Abstract
Alpha klotho (α-Klotho) is an anti-aging molecule associated with aging and several diseases. Previous studies have reported inconsistent levels of α-Klotho in smokers. This study aimed to demonstrate serum α-Klotho levels in smokers among the US population. This cross-sectional study recruited 11,559 participants (aged 40–79 years; 48.2% males). All data were collected from the 2007–2016 National Health and Nutrition Examination Survey. The study comprised adults with reliable Klotho and smoking questionnaire results. The relationship between smoking and serum α-klotho levels was assessed using multivariate linear regression models after adjusting for potential confounders. We also performed a stratified analysis of clinically important variables. The mean serum α-klotho level among the 11,559 participants was 843.85 pg/mL. After full adjustment, habitual smoking was significantly associated with decreased serum levels of α-klotho level (β = − 34.89; 95% CI − 54.97, − 14.81; P = 0.0013) in the total study population. Furthermore, the stratified analysis indicated that the association was insignificant in the 60–79 age group. Quitting smoking was not significantly associated with serum levels of α-klotho as expected (P = 0.1148) in the total study population. However, stratified analyses showed a significant inversed association in the male, those with chronic kidney disease, or those with cancer who quit smoking (all P < 0.05). Cigarette smoking was inversely associated with serum α-Klotho levels among US adults.
Similar content being viewed by others
Introduction
In 2019, there were 1.14 billion people who smoked cigarettes regularly, and smoking is responsible for 7.69 million deaths and 200 million disability-adjusted life-years1. Smoking is considered to have a great impact on life expectancy and makes major contribution to the global burden of morbidity and mortality2. Smoking is one of the most prevalent risk factors for a range of diseases, including cardiovascular disease (CVD)3, chronic obstructive pulmonary disease (COPD)4, and cancer1. Meanwhile, smoking is also considered to be responsible for aging, which is a normal physiological process but can be accelerated by cigarette consumption5.
Klotho is an important aging suppressor protein that emerged in 19976. Alpha klotho (α-Klotho) protein is a Klotho-encoded transmembrane protein that is highly expressed in the kidney7. α-klotho has been reported as a hormone with anti-inflammatory properties. It was also thought to play a vital role in the antioxidative process8. Differential expression of α-klotho was also discovered in various diseases, including cardiovascular disease9, chronic kidney disease (CKD)10, diabetes11, systemic lupus erythematosus12, and some types of cancer13. This may suggest α-klotho may have been involved in these diseases mentioned above.
Several single-center studies with small sample sizes have shown controversial results about the association between cigarette smoking and α-klotho levels14,15,16. Previous studies have demonstrated that serum α-klotho levels were significantly higher in heavy smokers15 and decreased following smoking cessation17. Additionally, another study found that serum α-klotho levels were significantly higher in male smokers than never-smokers and slightly lower in female smokers14. Therefore, the purpose of the present study was to investigate in the relationship between cigarette smoking and serum α-klotho levels in a nationally representative sample of US adults.
Materials and methods
Study design and participants
The five continuous 2-year cycles of the National Health and Nutrition Examination Survey (NHANES) data between 2007 and 2016 were analyzed for this study. NHANES was conducted in the US by the National Center for Health Statistics of the Centers for Disease Control and Prevention (CDC). NHANES adopts a stratified, multistage, sophisticated probabilistic design to acquire a representative sample of civilians in the US to examine the health and nutritional status of noninstitutionalized citizens. All participant data is a compilation of household interviews, physical examinations in a mobile examination center (MEC), and laboratory testing performed by highly qualified medical experts. More details about NHANES can be found on the website (https://www.cdc.gov/nchs/nhanes/about_nhanes.htm). The NCHS Ethics Review Board authorized the original research, and all participants obtained written informed consent. The current study was deemed exempt by the Institutional Review Board at the author's institution, as the dataset used in the research was deidentified entirely.
