Abstract
The growing interest in microRNAs (miRNAs) over recent years has led to their characterization in numerous organisms. However, there is currently a lack of data available on miRNAs from triatomine bugs (Reduviidae: Triatominae), which are the vectors of the protozoan parasite Trypanosoma cruzi, the causative agent of Chagas disease. A comprehensive understanding of the molecular biology of vectors provides new insights into insect-host interactions and insect control approaches, which are key methods to prevent disease incidence in endemic areas. In this work, we describe the miRNome profiles from gut, hemolymph, and salivary gland tissues of the Rhodnius prolixus triatomine. Small RNA sequencing data revealed abundant expression of miRNAs, along with tRNA- and rRNA-derived fragments. Fifty-two mature miRNAs, previously reported in Ecdysozoa, were identified, including 39 ubiquitously expressed in the three tissues. Additionally, 112, 73, and 78 novel miRNAs were predicted in the gut, hemolymph, and salivary glands, respectively. In silico prediction showed that the top eight most highly expressed miRNAs from salivary glands potentially target human blood-expressed genes, suggesting that R. prolixus may modulate the host’s gene expression at the bite site. This study provides the first characterization of miRNAs in a Triatominae species, shedding light on the role of these crucial regulatory molecules.
Similar content being viewed by others
Introduction
Insects of the Triatominae subfamily (Hemiptera: Reduviidae) are vectors of the Chagas disease agent Trypanosoma cruzi. While the impact of Chagas disease has decreased over the years, it continues to cause disability and death in Latin America, an endemic region where transmission primarily occurs during the blood meal of an infected insect or through the ingestion of contaminated food1. Moreover, due to migratory flows, the disease has been reported in non-endemic areas, where alternative routes such as blood transfusion, organ transplantation, and vertical transmission take place1,2,3. Estimates indicate a range of 6–7 million people worldwide suffer from disease-related morbidity, resulting in over 10,000 deaths annually4,5,6,7,8. Rhodnius prolixus is considered one of the main vectors of Chagas disease in Central America and northern South America.
The vector's bite causes vascular and tissue damage in the host, triggering a series of physiological defense mechanisms that act against pathogen invasion and blood loss, including immune response, inflammation, and hemostasis. Many studies have reported on the key effects of salivary molecules from blood-sucking insects, which are secreted during the bite to assist in the feeding process, counteracting the host's hemostasis, and modulating its immune responses9,10,11. In this context, transcriptomic and proteomic studies have unveiled the complexity of salivary content12. The blood ingested from the host is stored, digested, and absorbed in the gut compartments of the insect, where, upon infection, T. cruzi develops and replicates13. However, triatomine immune pathways and the details of their interactions with T. cruzi remain poorly understood. Insect hemolymph is a dynamic circulating tissue that mediates hormone, nutritional, and immunological homeostasis. Its composition varies depending on factors such as nutrient availability, stress conditions, and current physiological needs14. Hemolymph components are involved in the dynamics of the insect immunity, and in this regard, hemocytes and multiple soluble effectors work together towards a coordinated response to injury and/or control of pathogen development15.
Studies on gene regulation have revealed microRNAs (miRNAs) as a critical research topic. Insect miRNAs may coordinate the regulation of many protein-coding genes related to various biological processes16. Functional investigations have established their roles in metabolism17,18,19, development, apoptosis20,21,22,23, and immune responses24,25. MiRNAs are small non-coding RNAs (sncRNAs) with ~ 22 nucleotides in length that regulate gene expression by inhibiting translation or targeting mRNAs for cleavage at the posttranscriptional level through base pairing with the complementary untranslated regions (3′UTR) of a target mRNA sequence16,26,27,28. Conversely, miRNAs have also been reported to upregulate the expression of target genes29,30,31.
Knowledge on the roles of miRNA in arthropod hematophagous vectors is also increasing. Analyses of miRNA expression profiles in mosquitoes suggests that they are induced upon blood feeding and may regulate development and immunity32,33,34,35,36,37,38,39. Interactions of aae-miR-375 with Aedes aegypti immune-related genes have been documented and enhance Dengue virus serotype 2 infection in Ae. aegypti cell lines38. Mosquito miRNAs are also differentially expressed in the presence of the bacterial endosymbiont Wolbachia, where aae-miR-12 suppresses Mct1 and Mcm6 target genes, playing a crucial role in controlling Wolbachia density in mosquito cells40,41. Further studies have demonstrated that aae-miR-2940 downregulates the transcript levels of DNA methyltransferase (AaDnmt2), inhibiting Dengue virus replication in Wolbachia-infected mosquito cells42. Moreover, the expression of aae-miR-2940 is selectively downregulated in Aedes albopictus (C6/36) cells in response to the West Nile virus43.
