q0257, Q0258, Q0259, Q2530, Q2532, Q2533, Q2534, Q2535, Q2536, Q2537, Q2591, Q2592, Q2594, Q2595, Q2597, Q2598, QA533, QA534, QA535, QA536, QA595, QA597, QA598) or endoscopic submucosal dissection (ESD) (Procedure code Q7652, Q7653, Q2052, QX701, QX704, QZ933) and who were followed for 5 years after surgery or ESD were included. As an indicator of long-term renal impairment, patients who developed chronic kidney disease (CKD, KCD-7 N18) during the follow-up period were reviewed. Then, the following exclusion criteria were applied: (1) patients who were diagnosed with other malignancies within 2 years before gastric cancer diagnosis (washout period), (2) patients who had an underlying renal disease diagnosed (KCD-7 N00–N19, N25–N29, N39) before gastric cancer diagnosis, (3) patients who underwent any kind of chemotherapy in any time frame, (4) patients who underwent repeated gastric surgery/ESD due to recurring tumors, and (5) patients who were diagnosed with CKD within 2 years after cancer diagnosis to minimize the possibility of CKD developing due to other causes such as subclinical kidney disease rather than contrast media.
Patient and public involvement
No patients or public were involved in setting the research question or the outcome measures, nor in the interpretation of results.
Data analysis
In order to control for confounding factors, the presence or absence of diseases known to affect renal function including hypertension (KCD-7 I10.9, I15), diabetes (KCD-7 E10-E14), heart failure (KCD-7 I50), coronary artery occlusive disease (KCD-7 I20-25), stroke (KCD-7 I64), hyperlipidemia (KCD-7 E78.5), and chronic hepatitis virus infection (KCD-7 B18) were investigated. Data on prescription drugs that can affect renal function such as non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen, loop diuretics, angiotensin II receptor blockers (ARBs), angiotensin-converting-enzyme inhibitors (ACEi), and statins were also reviewed. If medications were prescribed within 1 month of cancer diagnosis and used for more than 2 weeks, they were included. The number of CTs taken using iodinated contrast media (Procedure code HA402, HA403, HA406, HA407, HA410, HA411, HA414, HA415, HA461, HA463, HA464, HA465, HA466, HA467, HA468, HA469, HA471, HA473, HA474, HA475, HA476, HA477, HA478, HA479) during the follow-up period was also analyzed. We compared the number of CTs taken using iodinated contrast media between patients who developed CKD 2 years after cancer diagnosis and patients who did not develop CKD to evaluate the long-term effect of repeated contrast media administration on renal function.
Statistical analysis
To investigate whether repetitive exposure to iodine CT contrast media affects the long-term renal function of patients who had EGC, a nested case–control study design was chosen. We identified case patients who developed CKD 2 years or later after gastric cancer diagnosis and then identified 4 control patients for each case patient among patients who did not have CKD at the time CKD was diagnosed in the case patient. These control patients were matched so that they were of the same sex and age at cancer diagnosis (within 1 year difference) and were diagnosed in the same year and month as the case patient.
After matching, baseline characteristics between the case group and control group were compared using appropriate statistical methods considering data dependency. A conditional logistic regression was performed to compare baseline characteristics between the case group and control group and to evaluate any existing associations between the number of CTs taken and CKD diagnosis after gastric cancer diagnosis (univariate analysis, Model 1 in Table 2). We further adjusted for prescribed drugs and underlying diseases (multivariate analysis, Model 2 in Table 2). All statistical analyses were performed using SAS Enterprise Guide version 9.4 (SAS Institute Inc., Cary, NC, USA). P-values < 0.05 were considered statistically significant.
Results
Patients
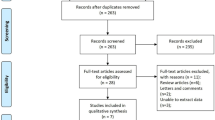
There were 254,422 patients who were 20 years old or more and diagnosed with gastric cancer from 2010 to 2013. Among these patients, 128,760 were diagnosed with another cancer during or before the washout period and therefore excluded. Other reasons for exclusion were underlying kidney disease (n = 23,651), chemotherapy (n = 38,876), recurrent surgery or ESD (n = 2605), and a diagnosis of CKD within 2 years after the gastric cancer diagnosis (n = 559). Finally, 59,971 gastric cancer patients were retrieved from the database. Among them, 1021 patients were diagnosed with CKD 2 years after their cancer diagnosis (the CKD group). After 1:5 matching, all CKD patients were matched to at least 1 patient in the control group and 5,097 patients were finally matched to the CKD patients to consist the control group. A detailed flow chart for patient selection is presented in Fig. 1. Baseline characteristics are compared between the CKD and control group after matching in Table 1. Time intervals between CKD diagnosis and EGC diagnosis are presented in Fig. 2.