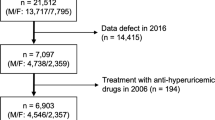
We restricted this cross-sectional analysis to participants from the NHANES database from 2007 to 2016 (50,588 individuals in total) because serum α-klotho levels were only tested in NHANES 2007–2016 among participants aged 40–79 who agreed to surplus serum collection for future research. At the time of recruiting, 19,344 were aged 40–79. Of these participants, 5580 were excluded because of missing data on α-klotho, smoking status (n = 7), pregnancy (n = 9), or other covariates (i.e., demographic variables, behavioral factors, history of diseases, n = 2189), resulting in a final analytical sample of 11,559 participants. The flowchart of participant selection was shown in Fig. 1.
Cigarette smoking
The smoking questions were asked by trained interviewers using the Computer-Assisted Personal Interviewing system at the MEC during the MEC Interview. Survey participants were categorized into 3 groups using self-reported responses for these questionnaires. These are classified as (1) never smoker (less than 100 cigarettes in entire life); (2) quit smoker (100 cigarettes or more in entire life but no smoking now); (3) habitual smoker (smoked 100 cigarettes or more in entire life while smoking now).
Serum α-klotho level
NHANES Laboratory/Medical Technologists Procedures Manual18 contains laboratory methodology and protocol information. Serum α-klotho in frozen serum samples taken during NHANES 2007–2016 were studied in 2019–2020. Fresh-frozen serum samples, stored at -80◦C, were measured using an enzyme-linked immunosorbent assay kit manufactured by IBL International, Japan, per the manufacturer's instructions. All samples were analyzed twice, and the average value was calculated. The mean serum α-klotho level was 698.0 pg/mL, ranging from 285.8 to 1638.6 pg/mL19.
Study covariates
The sociodemographic characteristics of each participant, including age, sex, race/ethnicity, marital status, ratio of family income to poverty level, and education level, were self-reported via computer-assisted interviews. Race/ethnicity was collected in five categories: Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and Others. Ratio of family income to poverty (PIR) (< 1.30, 1.30–2.99, and ≥ 3.00)20. Education level was divided into (1) Less than high school; (2) High school or GED; (3) Above high school. Body mass index (BMI) was computed using weight and height (kg/m2) and classified into the following WHO classes21: Normal weight (< 25), Overweight (25–30), and Obese (≥ 30). Alcohol consumption status were collected as a potential confounder classified as22,23: (1) never drinker (had < 12 drinks in a lifetime); (2) former drinker (had ≥ 12 drinks in 1 year and did not drink last year, or did not drink last year but drank ≥ 12 drinks in lifetime); (3) light-to-moderate drinker (< 3 drink per day for females, < 4 drinks per day for males on average over the past year, or binge drinking [≥ 4 drinks on same occasion for females, ≥ 5 drinks on same occasion for males] < 5 days per month over the past year); (4) heavy drinker (≥ 3 drinks per day for females, ≥ 4 drinks per day for males on average over the past year, or binge drinking ≥ 5 days per month over the past year). Comorbidities include diabetes, hypertension, CKD, COPD, and CVD. Diabetes was defined as the presence of any of the following conditions: (1) A self-reported diagnosis of diabetes; (2) Use of anti-diabetic drugs; (3) Hemoglobin A1c (HbA1c) level ≥ 6.5%; (4) Fasting plasma glucose level ≥ 7.0 mmol/L; (5) Random plasma glucose level ≥ 11.1 mmol/L. Borderline was defined as (1) Impaired fasting glycemia: Fasting plasma glucose level 6.11–7.0 mmol/L; (2) Impaired glucose tolerance: Random plasma glucose level 7.7–11.1 mmol/L. Hypertension was defined as systolic blood pressure (SBP) value ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg or habitually taking antihypertensive drugs24. CKD was defined according to the KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases25. COPD was defined if any of the following: (1) FEV1/FVC < 0.7; (2) A self-reported diagnosis of emphysema; (3) Age above 40 with smoke history or chronic bronchitis and use of phosphodiesterase-4 inhibitors, mast cell stabilizers, leukotriene modifiers, or inhaled corticosteroids. The presence of CVD (including congestive heart failure, coronary heart disease, angina pectoris, heart attack, and stroke) and cancer depended on whether a doctor had told participants they had such disease.
