Abstract
In most legumes, the rhizobial symbionts exhibit diversity across different environments. Although common bean (Phaseolus vulgaris L.) is one of the important legumes in southern Africa, there is no available information on the genetic diversity and N2-fixing effectiveness of its symbionts in Malkerns, Eswatini. In this study, we assessed the phylogenetic positions of rhizobial microsymbionts of common bean from Malkerns in Eswatini. The isolates obtained showed differences in morpho-physiology and N2-fixing efficiency. A dendrogram constructed from the ERIC-PCR banding patterns, grouped a total of 88 tested isolates into 80 ERIC-PCR types if considered at a 70% similarity cut-off point. Multilocus sequence analysis using 16S rRNA, rpoB, dnaK, gyrB, and glnII and symbiotic (nifH and nodC) gene sequences closely aligned the test isolates to the type strains of Rhizobium muluonense, R. paranaense, R. pusense, R. phaseoli and R. etli. Subjecting the isolates in this study to further description can potentially reveal novel species. Most of the isolates tested were efficient in fixing nitrogen and elicited greater stomatal conductance and photosynthetic rates in the common bean. Relative effectiveness (RE) varied from 18 to 433%, with 75 (85%) out of the 88 tested isolates being more effective than the nitrate fed control plants.
Similar content being viewed by others
Introduction
Common bean (Phaseolus vulgaris L.) is an important source of protein to millions of people around the world1,2. In low-income farming systems, legumes such as common bean are grown as secondary crops after staples like maize. However, most of these legumes are usually grown under adverse growing conditions with limited water and poor soil fertility, all of which negatively affect their growth and yield3,4. To increase yields, synthetic N fertilizers usually offer immediate solution to soil N deficiency5. Owing to the high cost and negative environmental footprints of these synthetic fertilizers6,7, it has become necessary to explore safer and sustainable biological means to meet legume N demand by identifying indigenous soil microsymbionts that can improve the efficiency of the legume-rhizobium symbiosis.
Even though common bean can fix N2 through their symbiotic relationship with rhizobia, the crop is known to derive relatively lower proportion of its N requirement from symbiosis when compared to other legumes8. Aside from screening the crop’s diverse germplasm for N2 fixation, bioprospecting its microsymbionts can also lead to the identification of strains that can be used to boost this important biological process. Common bean can be nodulated by different rhizobial species from the genera Rhizobium, Ensifer, and Burkholderia, and is therefore considered to be a promiscuous host9. Because the rhizobial symbionts of legumes have been shown to exhibit some level of biogeographic distribution10, it is important to explore their phylogenetic diversity across different environments to unravel potential new species. Despite the importance of common bean in diets and in the Swati (of Eswatini) cropping systems, no information is readily available on the phylogenetic positions of the crop’s native symbionts in the country relative to known reference strains. In this study, we explored the genetic diversity of rhizobia isolated from root nodules of different common bean genotypes grown in the field at the Malkerns Research station in Eswatini using ERIC PCR fingerprinting. The study further assessed phylogenetic relationships between the native rhizobia and reference strains reported from various parts of the globe using the sequence analysis of the 16S rRNA, symbiotic genes (nifH and nodC) and housekeeping genes (glnII, rpoB, dnaK and gyrB). In addition, the symbiotic effectiveness of the rhizobial isolates was assessed under glasshouse conditions.
Results
Morphophysiological characteristics of rhizobial isolates
A total of 162 bacterial isolates were obtained from root nodules of common bean collected from Malkerns in Eswatini. Of this number, 129 isolates were able to form nodules on the common bean host (Kranskop) in an authentication study in the glasshouse. From the 129 rhizobial isolates, 88 elicited effective nodules while 41 formed ineffective nodules on the host. As expected, there were no nodules on the roots of the control (uninoculated) plants as well as the nitrate fed seedlings.
The effective rhizobial isolates were phenotypically different in terms of colony appearance, shape, size, colour, opacity and texture (Table 1). For example, 60 isolates were fast growers (taking 1–3 days to appear on YMA plates), 27 were intermediate (between 4 and 8 days) and one isolate exhibited slow growth rate and took 10 days to appear on YMA plate. Moreover, 35 of the total isolates had small colony sizes of ≤ 2 mm diameter while 53 isolates had colony diameter between 3 and 7 mm. The colonies were either opaque or translucent in appearance. A greater proportion of the isolates were opaque (68%), having a gummy texture (94%), white in appearance (67%) and flat in shape (69%) (Table 1).