CT surveillance protocol for gastric cancer
The mean number of CTs taken was significantly higher in the CKD group than the control group (6.02 ± 3.88, 4.99 ± 3.73, respectively, p < 0.001) The number of CTs taken according to days from gastric cancer diagnosis is presented in Fig. 3. As expected, the number of CTs being performed peaked repeatedly 6 months, 1 year, 2 years, 3 years, 4 years and 5 years after diagnosis. The mean number of CTs taken for each patient was 6.12 ± 3.62 (mean ± standard deviation) and the median number was 6 (interquartile range 3–8). The number of CTs taken according to days from gastric cancer diagnosis for all patients before 1:5 matching (n = 59,971) is presented in Supplement Fig. 1.
Number of CTs taken from cancer diagnosis according to days from cancer diagnosis in the nested case–control study (the total number of cases was 1021, and the total number of controls was 5097. The left Y-axis indicates the case group, and the right Y-axis indicates the control group) CT scans peaked on the day of cancer diagnosis, and 6 months, 1 year, 1.5 year, 2 years, 3 years, 4 years, and 5 years after cancer diagnosis.
Relationship between CKD incidence and the number of CTs taken
Univariate analysis using the conditional logistic regression analysis (Model 1 in Table 2) showed that a higher number of CTs taken with ICM increased the odds of CKD (Odds Ratio (OR) = 1.084; 95% confidence interval (CI) 1.064, 1.104; P < 0.001). The conditional logistic regression analysis for multivariate analysis (Model 2 in Table 2) found that a higher number of CTs taken with ICM slightly increased the odds of CKD (OR = 1.080; 95% CI 1.059, 1.100; P < 0.001) even after adjusting for other confounding factors that are listed in Table 2. Known risk factors for CKD such as hypertension (OR = 1.584; 95% CI 1.312, 1.911; P < 0.001), diabetes mellitus (OR = 1.996; 95% CI 1.714, 2.324; P < 0.001), and hyperlipidemia (OR = 1.349 95% CI 1.109, 1.641; P = 0.003) also increased the odds of CKD developing.
Discussion
Our findings indicate that the use of ICM during CT scans may pose a potential risk factor for CKD development, albeit with a relatively low odds ratio of 1.080 for a single CT scan. However, patients diagnosed and treated for malignancies, including EGC, often undergo repeated contrast-enhanced CT scans for a minimum of five years. Thus, the significance of even the modest odds ratio associated with CKD development cannot be understated.
In previous research, iodinated contrast media was considered the cause of post-contrast acute kidney injury, and was linked to negative short-term and long-term outcomes, including prolonged hospitalizations, higher rates of cardiac and neurologic incidents, and increased mortality5,6,9. Nevertheless, recent studies have challenged the notion that contrast media significantly impacts renal function, even with repetitive exposure. These studies primarily focused on post-contrast acute kidney injury or the long-term effects of iodinated contrast media over a shorter time frame of 3–6 months2,3,4. In contrast, our study examined the long-term effects of iodinated contrast media over a span of 5 years. As a result, our study demonstrated that exposure to ICM increases the subtle yet statistically significant odds of developing CKD.
Post-contrast acute kidney injury due to iodinate contrast media can lead to the acute deterioration of renal function and this can happen within 48 hours to 7 days after the contrast media administration37,38. Traditionally, although acute kidney injury increases both morbidity and mortality, it is considered as a single independent episode and patients who recover from acute kidney injury are thought to have benign long-term outcomes39,40. However, recent observational studies have shown that acute kidney injury and CKD are related and that acute kidney injury is an independent risk factor for CKD. Furthermore, the severity, duration and frequency of acute kidney injury are important predictors of poor outcomes39,40. If an event of acute kidney injury breaks the balance between adaptive and maladaptive repair mechanisms, acute kidney injury can progress to CKD39,41,42. In our study, the odds ratio for CKD development from a single CT scan was relatively small (1.080). Nevertheless, for patients who require repeated CT scans to monitor disease progression or evaluate treatment response, this odds ratio cannot be considered negligible.