Statistical analysis
Data was acquired from the NHANES project's nhanesR (http://ckr123.synology.me:3838/nhanesR/). EmpowerStats (http://www.empowerstats.com/cn/, X&Y solutions, Inc., Boston, MA) and R software (The R Foundation; http://www.r-project.org; version 4.2.0) were used for all analyses. P < 0.05 is regarded as statistically significant. Strata, primary sampling units, and sample adult weights were used in studies to account for the National Health Interview Survey's complicated design. Weighted linear regression and weighted chi-square test were used to compare baseline characteristics for continuous and categorical variables, respectively. Finally, data were expressed as weighted proportions [95% Confidence interval (CI)] for categorical variables and as weighted means ± Standard Error (SE) for continuous variables. Multivariate linear regression models were set up to test the relationship between smoking and serum α-klotho levels. Following the recommendations for Strengthening the Reporting of Observational Studies in Epidemiology (STROBE), tested four models: Model 1 had no adjustment. Model 2 minimally adjusted for some demographic factors (age, sex, and race/ethnicity). Model 3 additionally adjusted for lifestyle variables (BMI, marital status, PIR, education level, and alcohol consumption status). Model 4 additionally adjusted for clinical variables (diabetes, hypertension, CKD, CVD, COPD, and cancer). Furthermore, stratified subgroup and interaction analyses were performed according to age (< / ≥ 60 years), sex, hypertension, CKD, CVD, and cancer using multivariate linear regression with full adjustment.
Ethical approval
The ethical approval to conduct the NHANES 2007–2016 was granted by the NHANES Institutional Review Board. The study procedures were structured in line with the Declaration of Helsinki. All participants provided informed consent. The patients/participants provided their written informed consent to participate in this study.
Results
Population characteristics
Among 11,559 participants (mean age, 56.2 years; 48.2% male), the mean level of serum α-klotho was 843.85 ± 5.34 pg/mL. The baseline characteristics were presented in Table 1 according to cigarette smoking status. Among all participants, 51.2% have never smoked, 30.4% have quitted smoked, and 18.4% have habitually smoked.
Briefly, participants in the quit smoking group were older, more likely to be male, Non-Hispanic White, lived alone, had lower α-klotho levels, and were poorer educated than those in the never smoking group. In addition, quit smokers seemed had higher BMI, more alcohol consumption, and comorbidities. Compared to the never smoking group, participants in the habitual smoking group presented lower serum α-klotho levels. We also noticed that the habitual smoking group was younger, most were male, Non-Hispanic Black, lived alone, and were poorer educated than those in the never smoking group. In addition, the habitual smoking group showed lower BMI, more alcohol consumption, and comorbidities (Table 1).
Univariate analysis of serum α-klotho levels
The univariate analysis of serum α-klotho levels varied in smoking status, age, sex, race/ethnicity, BMI, alcohol consumption, hypertension, CKD, CVD, and cancer (all P < 0.01, Supplementary Table 1).
Association between smoking and serum α-klotho levels
Four models were conducted to assess the association between smoking and serum α-klotho level, as shown in Table 2. Compared to the never smoking group, the serum α-klotho level was significantly decreased in the habitual smoking group (model 1, β = − 43.53; 95% CI − 62.79, − 24.27; P < 0.0001). Interestingly, significantly decreased serum α-klotho level was still observed in the population who has quit smoking (model 1, β = − 34.76; 95% CI − 50.02, − 19.49; P < 0.0001), the trend was significant among the different smoking groups (P < 0.0001), which may indicate a negative correlation between serum α-klotho levels and smoking habit. A significantly negative correlation in the habitual smoking group was still observed after adjustment in model 2 (age, sex, race/ethnicity; β = − 46.59; 95% CI − 66.37, − 26.80; P < 0.0001), model 3 (Further adjusted for BMI, marital status, PIR, education level, alcohol consumption; β = − 35.30; 95% CI − 56.75, − 13.85; P = 0.002), and model 4 (all covariates; β = − 34.89; 95% CI − 54.97, − 14.81; P = 0.0013). In the quit smoking group, the decreased serum α-klotho level was still significant in model 2 (β = − 19.73; 95% CI − 34.43, − 5.03; P = 0.0104). In model 3 (β = − 12.16; 95% CI − 27.99, 3.68; P = 0.1377) and model 4 (β = − 12.54; 95% CI − 27.86, 2.79; P = 0.1148), the serum α-klotho level was decreased without statistical significance. The trend remained significant among the different smoking groups in model 4 (P = 0.0011).