ERIC-PCR fingerprints of common bean isolates
The ERIC-PCR profiles grouped the isolates of common bean into eleven (11) major clusters if considered at 20% similarity level. However, these isolates represented 80 ERIC-PCR types when considered at a 70% similarity level (Fig. 1). In terms of the number of isolates, Cluster IV was the largest and contained 18 isolates while Cluster XI contained the least number of isolates, namely, TUTPvES 44(1), TUTPvES 4(4) and TUTPvES 42(2) (Fig. 1). Some clusters grouped isolates from different common bean genotypes; for example, Cluster II grouped isolates TUTPvES 11(3), TUTPvES 15(1), TUTPvES 41(3), TUTPvES (2) and TUTPvES 4(1) which were isolated from the root nodules of genotypes DAB 470, NUC 134, KG 98-36, NUC 134 and DAB 387, respectively (Fig. 1; Table 1).
Phylogeny of isolates based on 16S rRNA gene sequences
The 16S rRNA phylogeny grouped a total of 14 isolates into four clusters (Cluster I, II, III and IV) which were closely related to known Rhizobium type strains, except for isolate TUTPvES 7(3) which stood separately from any of the type strains (Fig. 2). In Cluster I, isolates TUTPvES 15(1), TUTPvES 26(1) and TUTPvES 30(1) (with 99.4–99.7% sequence similarities) shared 99.5–99.9% sequence similarity with the type strains R. paranaense and R. jaguaris. Isolate TUTPvES 54(3) formed an outgroup of isolates in Cluster I. Furthermore, isolates TUTPvES 42(3) and TUTPvES 57(2) (with 99.9% sequence similarity) respectively shared 99.7% and 100% sequence similarity with R. jaguaris and R. paranaense in Cluster II (Fig. 2). Isolate TUTPvES 14(1) shared 99.4% to 99.5% sequence similarity with R. freirei, R. hainanense, R. multihospitum and R. tropici with 95.5% bootstrap support in Cluster III (Fig. 2). Moreover, isolates TUTPvES 4(3), TUTPvES 7(2), TUTPvES 37(1), TUTPvES 40(1), TUTPvES 43(2) and TUTPvES 57(3) grouped together in Cluster IV with 98.1–100% sequence similarity; within this Cluster IV, isolates TUTPvES 4(3) and TUTPvES 57(3) were identical to the type strain Candidatus Rhizobium massiliae while the remaining isolates shared 98.1–99.9% sequence similarities with R. pusense and R. pongamiae (Fig. 2).
Phylogenies based on housekeeping genes (dnaK, glnII, gyrB and rpoB)
Four housekeeping genes (dnaK, glnII, gyrB and rpoB) were selected for a robust multilocus sequence phylogenetic analysis. The PCR amplification of the dnaK, glnII, rpoB, and gyrB genes yielded bands of about 650, 700, 700 and 500 bp, respectively for the selected rhizobial population. The primers used and temperature conditions are listed in supplementary Table S3. The maximum likelihood phylogenetic trees based on the individual gene sequences placed the isolates in different clusters within the genus Rhizobium. Some isolates consistently grouped together in the 16S rRNA phylogram as well as in the phylograms based on individual housekeeping genes, while others showed discrepancies. For example, isolates such as TUTPvES 7(2) and TUTPvES 43(2) which grouped with other isolates in Cluster IV in the 16S rRNA gene phylogeny showed a high divergence from those isolates in the dnaK, glnII, gyrB and rpoB phylogenies (Fig. 2; Supplementary Fig. S1–S4). Nevertheless, the grouping of isolates in the individual housekeeping genes were largely consistent. For example, isolate TUTPvES 14(1) aligned with R. tropici in the glnII, gyrB and rpoB phylogenies, but stood separately in the dnaK phylogeney due to the absence of the type strain in that phylogram (Supplementary Fig. S1–S4).
Phylogeny based on concatenated gene sequences
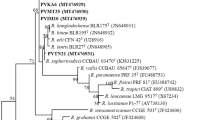
A concatenated gene phylogeny based on the combined sequences of glnII + dnaK + rpoB grouped a total of eleven (11) isolates into two main clusters (Cluster I and II) (Fig. 3). In Cluster I, isolates TUTPvES 15(1), TUTPvES 30(1) and TUTPvES 42(3) (with 99.2–99.6% sequence similarity) shared 98.3–98.9% sequence similarity with R. paranaense with 99% bootstrap support (Fig. 3). On the other hand, isolate TUTPvES 14(1) was highly divergent from the reference type strains and shared only 93.2% sequence similarity with R. multihospitium, the closest related type strain. Similarly, isolates TUTPvES 7(2) and TUTPvES 43(2) stood separately and shared only 91.5% and 92.4% sequence similarity with R. pusense and R. paranaense, respectively, the closest related type strains (Fig. 3). In Cluster III, isolates TUTPvES 37(1), TUTPvES 40 (1) and TUTPvES 57(3) (with 99.2–99.8% sequence similarity) aligned with R. pusense with 99.1–99.5% sequence similarity (Fig. 3).