The patients in our case group exhibited a notably higher prevalence of comorbid conditions, including hypertension and diabetes mellitus, in comparison to our control group, as detailed in Table 1. Hypertension and diabetes mellitus are widely recognized as risk factors for CKD43. Consequently, these baseline characteristics can potentially influence our findings. Therefore, to mitigate the impact of these confounding factors, we conducted a multivariate analysis employing conditional logistic regression. The multivariate analysis demonstrated that both hypertension and diabetes mellitus were associated with an increased odds ratio, in addition to the small but significant impact of ICM exposure. Future studies about the utilization of contrast-enhanced CT scans in patients known to possess risk factors for CKD, such as hypertension and diabetes mellitus are warranted.
Usually, endoscopy is regularly performed to detect mucosal recurrence or metachronous gastric cancer during surveillance44. The role of CT in gastric cancer follow-up is to detect extragastric recurrence, but Seo et al. stratified the risk for extragastric recurrence as high and low in EGC patients based on five risk factors which were male sex, lymph node metastasis, positive lymphovascular invasion, indications for endoscopic resection, and elevated macroscopic type and reported that only 0.265% of EGC patients at low risk of extragastric recurrence suffered extragastric recurrence in a 10-year period and that the 10-year extragastric recurrence-free survival was very high at 99.7%. Thus, they concluded that CT surveillance to detect extragastric recurrence should be avoided in the low risk group24. For patients with a very low risk of cancer recurrence, there is a need to weigh the diagnostic value of CT against the harmful effect of contrast media exposure to long-term renal function. In our study, there was significant difference of CKD development between case and control groups, albeit, the difference of mean number of CT taken was just approximately one scan (6.02 versus 4.99). Thus, the surveillance period and CT scan interval should be adjusted and redesigned based on future research.
According to a previous study, approximately 44% of elderly adults 65 years or older have CKD, but only 7% have been diagnosed in the United States45. These numbers are comparable with our results as 229 patients among 1021 patients (14.5%, Fig. 2) were diagnosed with CKD at the same time gastric cancer was diagnosed, which suggests that these patients already had CKD, but did not undergo relevant examinations until perioperative renal function assessment. Thus, CKD was undetected and overlooked in many cases. The washout period has been widely used in national databases to define new incident cases and eliminate any potential prevalent cases46. In a similar manner, we excluded patents who were diagnosed with CKD within 2 years of cancer diagnosis to minimize the possibility of CKD developing from other underlying medical conditions rather than contrast media. In patients who have not yet been diagnosed with CKD but may be experiencing deteriorating renal function due to etiologies other than those related to contrast media, we must consider these patients as confounding variables if CKD is diagnosed later after the administration of contrast media. Given that CKD is a condition that progresses gradually over a long period of time, we established a substantial washout period of two years to mitigate the inclusion of such patients in our study.
Global guidelines usually recommend a CT follow-up every 6 months for 2 years after surgery, then an annual CT follow-up thereafter for 5 years22,23,47. This general protocol is reflected in our study with CT follow-up examinations peaking at 6 months, 1 year, 2 years, 3 years, 4 years and 5 years after diagnosis as shown in Fig. 3 and Supplement Fig. 1. In our study, the number of CTs taken was the highest just after diagnosis and we assumed that this was because CT scans were performed to check for postoperative complications in the perioperative period.
There are several limitations to this study. First, the HIRA data did not included specifics on the iodinated contrast media such as the administered volume and osmolality of the contrast media which have also been reported as risk factors for kidney injury12,48. Also, eGFR data was not available and CKD was defined with the KCD-7 codes. Furthermore, specific pathologic stages were not recorded in the HIRA database. Second, heterogeneity due to different institutional guidelines and CT protocols needs to be considered when analyzing the HIRA data. Third, a selection bias might exist because physicians try to minimize the use of contrast media in patients who at higher risk of renal injury. Fourth, more severe postoperative complications may lead to more CT scans, which might subsequently have affect renal function. However, the HIRA database referred to in this study does not store detailed information on complications, so the severity of postoperative complications on renal function could not be analyzed in this study. Finally, we could not collect follow-up data that extended past 10 years of the ICM administration. Studies based on data collected from a longer follow-up period are warranted.