Subgroup analyses
In age, sex, hypertension, CKD, CVD, and cancer subgroup, most subgroups showed the serum α-klotho levels were significantly decreased in the habitual smoking group but not in the quit smoking group (Fig. 2). However, no significant correlation was observed in the habitual smoking group aged 60–79 years old, those with hypertension or CVD (all P > 0.05). Serum α-Klotho levels were more significantly reduced in male, participants with CKD or cancer in the quit smoking groups than in the never smoking groups (all P < 0.05). We found evidence of a significant interaction between cancer and smoking exposure (P for interaction = 0.0165).
Subgroup analyses of the association between smoking and serum α-Klotho level (pg/mL). Each stratification adjusted for all factors (age, sex, race/ethnicity, BMI, marital status, PIR, education level, alcohol consumption, diabetes, hypertension, CKD, CVD, COPD, and cancer) except the stratification factor itself. BMI, body mass index; PIR, Ratio of family income to poverty; CKD, chronic kidney disease; CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease.
Discussion
Smoking is considered contributed to the shortening of life expectancy. Our study showed that the serum level of α-klotho, a potential aging biomarker, was negatively correlated with cigarette smoking.
Numerous studies have supported the association between smoking and α-Klotho levels. A previous study has shown that the serum levels of soluble a-klotho were significantly higher in heavy smokers among 40 smokers (40–60 years, ≥ 6 pack cigarette per year) without COPD15. In another study that enrolled 230 health participants (90 men and 140 women), the serum α-Klotho level was significantly higher in smokers than in never-smokers in males. However, the serum α-Klotho level was slightly lower in the female smokers14. Interestingly, α-Klotho levels decreased for smoking cessation (n = 28)17. However, most of these studies are based on small samples, and the results are inconsistent14,15,17,26. In our research, we found the serum α-Klotho level was significantly lower in habitual smokers than in never smokers, which was consistent with a previous study26.
Our study found no significant relationship between habitual smoking and levels of α-Klotho in individuals over 60 years old. It is important to mention that α-Klotho is acknowledged as an anti-aging protein, and its serum levels tend to diminish as individuals progress in age27. The findings of our study align with previous report28, which could explain why significant results were observed only in the 40–59 age group. In the male, CKD and cancer population, smoking cessation is related to lowering the serum α-Klotho. These results may not indicate a protective role of smoking cessation in these subgroups as expected. However, previous studies29,30 showed that the α-Klotho level was more significantly correlated with the severity of these diseases than smoking. Furthermore, it is worth noting that the current study did not consider the duration of smoking cessation, which is an important factor in assessing the dose–response relationship. The time since quitting smoking can significantly influence the impact on α-Klotho levels17. Therefore, future research should consider examining the relationship between smoking cessation duration and α-Klotho levels to provide a more comprehensive understanding of the effects of quitting smoking on this biomarker.
These findings may indicate some association between smoking and aging. Cellular senescence includes inflammation, fibrosis, metabolic disorders, DNA damage, mitochondrial dysfunction, loss of protein homeostasis, failed autophagy, Reactive Oxygen Species generation, NAD + depletion, and stem/progenitor cell dysfunction31,32,33. A mixture of thousands of chemicals is created by burning or heating tobacco when people smoke. Reactive oxygen species (ROS), one of these chemicals, can activate epithelial intracellular cell signaling cascades and lead to inflammatory gene activation. 34 The downstream of the inflammatory cascades including the Tumor necrosis factor (TNF), interferon γ (IFN- γ), and interleukin-6 (IL-6)34,35 can downregulate the α-klotho gene expression36. Oxidative stress has been shown to decrease the expression of the α-Klotho gene37. Smoking-induced oxidative stress was also observed in humans, animal models, and in vitro cell culture systems38,39. α-Klotho deficiency was also directly or indirectly instrumental in lowering autophagic flux by causing phosphotoxicity40,41. Cigarette smoke exposure induces ROS-mediated autophagy by regulating sestrin, AMPK, and mTOR levels42,43. All the above molecules (ROS, TNF, IFN- γ, IL-6, oxidative stress, AMPK, and mTOR) were closely related to the biological process of aging44,45,46.