Maximum-likelihood phylogeny of microsymbionts of common bean from Eswatini inferred from concatenated glnII + dnaK + rpoB gene sequences. Phylogenetic trees were inferred using MEGA 7 software 35. The Kimura 2-paramete model with uniform rates among the sites was used to calculate evolutionary distances.
Phylogeny based on symbiotic gene sequences (nodC and nifH)
The phylogeny based on nodC gene grouped all four isolates, TUTPvES 30(1), TUTPvES 26(1), TUTPvES 15(1) and TUTPvES 14(1) with R. tropici, R. freirei, R. lusitanum and R. leucaenae with 100% similarity (Fig. 4). The phylogeny based on the nifH gene also grouped 12 identical isolates with the type strains R. tropici, R. freirei, R. leucaenae, R. lusitanum, R. miluonense and R. multihospitium with 100% sequence similarity and 95% bootstrap support; however, isolate TUTPvES 40(1) formed an out group of the Cluster and shared only 96.6% sequence similarity with the type strains (Fig. 5).
Nodulation and plant growth induced by isolates
The results showed significant differences (p ≤ 0.05) in the nodule number, nodule weight and shoot dry matter (SDM) of common beans inoculated with the different rhizobial isolates (Table 2). Isolates TUTPvES 34(2), TUTPvES 20(1), TUTPvES 2(3) and TUTPvES 22(1) had significantly higher nodule numbers than the remaining isolates, recording 756, 750, 646 and 537 nodules per plant, respectively, whereas isolates TUTPvES 48(1), TUTPvES 13(3), TUTPvES 34(1) and TUTPvES 7(1) recorded the least nodule numbers, with values of 5, 20, 22, 31 nodules per plant, respectively. Isolates TUTPvES 34(2), TUTPvES 20(1) and TUTPvES 6(1) coupled higher nodule number with high nodule weight; however, isolates TUTPvES 62(2) and TUTPvES 43(2) had lower nodule number but elicited greater nodule dry matter (Table 2).
Nevertheless, there was a significant positive correlation between nodule number and nodule dry matter induced by the isolates (Fig. 6a). Generally, the rhizobia that induced higher nodule numbers, also elicited greater shoot dry matter in the common bean host plant, with a few exceptions. There was therefore a significant positive correlation when nodule number and nodule dry matter were each plotted against shoot DM (Fig. 6b and c). The values of shoot DM ranged from 0.23 g. plant-1 in plants inoculated with isolate TUTPvES 53(4) to 5.77 g. plant-1 in plants inoculated with isolate TUTPvES 20(1). Of all tested isolates, 65 induced significantly higher shoot biomass than the nitrate fed plants, whereas 24 of the isolates induced lower shoot biomass than the nitrate fed plants.
Relative symbiotic effectiveness (RE) ranged from 18% in the plants inoculated with isolate TUTPvES 53(4) to 433% in the plants inoculated with isolate TUTPvES 20(1). The data showed that over 85% of the isolates were highly effective (with RE between 103 and 433%) while five isolates were less effective (Table 2).
Photosynthetic rates induced by rhizobial isolates
Inoculating common bean seedlings with the different rhizobial isolates induced varying levels of leaf photosynthetic rates (A), and other gas exchange parameters in the plant. Plants that were inoculated with isolates TUTPvES 24(1), TUTPvES 2(3), TUTPvES 5(1) and TUTPvES 11(3) induced significantly higher photosynthetic rates (20.71, 20.61, 20.60 and 20.47 µmol (CO2) m-2 s-1, respectively) than those inoculated with the remaining isolates (Table 2). With regards to stomatal conductance, plants that were inoculated by isolates TUTPvES 49(2), TUTPvES 6(1), TUTPvES 59(1) and TUTPvES 11(3) induced significantly higher values (0.45, 0.45, 0.44 and 0.42 µmol (CO2) m-2 s-1, respectively) than those inoculated with the remaining isolates. With few exceptions however, the isolates which induced lower stomatal conductance and transpiration rates (E) were generally found to induce lower photosynthetic rates in the host common bean, and vice versa (Table 2).
Discussion
Morpho-genetic diversity of rhizobial symbionts of common bean
While the diversity of rhizobia nodulating common bean has been studied worldwide9,11, little is known about the crop’s rhizobial microsymbionts in Swati (of Eswatini) soils. The differences in morphological features of bacteria can be used as a preliminary assessment of their diversity. In this study, the diversity of rhizobial symbionts of common bean from Eswatini was evidenced by differences in their growth rate and colony appearance. However, because distantly related rhizobia may share similar morpho-physiological characteristics, ERIC PCR fingerprinting is often used as a robust tool in distinguishing closely related species12.