Extracellular miRNAs (ex-miRNAs) have been observed in body fluids such as serum and saliva44,45. To protect and stabilize ex-miRNAs in the extracellular milieu, circulation occurs within vesicles or in miRNA/protein complexes46,47. Circulating ex-miRNAs can be delivered to target cells, mediating a direct cell–cell communication, or even a dynamic cross-species function, where the recipient cell takes up miRNAs to modulate protein production44,48. Regarding the vector-parasite-host interface, it was reported that a human-blood-derived miRNA, hsa-miR-150-5p, modulates mosquito immune response to facilitate flavivirus infection and transmission49. The specific mechanisms of ex-miRNA uptake by recipient cells, vesicles, or membrane receptors, as well as the control of this process, remain under investigation.
Although miRNAs have been described as important molecular regulators of other insect vectors, there is a lack of knowledge concerning miRNA expression in Triatominae. To address this gap, we described the miRNomes of the salivary glands (SG), gut (G), and hemolymph (H) from R. prolixus through sRNA-seq. Our analysis revealed transcriptional evidence of several known and novel miRNAs in R. prolixus tissues. This repertoire offers new insights into the dynamic interactions between vector/pathogens and vector/hosts and may be explored to expand our understanding of miRNA roles in triatomine biological functions.
Results and discussion
Overview of the sncRNAs from Rhodnius prolixus
To identify the miRNome from R. prolixus tissues, three independent libraries of small RNAs were constructed and used for high-throughput sequencing. These libraries generated over 63, 57, and 80 million raw reads from the G, H, and SG tissues, respectively. After size selection (≥ 18 nt) and filtering out of low-quality sequences and adapter contaminants, more than 61 (G), 38 (H), and 54 (SG) million processed reads were obtained (Supplementary Table S1).
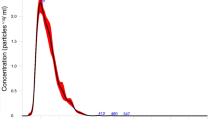
The processed reads within the three libraries (18–51 nt) were examined for read length distribution and abundance (Fig. 1). Most of the reads fell within the expected range of 20–36 nt, typical for small non-coding RNAs (sncRNAs)50. In the G and SG libraries, two major peaks were observed, one in the 21–23 nt range and a smaller peak in the 27–29 nt range. The length distribution of mapped reads in the H library showed smaller peaks, with the one in the 21–23 nt range being more prominent (Fig. 1). The read abundance profiles suggest that the H tissue has a relatively lower abundance of sncRNAs species. Given that the ~ 21–23 nt range corresponds to the typical size of mature miRNA, it can be concluded that miRNAs are present in a significant proportion in the three tissues analyzed. Additionally, the ~ 27 nt reads in G and SG libraries may represent piRNAs, a class of sncRNAs that typically range from 24 to 30 nt in length and can vary in abundance across different tissue types51,52. The two-peak feature (21–23 and 27–29 nt) has been previously reported in other insect miRNomes53,54,55.
The miRNomes from Rhodnius prolixus gut, hemolymph, and salivary gland possess conserved miRNAs
Genome-mapped reads were aligned against a collection of miRNA sequences from miRBase56 (see “Materials and methods” section); Table 1 presents the read counts and the number of the extracted R. prolixus miRNAs. Using the sequence similarity searching method, a total of 13,652,243 (G), 8,711,715 (H), and 21,489,863 (SG) reads were aligned, resulting in the annotation of 51 (G), 45 (H), and 43 (SG) known miRNAs. Notably, all the extracted R. prolixus miRNAs belonged to species within the phylum Arthropoda. The distribution of alignment hits per species and the corresponding abundance of reads from the extracted R. prolixus miRNAs are illustrated in Fig. 2. Overall, 52 miRNAs were identified. When comparing the resulting miRNAs extracted from the G, H, and SG tissues, a strong conservation across the tissues was observed, with an overlap of 39 miRNAs. Additionally, a small fraction of tissue-specific miRNAs was observed, with 3 miRNAs unique to G and 1 unique to H (Fig. 3). MiRBase serves as a comprehensive public repository of miRNA data, and its latest release (v.22, 2022) contains entries from 271 organisms, with a total of 48,860 mature miRNA sequences56. Due to the relatively limited diversity of insect sequences in miRBase, however, the recovery of conserved miRNAs in samples may have been somewhat restricted.