In conclusion, the administration of iodinated contrast media may be a potential risk factor for long-term renal impairment. Hence, the risks and benefits of contrast-enhanced CT need to be reevaluated in patients who undergo curative surgery and who are at low risk of recurrence such as those with EGC.
Data availability
The data that support the findings of this study are available from HIRA but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of HIRA.
Abbreviations
- HIRA:
-
Korean Health Insurance and Review Assessment
- EGC:
-
Early gastric cancer
- CKD:
-
Chronic kidney disease
- ICM:
-
Intravenous iodinated contrast media
References
Jee, H. B. et al. Is non-contrast CT adequate for the evaluation of hepatic metastasis in patients who cannot receive iodinated contrast media?. PLoS ONE 10, e0134133. https://doi.org/10.1371/journal.pone.0134133 (2015).
Davenport, M. S. et al. Use of intravenous iodinated contrast media in patients with kidney disease: Consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology 294, 660–668. https://doi.org/10.1148/radiol.2019192094 (2020).
Sudarski, D. et al. Risk of worsening renal function following repeated exposures to contrast media during percutaneous coronary interventions. J. Am. Heart Assoc. 10, e021473. https://doi.org/10.1161/jaha.121.021473 (2021).
Berglund, F. et al. Acute and long-term renal effects after iodine contrast media-enhanced computerised tomography in the critically ill—A retrospective bi-centre cohort study. Eur. Radiol. https://doi.org/10.1007/s00330-023-10059-7 (2023).
Lakhal, K. et al. Acute Kidney Injury Network definition of contrast-induced nephropathy in the critically ill: Incidence and outcome. J. Crit. Care 26, 593–599. https://doi.org/10.1016/j.jcrc.2011.05.010 (2011).
Seeliger, E., Sendeski, M., Rihal, C. S. & Persson, P. B. Contrast-induced kidney injury: Mechanisms, risk factors, and prevention. Eur. Heart J. 33, 2007–2015. https://doi.org/10.1093/eurheartj/ehr494 (2012).
Seo, N., Oh, H., Oh, H. J. & Chung, Y. E. Quantitative analysis of microperfusion in contrast-induced nephropathy using contrast-enhanced ultrasound: An animal study. Korean J. Radiol. 22, 801–810. https://doi.org/10.3348/kjr.2020.0577 (2021).
Rudnick, M. R. et al. The controversy of contrast-induced nephropathy with intravenous contrast: What is the risk?. Am. J. Kidney Dis. 75, 105–113. https://doi.org/10.1053/j.ajkd.2019.05.022 (2020).
Fahling, M., Seeliger, E., Patzak, A. & Persson, P. B. Understanding and preventing contrast-induced acute kidney injury. Nat. Rev. Nephrol. 13, 169–180. https://doi.org/10.1038/nrneph.2016.196 (2017).
Haase, M. et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am. J. Kidney Dis. 54, 1012–1024. https://doi.org/10.1053/j.ajkd.2009.07.020 (2009).
Oh, H. J. et al. The protective effect of klotho against contrast-associated acute kidney injury via the antioxidative effect. Am. J. Physiol. Renal Physiol. 317, F881–F889. https://doi.org/10.1152/ajprenal.00297.2018 (2019).
Mehran, R., Dangas, G. D. & Weisbord, S. D. Contrast-associated acute kidney injury. N. Engl. J. Med. 380, 2146–2155. https://doi.org/10.1056/NEJMra1805256 (2019).
Collaborators, G. B. D. S. C. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol. Hepatol. 5, 42–54. https://doi.org/10.1016/S2468-1253(19)30328-0 (2020).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Jung, K. W., Won, Y. J., Kong, H. J., Lee, E. S., Community of Population-Based Regional Cancer, R. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2015. Cancer Res. Treat. 50, 303–316. https://doi.org/10.4143/crt.2018.143 (2018).
Kim, Y. I. et al. Long-term survival after endoscopic resection versus surgery in early gastric cancers. Endoscopy 47, 293–301. https://doi.org/10.1055/s-0034-1391284 (2015).