The genetic diversity of common bean rhizobia in Swati soils was further assessed using ERIC-PCR fingerprinting. The dendrogram constructed from ERIC-PCR profiles placed a total of 88 common bean isolates in 11 major clusters when considering a 20% similarity level, which represented 80 ERIC PCR types if considered at a 70% similarity level. The diversity of bacteria nodulating various legumes, including common bean, have previously been analysed using ERIC-PCR profiles13. For example, the study by Zinga et al.9 observed high genetic diversity among rhizobial symbionts of common bean in South African soils. Although the number of authenticated rhizobia in this study varied between common bean genotypes, the isolates from genotype DAB 381 showed greater diversity, as they [e.g., TUTPvES 4(1), TUTPvES 4(2), TUTPvES 4(3) and TUTPvES 4(4)] were distributed in four different ERIC clusters (i.e., cluster II, IV, VII and X) (Fig. 1). Similarly, the isolates from genotype DAB 155 appeared in three of the clusters. Within the major clusters, there was a general tendency for isolates from the same field or genotype to group closely together. These observations are consistent with earlier reports that similarly reported the clustering of rhizobial symbionts of cowpea in Mozambique based on their geographic location of origin14. As the fields used to trap rhizobia in this study were in proximity at the Malkerns Research Station, the soil chemical properties did not show marked variation; except for the levels of available P which was slightly higher in the soil planted to SARBEN common bean genotypes (Supplementary Table S2). It may therefore be worthwhile to explore the symbionts of the test legume across contrasting agroecologies of Eswatini to provide more insights into the impact of environmental variables on the diversity of the crop’s symbionts.
Phylogenetic positions of rhizobia nodulating common bean in Eswatini
Common bean has been widely reported to be promiscuous to a wide range of rhizobia and can be nodulated by different species including R. etli, R. mayense, R pusense, R lusitanum and R. fabae9,15. The fields in Eswatini used in this study had no history of inoculation; thus, the rhizobial isolates obtained were considered indigenous to the area, even though common bean seeds may carry viable rhizobial cells from different geographic environments16. The rhizobial isolates in this study aligned with different species belonging to the Rhizobium genus based on the 16S rRNA gene sequence analysis. As shown in Fig. 2, some of the indigenous isolates shared close relations with R. freirei, R. paranaense, R. pusense and R. tropici. However, because the 16S rRNA gene tends to show low resolution at the species/genus level, different housekeeping genes are often used to decipher closely related species17,18. In this study, the phylogeny of rhizobial isolates based on the 16S rRNA gene was mostly congruent with those of the housekeeping genes (dnaK, glnII, gyrB and rpoB), with the test isolates aligning closely with Rhizobium type strains in the different phylograms. As a result, the phylogram based on concatenated glnII + dnaK + rpoB was also congruent with those of individual housekeeping genes and showed close similarity with the type strains R. paranaense and R. pusense (Fig. 3). However, although isolate TUTPvES 14(1) consistently aligned with R. tropici in the 16S rRNA phylogeny and in the phylograms based on the individual housekeeping genes, the isolate stood away from the other type strains in the concatenated gene phylogeny due to the absence of R. tropici in that tree. These findings are similar to those of Zinga et al.9 who also found close relatives of R. tropici in the root nodules of common bean in South African soils.
Interestingly, although R. phaseoli and R. etli are known symbionts of common bean19, the indigenous isolates in this study showed low similarity with those type strains. Rhizobium tropici is a well-known microsymbiont for common bean and has been reported as the main symbiont of the crop in Latin America and in East, West and southern Africa20. However, as observed in this study, R. paranaense has previously been isolated from root nodules of common bean in sub-Saharan Africa20. The other test isolates which did not align closely with the known type strains will require a detailed description via whole genome sequencing.
As to be expected, the phylograms based on the two symbiotic genes (nifH and nodC) were incongruent with the 16S rRNA and the housekeeping genes phylogenies. For example, although the isolates grouped in different clusters in the housekeeping gene phylogenies, they formed a single cluster in the nifH and nodC phylogenies, an observation that could be attributed to the acquisition of those genes via horizontal gene transfer from a common ancestor since they are located on transmissible plasmids in some Rhizobium species21,22. A similar observation was made by Zinga et al.22 in a study on common bean symbionts from South Africa and Mozambique. Aserse et al.23 also reported incongruency between the phylogenies inferred from two symbiotic genes and that of the 16S rRNA gene in Ethiopia due to possible inter strain gene transfer and gene recombination.
Dlamini et al.24 had earlier reported the influence of soil pH and nutrient levels of the distribution of rhizobia associated with Bambara groundnut from different locations in Eswatini. Since the present study focused on the symbionts of common beans grown at one location, it may be worthwhile to explore the diversity of the crop’s symbionts across contrasting agroecologies of Eswatini in subsequent works.