We revealed lower serum α-klotho in smokers which may suggest smoking may accelerate aging by decreasing the anti-aging factor klotho. This study’s large sample size and use of a representative multiethnic population allows for better generalization to the US population. Further studies are required to prove this hypothesis.
Nevertheless, the present study has several limitations. First, a bias may exist for the self-reporting smoking status, which may be discrepancy with the real smoking status in the database. Second, we can only observe the correlation between smoking and α-klotho but not affirm the causal relationship between the two factors in an observational study. Third, we can't acquire complete information on the cigarette type and accurate dose of cigarette consumption in the NHANES database, which may prevent a further study between cigarette consumption and α-Klotho levels. Additionally, it is important to acknowledge that the current study did not investigate the time dose–response relationship in relation to smoking cessation, which could potentially influence the findings.
Conclusions
In conclusion, we proved habitual smoking is associated with a lower serum level of α-Klotho, which was considered an anti-aging factor. The result may indicate that smoking may accelerate aging by affecting α-Klotho levels. Further studies are required to confirm these results and convince the underlying mechanism.
Data availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Collaborators, G. B. D. T. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet 397, 2337–2360. https://doi.org/10.1016/S0140-6736(21)01169-7 (2021).
Peacock, A. et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 113, 1905–1926. https://doi.org/10.1111/add.14234 (2018).
Duncan, M. S. et al. Association of smoking cessation with subsequent risk of cardiovascular disease. JAMA 322, 642–650. https://doi.org/10.1001/jama.2019.10298 (2019).
Christenson, S. A., Smith, B. M., Bafadhel, M. & Putcha, N. Chronic obstructive pulmonary disease. Lancet 399, 2227–2242. https://doi.org/10.1016/S0140-6736(22)00470-6 (2022).
Beach, S. R. et al. Methylomic aging as a window onto the influence of lifestyle: Tobacco and alcohol use alter the rate of biological aging. J. Am. Geriatr. Soc. 63, 2519–2525. https://doi.org/10.1111/jgs.13830 (2015).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51. https://doi.org/10.1038/36285 (1997).
Bergmark, B. A. et al. Klotho, fibroblast growth factor-23, and the renin-angiotensin system—An analysis from the PEACE trial. Eur. J. Heart Fail 21, 462–470. https://doi.org/10.1002/ejhf.1424 (2019).
Kuro-o, M. Klotho as a regulator of oxidative stress and senescence. Biol. Chem. 389, 233–241. https://doi.org/10.1515/BC.2008.028 (2008).
Donato, A. J., Machin, D. R. & Lesniewski, L. A. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ. Res. 123, 825–848. https://doi.org/10.1161/CIRCRESAHA.118.312563 (2018).
Neyra, J. A., Hu, M. C. & Moe, O. W. Klotho in clinical nephrology: Diagnostic and therapeutic implications. Clin. J. Am. Soc. Nephrol. 16, 162–176. https://doi.org/10.2215/CJN.02840320 (2020).
Hua, S. et al. Beta-klotho in type 2 diabetes mellitus: From pathophysiology to therapeutic strategies. Rev. Endocr. Metab. Disord. 22, 1091–1109. https://doi.org/10.1007/s11154-021-09661-1 (2021).
Martin-Gonzalez, C. et al. Alpha-Klotho protein in systemic lupus erythematosus. Clin. Exp. Rheumatol. https://doi.org/10.55563/clinexprheumatol/salqon (2022).