Kim, S. G., Seo, H. S., Lee, H. H., Song, K. Y. & Park, C. H. Comparison of the differences in survival rates between the 7th and 8th editions of the AJCC TNM staging system for gastric adenocarcinoma: A single-institution study of 5,507 patients in Korea. J. Gastric Cancer 17, 212–219. https://doi.org/10.5230/jgc.2017.17.e23 (2017).
Pita-Fernández, S. et al. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: A systematic review and meta-analysis. Ann. Oncol. 26, 644–656. https://doi.org/10.1093/annonc/mdu543 (2015).
Renehan, A. G., Egger, M., Saunders, M. P. & O’Dwyer, S. T. Impact on survival of intensive follow up after curative resection for colorectal cancer: Systematic review and meta-analysis of randomised trials. BMJ 324, 813. https://doi.org/10.1136/bmj.324.7341.813 (2002).
Sisic, L. et al. Postoperative follow-up programs improve survival in curatively resected gastric and junctional cancer patients: A propensity score matched analysis. Gastric Cancer 21, 552–568. https://doi.org/10.1007/s10120-017-0751-4 (2018).
Tan, I. T. & So, B. Y. Value of intensive follow-up of patients after curative surgery for gastric carcinoma. J. Surg. Oncol. 96, 503–506. https://doi.org/10.1002/jso.20823 (2007).
Hur, H., Song, K. Y., Park, C. H. & Jeon, H. M. Follow-up strategy after curative resection of gastric cancer: A nationwide survey in Korea. Ann. Surg. Oncol 17, 54–64. https://doi.org/10.1245/s10434-009-0676-1 (2010).
Japanese Gastric Cancer, A. Japanese gastric cancer treatment guidelines 2014 (ver 4). Gastric Cancer 20, 1–19. https://doi.org/10.1007/s10120-016-0622-4 (2017).
Seo, N. et al. Stratification of postsurgical computed tomography surveillance based on the extragastric recurrence of early gastric cancer. Ann. Surg. 272, 319–325. https://doi.org/10.1097/Sla.0000000000003238 (2020).
Lee, Y. J. et al. Cumulative radiation exposure during follow-up after curative surgery for gastric cancer. Korean J. Radiol. 13, 144–151. https://doi.org/10.3348/kjr.2012.13.2.144 (2012).
Solomon, R. & DuMouchel, W. Contrast media and nephropathy—Findings from systematic analysis and food and drug administration reports of adverse effects. Investig. Radiol. 41, 651–660. https://doi.org/10.1097/01.rli.0000229742.54589.7b (2006).
McDonald, R. J. et al. Intravenous contrast material-induced nephropathy: Causal or coincident phenomenon?. Radiology 267, 106–118. https://doi.org/10.1148/radiol.12121823 (2013).
McDonald, J. S. et al. Frequency of acute kidney injury following intravenous contrast medium administration: A systematic review and meta-analysis. Radiology 267, 119–128. https://doi.org/10.1148/radiol.12121460 (2013).
Aycock, R. D. et al. Acute kidney injury after computed tomography: A meta-analysis. Ann. Emerg. Med. 71, 44-53.e44. https://doi.org/10.1016/j.annemergmed.2017.06.041 (2018).
Hsieh, M. S. et al. Contrast medium exposure during computed tomography and risk of development of end-stage renal disease in patients with chronic kidney disease: A nationwide population-based, propensity score-matched, longitudinal follow-up study. Medicine 95, e3388. https://doi.org/10.1097/MD.0000000000003388 (2016).
Kim, J. A., Yoon, S., Kim, L. Y. & Kim, D. S. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: Strengths, limitations, applications, and strategies for optimal use of HIRA data. J. Korean Med. Sci. 32, 718–728. https://doi.org/10.3346/jkms.2017.32.5.718 (2017).
Launay-Vacher, V. et al. Renal insufficiency and anticancer drugs in elderly cancer patients: A subgroup analysis of the IRMA study. Crit. Rev. Oncol. Hematol. 70, 124–133. https://doi.org/10.1016/j.critrevonc.2008.09.012 (2009).
Aapro, M. & Launay-Vacher, V. Importance of monitoring renal function in patients with cancer. Cancer Treat. Rev. 38, 235–240. https://doi.org/10.1016/j.ctrv.2011.05.001 (2012).