Symbiotic efficiency of common bean isolates from Eswatini
Aside from variability in morpho-genetic characteristics, the diversity of common bean isolates in this study were also shown by the variable nodulation, plant growth and photosynthetic parameters they elicited in the host plants. Whereas some isolates induced both greater nodule number and nodule weight in the host, others elicited greater nodule weight despite inducing fewer nodules in the host; these observations were probably due to differences in nodule size as well as the N2-fixing efficiency of the rhizobial symbionts25. For example, despite forming few nodules, isolate TUTPvES 45(1) induced higher nodule dry matter, which led to higher photosynthetic rates and shoot dry matter when compared to the 5 mM nitrate fed plants. In some instances, nodule symbionts elicited lower plant growth and photosynthesis in the host despite inducing high nodulation, an indication that some of the nodule symbionts were less effective in fixing nitrogen (N2). Some studies have observed that legumes can sometimes form ineffective nodules that harbour low N2-fixing rhizobia26. Nevertheless, nodule number and nodule weight were both positively correlated with shoot biomass, indicating that the nodule symbionts contributed to plant growth promotion.
As the uninoculated control plants expectedly showed the least plant growth, the effectiveness of isolates was assessed by comparing the biomass of inoculated plants with that of nitrate fed plants27. Of the 88 isolates, 74% elicited greater shoot biomass in the host common bean when compared to the 5 mM nitrate-fed plants, with relative effectiveness values ranging from 103 to 433%. Thus, the soils in Eswatini are home to highly effective rhizobia that can potentially be used to formulate commercial inoculants for increased common bean cultivation upon extensive testing in the field to assess their adaptation to prevailing abiotic conditions.
Conclusion
Based on their ERIC PCR banding patterns, this study revealed a high genetic diversity among common bean symbionts in Eswatini, an observation consistent with several reports on the diversity of rhizobia in African soils. Moreover, multilocus sequence analysis based on the sequences of 16S rRNA, rpoB, dnaK, gyrB, and glnII and symbiotic (nifH and nodC) genes aligned the test isolates with the type strains of Rhizobium muluonense, R. paranaense, R. pusense, R. phaseoli and R. etli. Some of the indigenous isolates showed a high divergence from the known reference type strains, and may require further description via whole genome sequencing. Aside from their diversity, a glasshouse experiment showed that most of the isolates were efficient in fixing nitrogen, and elicited greater stomatal conductance and photosynthetic rates in the common bean host. Relative symbiotic effectiveness (RE) of the isolates varied from 18 to 433%, with 75 out of the 80 tested isolates producing greater shoot biomass than the nitrate fed control plants.
Materials and methods
Nodule collection and bacterial isolation
Root nodules were collected from different common bean genotypes grown at the Malkerns Research Station in Eswatini during the 2017/2018 cropping season. The soil chemical properties as well as the climatic data of the Malkerns site during the 2017/2018 cropping season are presented in Supplementary Tables S1 and S2. For this, the plants were carefully dug out at the early podding stage and the nodulated roots separated from the shoots. The roots were then transported to the laboratory in prelabelled zip-lock plastics in a cooler box with ice. The roots were rinsed in running tap water to remove debris, and the nodules attached to small root segments removed and preserved on silica gel in plastic vials prior to bacterial isolation. Bacteria were isolated from the nodules according to the procedure described by Somasegaran and Hoben28. For this, surface sterilized nodules were crushed in a loop of sterile water in a petri dish, and the nodule macerate streaked on yeast mannitol agar (YMA) plates and incubated at 28 °C. The plates were monitored daily for colony appearance. The number of days taken for colonies to appear as well as other morphological characteristics (colony size/shape, colour, and texture) were recorded29,30.
Nodulation bioassay in the glasshouse
A total of 162 bacterial isolates were obtained from the root nodules of the different common bean genotypes. To fulfil Koch’s postulates, single-colony cultures from these bacterial isolates were assessed for their ability to elicit root nodules on their homologous Kranskop common bean genotype in a naturally lit glasshouse under aseptic conditions. For this, the common bean seeds were surface sterilized as described by29 and planted in sterile (autoclaved) sand which were contained in sterile plastic pots. After germination, the seedlings were inoculated with 1 mL broth culture of the different bacterial isolates grown to the exponential phase, with three replicate pots per isolate. The plants were watered with autoclaved nitrogen-free nutrient solution and sterile distilled water in alternation. Uninoculated plants and plants treated with 5 mM KNO3 were used as negative and positive controls, respectively. At 60 days after planting, the plants were harvested and assessed for nodulation; isolates which elicited nodules on three replicate plants were considered as rhizobia. The use of plant materials in different aspects of this study complied with relevant institutional, national and/or institutional guidelines.