Ligumsky, H., Merenbakh-Lamin, K., Keren-Khadmy, N., Wolf, I. & Rubinek, T. The role of alpha-klotho in human cancer: Molecular and clinical aspects. Oncogene https://doi.org/10.1038/s41388-022-02440-5 (2022).
Nakanishi, K. et al. An implication of Klotho-related molecules in different smoking-related health outcomes between men and women. Clin. Chim. Acta 476, 44–48. https://doi.org/10.1016/j.cca.2017.11.007 (2018).
Verde, Z. et al. A paradox: Alpha-klotho levels and smoking intensity. Lung 195, 53–57. https://doi.org/10.1007/s00408-016-9944-6 (2017).
Kureya, Y. et al. Down-regulation of soluble alpha-klotho is associated with reduction in serum irisin levels in chronic obstructive pulmonary disease. Lung 194, 345–351. https://doi.org/10.1007/s00408-016-9870-7 (2016).
Kamizono, Y. et al. Impact of cigarette smoking cessation on plasma alpha-klotho levels. Medicine (Baltimore) 97, e11947. https://doi.org/10.1097/MD.0000000000011947 (2018).
Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey. Laboratory procedures manual. (2011).
Yamazaki, Y. et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem. Biophys. Res. Commun. 398, 513–518. https://doi.org/10.1016/j.bbrc.2010.06.110 (2010).
Wang, L. et al. Trends in consumption of ultraprocessed foods among US youths aged 2–19 years, 1999–2018. JAMA 326, 519–530. https://doi.org/10.1001/jama.2021.10238 (2021).
James, W. P. T. Obesity: A global public health challenge. Clin. Chem. 64, 24–29. https://doi.org/10.1373/clinchem.2017.273052 (2018).
Rattan, P. et al. Inverse association of telomere length with liver disease and mortality in the US population. Hepatol. Commun. 6, 399–410. https://doi.org/10.1002/hep4.1803 (2022).
Jiang, M., Tang, X., Wang, P., Yang, L. & Du, R. Association between daily alcohol consumption and serum alpha klotho levels among US adults over 40 years old: A cross-sectional study. BMC Public Health 23, 1901. https://doi.org/10.1186/s12889-023-16830-1 (2023).
Whelton, P. K. & Carey, R. M. The 2017 American college of cardiology/American heart association clinical practice guideline for high blood pressure in adults. JAMA Cardiol. 3, 352–353. https://doi.org/10.1001/jamacardio.2018.0005 (2018).
Kidney Disease: Improving Global Outcomes Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 100, S1–S276. https://doi.org/10.1016/j.kint.2021.05.021 (2021).
Lam-Rachlin, J. et al. Infection and smoking are associated with decreased plasma concentration of the anti-aging protein, alpha-klotho. J. Perinat. Med. 41, 581–594. https://doi.org/10.1515/jpm-2013-0084 (2013).
Kuro, O. M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 15, 27–44. https://doi.org/10.1038/s41581-018-0078-3 (2019).
Yao, Y., Long, Y., Du, F. W., Zhao, Y. & Luo, X. B. Association between serum cotinine and alpha-Klotho levels among adults: Findings from the National Health and Nutrition Examination Survey 2007–2016. Tob. Induc. Dis. 20, 57. https://doi.org/10.18332/tid/144622 (2022).
Zhu, X. et al. Renal function mediates the association between klotho and congestive heart failure among middle-aged and older individuals. Front. Cardiovasc. Med. 9, 802287. https://doi.org/10.3389/fcvm.2022.802287 (2022).
Qiao, Y. et al. Association of serum klotho levels with cancer and cancer mortality: Evidence from national health and nutrition examination survey. Cancer Med. 12, 1922–1934. https://doi.org/10.1002/cam4.5027 (2023).
Shvedova, M., Samdavid Thanapaul, R. J. R., Thompson, E. L., Niedernhofer, L. J. & Roh, D. S. Cellular Senescence in aging, tissue repair, and regeneration. Plast. Reconstr. Surg. 150, 4S-11S. https://doi.org/10.1097/PRS.0000000000009667 (2022).
Wang, C., Hao, X. & Zhang, R. Targeting cellular senescence to combat cancer and ageing. Mol. Oncol. 16, 3319–3332. https://doi.org/10.1002/1878-0261.13266 (2022).