Perazella, M. A. Onco-nephrology: Renal toxicities of chemotherapeutic agents. Clin. J. Am. Soc. Nephrol. 7, 1713–1721. https://doi.org/10.2215/cjn.02780312 (2012).
Malyszko, J., Tesarova, P., Capasso, G. & Capasso, A. The link between kidney disease and cancer: Complications and treatment. Lancet 396, 277–287. https://doi.org/10.1016/S0140-6736(20)30540-7 (2020).
Guideline Committee of the Korean Gastric Cancer Association, D.W.G., Review, P. Korean practice guideline for gastric cancer 2018: An evidence-based, multi-disciplinary approach. J. Gastric Cancer 19(1–48), 2019. https://doi.org/10.5230/jgc.2019.19.e8 (2018).
ACR. ACR Manual on Contrast media 2021. (2021).
Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 120, c179-184. https://doi.org/10.1159/000339789 (2012).
Chawla, L. S. & Kimmel, P. L. Acute kidney injury and chronic kidney disease: An integrated clinical syndrome. Kidney Int. 82, 516–524. https://doi.org/10.1038/ki.2012.208 (2012).
Chawla, L. S., Eggers, P. W., Star, R. A. & Kimmel, P. L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 371, 58–66. https://doi.org/10.1056/NEJMra1214243 (2014).
Venkatachalam, M. A. et al. Acute kidney injury: A springboard for progression in chronic kidney disease. Am. J. Physiol. Renal Physiol. 298, F1078-1094. https://doi.org/10.1152/ajprenal.00017.2010 (2010).
Basile, D. P. et al. Progression after AKI: Understanding maladaptive repair processes to predict and identify therapeutic treatments. J. Am. Soc. Nephrol. 27, 687–697. https://doi.org/10.1681/asn.2015030309 (2016).
Lea, J. P. & Nicholas, S. B. Diabetes mellitus and hypertension: Key risk factors for kidney disease. J. Natl. Med. Assoc. 94, 7s–15s (2002).
Park, C. H. et al. Clinical practice guideline for endoscopic resection of early gastrointestinal cancer. Korean J. Gastroenterol. 75, 264–291. https://doi.org/10.4166/kjg.2020.75.5.264 (2020).
Stevens, L. A. et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: Results from the Kidney Early Evaluation Program (KEEP). Am. J. Kidney Dis. 55, S23-33. https://doi.org/10.1053/j.ajkd.2009.09.035 (2010).
Ahn, I. M. et al. Incidence, prevalence, and survival of moyamoya disease in Korea: A nationwide, population-based study. Stroke 45, 1090–1095. https://doi.org/10.1161/strokeaha.113.004273 (2014).
Ajani, J. A. et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 14, 1286–1312. https://doi.org/10.6004/jnccn.2016.0137 (2016).
Amsterdam, E. A. et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 64, e139–e228. https://doi.org/10.1016/j.jacc.2014.09.017 (2014).
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government. (MIST) (2022R1A2C101141111).
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.E.C., I.K.J., M.J.L., J.H.K., J.S.L., W.J.H., H.I.Y. Data curation: J.H.K., M.J.L., E.H.K. Formal analysis: J.H.K., M.J.L., E.H.K., I.K.J., Y.E.C. Funding acquisition: Y.E.C. Investigation: J.H.K., M.J.L., E.H.K., H.J.O., I.K.J., Y.E.C. Methodology: J.H.K., M.J.L., E.H.K., I.K.J., Y.E.C. Project administration: H.J.O., J.S.L., W.J.H., H.I.Y., I.K.J., Y.E.C. Supervision: H.J.O., J.S.L., W.J.H., H.I.Y., I.K.J., Y.E.C. Validation: J.H.K., M.J.L., H.J.O., J.S.L., W.J.H., H.I.Y., I.K.J., Y.E.C. Visualization: J.H.K., M.J.L., E.H.K. Writing: J.H.K., M.J.L., I.K.J., Y.E.C. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koo, J.H., Lee, M., Kim, E.H. et al. Harmful effect of repetitive intravenous iodinated contrast media administration on the long-term renal function of patients with early gastric cancer. Sci Rep 13, 19448 (2023). https://doi.org/10.1038/s41598-023-46773-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46773-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.