Assessment of plant growth and photosynthetic rates induced by isolates
The authenticated rhizobia were further assessed for their symbiotic efficiency using plant nodulation, growth, and photosynthetic rates as reference parameters. For this, photosynthetic rates (A), stomatal conductance (gs) and transpiration rates (E) were measured on the youngest fully expanded trifoliate leaves of common bean plants inoculated with the different isolates using a portable infrared red gas analyser, version 6.2 (LI 6400XT, Lincoln, Nebraska, USA). The chamber conditions were set as follows: photosynthetic flux density of 1000 μmolm-2 s-1, reference CO2 concentration of 400 μmolmol-1 and flow rate of 500 μmols-1. The gas exchange measurements were performed between 9.00 am and 12.00 pm at 60 days after planting31. Thereafter, the plants were harvested, separated into shoots and nodulated roots, and the nodules collected from the roots. The shoots and nodules were separately oven-dried in brown paper envelopes, and weighed to determine shoot and nodule dry matter, respectively. The relative effectiveness (RE) of isolates was calculated by expressing the shoot biomass of plants inoculated with the rhizobial isolates as a percentage of the shoot biomass of plants treated with the 5 mM KNO332.
The isolates were categorized as highly effective (> 80% RE), moderately effective (50 to 80% RE), lowly effective (35 to 49% RE) and ineffective (< 35% RE).
Molecular characterization of isolates
Genomic DNA extraction and ERIC PCR fingerprinting
Bacterial genomic DNA extraction was carried out using the GenElute bacterial DNA isolation kit by following the manufacturer’s instructions (Sigma Aldrich, USA). The quality of DNA was assessed on a 1% agarose gel stained with ethidium bromide. The genomic DNA of rhizobial isolates were subjected to ERIC-PCR fingerprinting. The final PCR reaction volume was 25 μL and contained 1 μL of genomic DNA,12.5 μL 2× myTaq PCR master mix (Bioline USA), 1 μL each of the forward and reverse ERIC primers and 9.5 mL double distilled water. The DNA amplification was carried out in a Thermal cycler (T100 Bio-Rad, USA) using standard temperature profiles24,33, and the PCR products were subjected to gel electrophoresis on a 1% agarose gel at 85 V for 5 h. A cluster analysis to determine the similarities among isolates using the Jaccard’s similarity coefficient was performed with the Bionumerics software (version 8.1).
PCR amplification of 16S rRNA, housekeeping (rpoB, dnaK, gyrB, and glnII) and symbiotic (nifH and nodC) genes
The amplification of the 16S rRNA, housekeeping genes (rpoB, dnaK, gyrB, and glnII), and symbiotic (nifH and nodC) genes were carried out in a 25 μL PCR reaction mixture which contained 1 μL bacterial DNA, 3 μL of 5× myTaq buffer, 1 μL each of the forward and reverse primers of the gene of interest, 0.1 μL Taq polymerase (Bioline, USA), and 18.9 μL sterile ultrapure water using standard temperature profiles (Supplementary Table S3) in a T100 Bio-Rad Thermal Cycler, USA. The amplified gene products were confirmed by gel electrophoresis in a 1% agarose gel and the image captured using the GeldocTm XR + system (Bio-Rad, USA). The primers used and their temperature profiles are shown in Supplementary Table S3.
Sequencing and Phylogenetic analysis of amplified genes
The PCR-amplified gene products were purified using a PCR cleanup kit (NEB, USA) following the manufacturer’s instructions. Purified DNA was sequenced at Macrogen laboratories (The Netherlands). The software BioEdit 7.0.9.0 was used to confirm the quality of sequences34. The sequences of each gene were subjected to BLASTn in the National Centre Biotechnology Information (NCBI) database to identify closely related rhizobial species. The alignment of the reference strain sequences with the test rhizobial isolates were done with CLUSTAL W and phylogenetic trees were inferred using MEGA 7 software35. The Kimura 2-paramete model with uniform rates among the sites was used to calculate evolutionary distances and evolutionary history was inferred using the maximum likelihood method. The robustness of tree branching was estimated using 1000 bootstrap replicates of the sequence36.
Statistical analysis
All quantitative data collected from the glasshouse experiment were subjected to a one-way ANOVA using the STATISTICA program (Version 10). The quantitative datasets such as nodule number, nodule dry matter, shoot dry matter, photosynthetic rates (A), stomatal conductance (gs), leaf transpiration (E) and relative symbiotic effectiveness (RE) were tested for normality by calculating skewness and kurtosis values using the Data Analysis component of Excel. The skewness and kurtosis values ranged from − 0.10 to + 1.51 and −1.67 to + 2.30, respectively, and are consistent with values of a normal distribution37. Where there were significant treatment differences, the Duncan’s multiple range test was used to separate the means at p ≤ 0.05. Correlation analyses were done to assess the relationship between measured parameters.
Data availability
The nucleotide sequences of all the tested genes were submitted to the NCBI GenBank database to obtain the accession numbers: OP537152–OP537165 (16S rRNA), OP654835–OP654843 (gyrB), OP654807–OP654820 (dnaK), OP654821–OP654834 (glnII), OP654844–OP654855 (rpoB), OP654856–OP654868 (nifH) and OP654869–OP654872 (nodC).