Gao, X. et al. Telomeres and mitochondrial metabolism: Implications for cellular senescence and age-related diseases. Stem Cell Rev. Rep. 18, 2315–2327. https://doi.org/10.1007/s12015-022-10370-8 (2022).
Huang, M. F., Lin, W. L. & Ma, Y. C. A study of reactive oxygen species in mainstream of cigarette. Indoor Air 15, 135–140. https://doi.org/10.1111/j.1600-0668.2005.00330.x (2005).
Larsson, S. C. & Burgess, S. Appraising the causal role of smoking in multiple diseases: A systematic review and meta-analysis of Mendelian randomization studies. EBioMedicine 82, 104154. https://doi.org/10.1016/j.ebiom.2022.104154 (2022).
Zhang, C. et al. Association between Dietary Inflammatory Index and serum Klotho concentration among adults in the United States. BMC Geriatr. 22, 528. https://doi.org/10.1186/s12877-022-03228-8 (2022).
Xu, Y. & Sun, Z. Molecular basis of Klotho: From gene to function in aging. Endocr. Rev. 36, 174–193. https://doi.org/10.1210/er.2013-1079 (2015).
Filaire, E. et al. Lung cancer: What are the links with oxidative stress, physical activity and nutrition. Lung Cancer 82, 383–389. https://doi.org/10.1016/j.lungcan.2013.09.009 (2013).
Munzel, T. et al. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur. Heart J. 41, 4057–4070. https://doi.org/10.1093/eurheartj/ehaa460 (2020).
Shi, M. et al. alphaKlotho mitigates progression of AKI to CKD through activation of autophagy. J. Am. Soc. Nephrol. 27, 2331–2345. https://doi.org/10.1681/ASN.2015060613 (2016).
Fernandez, A. F. et al. Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature 558, 136–140. https://doi.org/10.1038/s41586-018-0162-7 (2018).
Morsch, A. et al. Cigarette smoke exposure induces ROS-mediated autophagy by regulating sestrin, AMPK, and mTOR level in mice. Redox Rep. 24, 27–33. https://doi.org/10.1080/13510002.2019.1601448 (2019).
Vij, N., Chandramani-Shivalingappa, P., Van Westphal, C., Hole, R. & Bodas, M. Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am. J. Physiol. Cell Physiol. 314, C73–C87. https://doi.org/10.1152/ajpcell.00110.2016 (2018).
Ureshino, R. P., Rocha, K. K., Lopes, G. S., Bincoletto, C. & Smaili, S. S. Calcium signaling alterations, oxidative stress, and autophagy in aging. Antioxid. Redox Signal 21, 123–137. https://doi.org/10.1089/ars.2013.5777 (2014).
Wennberg, A. M. V. et al. The cross-sectional and longitudinal associations between IL-6, IL-10, and TNFalpha and cognitive outcomes in the mayo clinic study of aging. J. Gerontol. A Biol. Sci. Med. Sci. 74, 1289–1295. https://doi.org/10.1093/gerona/gly217 (2019).
Ge, Y., Zhou, M., Chen, C., Wu, X. & Wang, X. Role of AMPK mediated pathways in autophagy and aging. Biochimie 195, 100–113. https://doi.org/10.1016/j.biochi.2021.11.008 (2022).
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
R.D., L.Y., and X.L.T., W.H. are responsible for the conception and design of the study. R.D., M.H.J., and L.Y. interpreted the analysis. R.D., X.Y.T., M.H.J., and S.L.Q. were responsible for the acquisition of data. R.D., X.Y.T., M.H.J., X.L.T., and W.H. wrote the first draft of the manuscript, interpreted the data, and wrote the final version. All authors critically revised the article for important intellectual content and approved the final version. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Du, R., Tang, X., Jiang, M. et al. Association between cigarette smoking and serum alpha klotho levels among US adults over 40-years-old: a cross-sectional study. Sci Rep 13, 19519 (2023). https://doi.org/10.1038/s41598-023-46698-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46698-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.