Abundance of Rhodnius prolixus miRNA reads aligned against known miRNAs database. The box plots illustrate hit species distribution results of R. prolixus genome-mapped reads aligned against a collection of known miRNAs from Ecdysozoa taxa. Features are based on sequence alignments and the abundance of reads from the extracted R. prolixus miRNAs. The box covers the interquartile interval, where 50% of the data is found. Each point represents a unique miRNA. Species are shown at the left for visualization.
In our workflow, a single library was sequenced per tissue, which can pose challenges when discussing abundance in data analysis. To minimize this issue, we increased the number of individuals batched together and employed deep sequencing to maximize biological variability and coverage in a unique RNA-seq dataset. The expression levels of R. prolixus miRNAs and their nucleotide sequence can be observed in Fig. 4. A detailed analysis revealed eight up-regulated miRNAs (with an AI > 70 cut-off) in G, two in H, and eight in SG, accounting for 85%, 65%, and 94% of the mature read counts respectively, suggesting their potential crucial regulatory roles (Supplementary Fig. S1). These abundant miRNAs (miR-8-5p; miR-184-3p; bantam-3p; miR-12a-5p; miR-276b-5p; miR-10-5p; miR-306-5p; miR-283; miR-281-5p) are shared among the three tissues, although exhibiting different expression patterns (Fig. 4). Specifically, miR-276b-5p is up-regulated across all three tissues, while miR-8-5p and bantam were up-regulated in G and SG, and miR-184-3p in G and H. It has been previously suggested that insects possess a conserved miRNA repertoire comprising 65 families with minimal variation57. Most miRNAs identified in this study belong to this insect-conserved collection, which includes the ubiquitously up-regulated miR-8-5p, bantam, and miR276b-5p. Deep-sequencing studies have consistently demonstrated a strong conservation of miRNAs across different insect species57.
Expression levels of Rhodnius prolixus miRNAs. The expression levels of miRNAs are based on normalization standards and AI levels, as described in the “Materials and methods” section. The blue color represents the highly expressed conserved miRNAs, the gray color represents the mild expressed, and yellow is the less expressed, all shown with color intensities degree. Colored circles indicate the presence of miRNA in the tissue as indicated: pink, gut; green, hemolymph; blue, salivary gland. The highlighted blue bars indicate the top expressed miRNAs. The miRNA sequences are shown.
Interestingly, the sum of AI values for these enriched miRNAs (G: AI = 1531; H: AI = 383; SG: AI = 3726) revealed that the highest expression levels are observed in SG, surpassing those in G by more than double and H by sevenfold. A relevant aspect to note is that miRNAs from cellular fractions and ex-miRNAs were extracted together as a single sample, making it challenging to determine the contribution of each miRNA to the dynamic miRNome profiles.
Determining the physiological concentration of miRNAs and elucidating the key players involved in changes in miRNAs levels depends on physiological state and environmental responses58,59. The expression pattern of a specific tissue miRNome delineates the mRNA collection under its influence, shedding light on the biological pathways that may be regulated. Over millions of years, triatomines have evolved blood-feeding behavior to ensure a successful acquisition of the blood meal, suggesting that miRNAs from the SGs may also play specific roles, contributing to gene regulation at a cross-species level within vector-host and even vector-pathogen-host interactions. Indeed, ex-miRNAs have been reported in mosquitoes acting in this interface, potentially contributing to the manipulation of vertebrate host, and influencing the transmission of pathogens60,61.
Drosophila has been used as a model organism to decipher miRNA functions due to the conservation of its genes and regulatory pathways throughout evolution. Some biological functions were proposed regarding the four ubiquitously up-regulated miRNAs in R. prolixus tissues (miR-8, bantam, miR-184 and miR-276). miR-8 is highly conserved and plays a variety of roles related to growth and development, regulating multiple peptide hormones of body development62,63. It was reported that miR-8 modulates neuronal development by regulating the morphology of presynaptic arbors at the Drosophila neuromuscular junction through a postsynaptic mechanism64. In addition, atrophin is a tuning target of miR-8. Silencing of miR-8 causes neurodegeneration and consequently development defects65. Moreover, miR-8 and miR-2 are critical for ecdysone-induced chitin biosynthesis in the hemipteran insect Nilaparvata lugens66. Here, R. prolixus miR-8 is up-regulated, a trait already demonstrated in the miRNomes of other hematophagous insects like Anopheles gambiae and An. funestus mosquitoes36,37.