References
Broughton, W. J. et al. Beans (Phaseolus spp.)-model food legumes. Plant and Soil vol. 252 http://www.phaseolus.net (2003).
Mwenda, G. M., O’Hara, G. W., de Meyer, S. E., Howieson, J. G. & Terpolilli, J. J. Genetic diversity and symbiotic effectiveness of Phaseolus vulgaris-nodulating rhizobia in Kenya. Syst. Appl. Microbiol. 41, 291–299 (2018).
Linnemann, A. R., Westphal, E. & Wessel, M. Photoperiod regulation of development and growth in bambara groundnut (Vigna subterranea). Field Crops Res. 40, 39–47 (1995).
Edje, O. T. & Mavimbela, E. K. Response of Bambara groundnuts [Vigna subterranea [L.] Verd] to inter-row spacing in Swaziland. Afr. J. Appl. Agric. Sci. Technol. 1, 91–104 (2014).
Kawaka, F. et al. Effect of nitrogen sources on the yield of common bean (Phaseolus vulgaris) in western Kenya. J. Plant. Nutr. 41, 1652–1661 (2018).
Vlassak, K. M., Vanderleyden, J. & Graham, P. H. Factors influencing nodule occupancy by inoculant rhizobia. Crit. Rev. Plant Sci. 16, 163–229 (1997).
Odori, C., Ngaira, J., Kinyua, J. & Nyaboga, E. N. Cogent Food & Morphological, genetic diversity and symbiotic functioning of rhizobia isolates nodulating cowpea (Vigna unguiculata L. Walp) in soils of Western Kenya and their tolerance to abiotic stress. Cogent Food Agric. 6(1), 1853009 (2020).
Kamfwa, K., Cichy, K. A. & Kelly, J. D. Genome-wide association analysis of symbiotic nitrogen fixation in common bean. Theor. Appl. Genet. 128, 1999–2017 (2015).
Zinga, M. K., Jaiswal, S. K. & Dakora, F. D. Presence of diverse rhizobial communities responsible for nodulation of common bean (Phaseolus vulgaris) in South African and Mozambican soils. FEMS Microbiol. Ecol. 93(2), fiw236 (2017).
Sprent, J. I., Ardley, J. & James, E. K. Biogeography of nodulated legumes and their nitrogen-fixing symbionts. New Phytol. 215, 40–56. https://doi.org/10.1111/nph.14474 (2017).
Martens, M. et al. Advantages of multilocus sequence analysis for taxonomic studies: A case study using 10 housekeeping genes in the genus Ensifer (including former Sinorhizobium). Int. J. Syst. Evol. Microbiol. 58, 200–214 (2008).
Xu, L. et al. Rhizobium qilianshanense sp. Nov., a novel species isolated from root nodule of Oxytropis ochrocephala Bunge in China. Antonie Van Leeuwenhoek 103, 559–565. https://doi.org/10.1007/s10482-012-9840-x (2013).
Wadhwa, K., Dudeja, S. S. & Yadav, R. K. Molecular diversity of native rhizobia trapped by five field pea genotypes in Indian soils. J. Basic Microbiol. 51, 89–97 (2011).
Chidebe, I. N., Jaiswal, S. K. & Dakora, F. D. Distribution and phylogeny of microsymbionts associated with cowpea (Vigna unguiculata) nodulation in three agroecological regions of Mozambique. Appl. Environ. Microbiol. 84(2), e01712-17 (2018).
Kaschuk, G., Hungria, M., Andrade, D. S. & Campo, R. J. Genetic diversity of rhizobia associated with common bean (Phaseolus vulgaris L.) grown under no-tillage and conventional systems in Southern Brazil. Appl. Soil Ecol. 32, 210–220 (2006).
Grange, L. & Hungria, M. Genetic diversity of indigenous common bean (Phaseolus vulgaris) rhizobia in two Brazilian ecosystems. Soil Biol. Biochem. 36, 1389–1398 (2004).
Martens, M. et al. Multilocus sequence analysis of Ensifer and related taxa. Int. J. Syst. Evol. Microbiol. 57, 489–503 (2007).
Adékambi, T., Drancourt, M. & Raoult, D. The rpoB gene as a tool for clinical microbiologists. Trends Microbiol. 17, 37–45. https://doi.org/10.1016/j.tim.2008.09.008 (2009).
Bongo, A. & Peitr, S. Biodiversity of nitrogen fixing indigenous rhizobia nodulating electronic polish agricultural pulses isolated from Sub-Saharan Africa soils. A. J. Pol. Agric. Univ. 22, 1–11 (2019).
Bongo, A. & Peitr, S. Biodiversity of nitrogen fixing indigenous rhizobia nodulating electronic polish agricultural pulses isolated from Sub-Saharan Africa soils. J. Pol. Agric. Univ 22, 1–11 (2009).
Martínez-Romero, E. & Palacios, R. The Rhizobium genome. Crit. Rev. Plant Sci. 9, 59–93 (1990).
Zinga, M. K., Jaiswal, S. K. & Dakora, F. D. Presence of diverse rhizobial communities responsible for nodulation of common bean (Phaseolus vulgaris ) in South African and Mozambican soils. FEMS Microbiol. Rev. 93, 1–16 (2017).
Aserse, A. A., Räsänen, L. A., Assefa, F., Hailemariam, A. & Lindström, K. Phylogeny and genetic diversity of native rhizobia nodulating common bean (Phaseolus vulgaris L.) in Ethiopia. Syst. Appl. Microbiol. 35, 120–131 (2012).
Dlamini, S. T., Jaiswal, S. K., Mohammed, M. & Dakora, F. D. Studies of phylogeny, symbiotic functioning and ecological traits of indigenous microsymbionts nodulating bambara groundnut (Vigna subterranea L. Verdc) in Eswatini. Microb. Ecol. https://doi.org/10.1007/s00248-021-01684-0 (2021).
Ibny, F. Y. I., Jaiswal, S. K., Mohammed, M. & Dakora, F. D. Symbiotic effectiveness and ecologically adaptive traits of native rhizobial symbionts of Bambara groundnut (Vigna subterranea L. Verdc) in Africa and their relationship with phylogeny. Sci. Rep. 9(1), 12666 (2019).
Simbine, M. G., Mohammed, M., Jaiswal, S. K. & Dakora, D. D. Functional and genetic diversity of native rhizobial isolates nodulating cowpea (Vigna unguiculata L. Walp.) in Mozambican soils. Sci. Rep. 11, 1–15 (2021).
Gyogluu, C., Jaiswal, S. K., Kyei-Boahen, S. & Dakora, F. D. Identification and distribution of microsymbionts associated with soybean nodulation in Mozambican soils. Syst. Appl. Microbiol. https://doi.org/10.1016/j.syapm.2018.05.003 (2018).
Somasegaran, P. H. & Hoben, H. J. Methods in Legume-Rhizobium Technology. (1985).
Somasegaran, P. & Hoben, H. J. Handbook for Rhizobia (Springer-Verlag, New York, 1994). https://doi.org/10.1007/978-1-4613-8375-8.
Howieson J.G & Dilworth M.J. Working with rhizobia. (Australian Centre for International Agricultural Research, 2016).
Mohammed, M., Jaiswal, S. & Dakora, F. Insights into the phylogeny, nodule function, and biogeographic distribution of microsymbionts nodulating the the orphan kersting’s groundnut [Macrotyloma geocarpum (Harms) Marechal & Baudet] in African Soils. Appl. Environ. Microbiol. 85, 1–21 (2019).
Chibeba, M. A., Kyei-boahen, S., Guimarães, M. D. F., Nogueira, A. M. & Hungria, M. Isolation, characterization and selection of indigenous Bradyrhizobium strains with outstanding symbiotic performance to increase soybean yields in Mozambique. Agric. Ecosyst. Environ. 246, 291–305. https://doi.org/10.1016/j.agee.2017.06.017 (2017).
Bruijn, F. J. D. E. Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergeneric consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl. Environ. Microbiol. 2180–2187(58), 2180–2187 (1992).
Hall, T. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 41, 95–98 (1991).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Felsentein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution (N Y) 39, 783–791 (1985).
Debbie, H., Peter, M., Diana, D. & Laurence, T. Research Methods for the Biosciences (Oxford University Press, Oxford, 2016).
Acknowledgements
We are grateful to the South African Research Chair in Agrochemurgy and Plant Symbioses, the National Research Foundation, and the Tshwane University of Technology (TUT) for financial support to FDD’s research, and for a competitive NRF Doctoral bursary to RPG. We are grateful to Mr Manana, Head of the Malkerns Research Station, for permission to sample common bean plants from their field trials, and to all the staff for helping us to sample the bean plants.
Author information
Authors and Affiliations
Contributions
R.P.G. collected root nodules, carried out bacterial isolation and characterization. M.M. took part in nodule collection, molecular characterization of isolates and drafted the manuscript with R.P.G.; S.K.J. took part in molecular characterization of isolates. F.D.D. is the doctoral supervisor of R.P.G., secured funding for the research and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gunununu, R.P., Mohammed, M., Jaiswal, S.K. et al. Phylogeny and symbiotic effectiveness of indigenous rhizobial microsymbionts of common bean (Phaseolus vulgaris L.) in Malkerns, Eswatini. Sci Rep 13, 17029 (2023). https://doi.org/10.1038/s41598-023-43634-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43634-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.