Conserved bantam also controls cell and tissue growth, mostly regulating the production of ecdysone, a hormone tightly controlled during larval development. This miRNA mediates the insulin-dependent regulation of ecdysone, acting as a buffering mechanism to adjust growth in response to nutrient availability67. Bantam might also be a positive hematopoietic regulator in the stem cell-like niche, by functioning upstream of the insulin signaling pathway68. Furthermore, it may prevent apoptosis by downregulating the apoptotic gene hid or even targeting the clk gene, regulating circadian rhythms in Drosophila69,70. MiR-184 regulates several distinct steps during oogenesis and early embryogenesis of Drosophila over multiple biological processes, targeting components involved in the regulation of development71.
A role in neural circuits of Drosophila underlying naive olfactory responses and conditioned odor memory was proposed for miR-276a. It was demonstrated that DopR, a type-one dopamine receptor, is a functional downstream effector of miR-276a72. Interestingly, miRNA expression profiles of infected and uninfected midguts from Aedes albopictus, an important vector for dengue virus, revealed miR-276 is up-regulated in infected mosquitoes and enhanced dengue virus replication in C6/36 cells73, a result that reinforces the possible unusual roles for arthropod vector miRNAs.
Validation of miRNA expression by qPCR
The enrichment of selected miRNAs was analyzed by RT-qPCR amplification. A subset of five highly- and two lowly-abundant miRNAs from the SGs was selected for correlation analysis using slRT-PCR approach74. The Ct values obtained for miR-283, miR-12a, miR-281, bantam, and miR-8 (up-regulated) were much lower than those for miR-315 and miR-92b (down-regulated). The correlation between miRNA abundance (read counts) and Ct values was measured and is presented in Supplementary Fig. S2. The obtained data show RT-qPCR Ct values that reflect the read abundance in small RNA-seq analysis.
The novel miRNAs from Rhodnius prolixus gut, hemolymph, and salivary glands
Prediction of novel miRNAs is based on secondary structures and lack of homology with miRNAs in other species75. Two hundred and one candidates were predicted as novel miRNAs in R. prolixus miRNomes. The subsets of novel miRNAs in G, H, and SG tissues consist of 112, 73, and 78 unannotated members, respectively. Supplementary Fig. S3 presents the predicted secondary structure of the two most abundant R. prolixus novel miRNAs precursors in each tissue. A comparative analysis among G, H, and SG tissues showed an overlap of 15 miRNAs. Furthermore, 71 were specific to G, 45 to H, and 37 to SG. In this regard, miRNA genes evolve easily, and targets may be also easily gained and lost76. Most of these novel non-conserved miRNAs were found to be expressed at low levels, a trend that may be related to more specific biological roles76. The read counts and the total number of the predicted novel miRNAs from R. prolixus samples can be seen in Supplementary Table S2. The overlap analysis of these three subsets can be observed in Fig. 5.
The sncRNA collection from Rhodnius prolixus is enriched with miRNA reads and possesses tRNA- and rRNA-derived RNA fragments
By aligning the genome-mapped reads against a collection of sncRNAs from Triatominae, we successfully classified the extracted sncRNA types, which included snoRNA, snRNA, tRNA- and rRNA-derived fragments. The R. prolixus miRNA sequences (both conserved and novel) obtained from our analysis were also included in this dataset to estimate the diversity and abundance distribution of sncRNA types within the three libraries. Supplementary Fig. S4 illustrates the diversity distribution of the identified sncRNA types from R. prolixus as a percentage-based representation. Tissues are mainly composed of 52% (G), 51% (H), and 54% (SG) rRNA, followed by 31% (G), 35% (H), and 20% (SG) tRNAs. With the advent of high-throughput sequencing, several sncRNA types, including tRNA- and rRNA-derived RNA fragments, have been identified77,78. For instance, rRNA-derived RNA fragments were reported in the small RNA sequencing library of the Amblyomma testudinarium tick79. The diversity of sncRNA types found here reflects these ongoing findings, suggesting these fragments are not random intermediates or degradation products.
Furthermore, we performed a second analysis to estimate the abundance of aligned reads, expressed as percentage, which reveals the relative frequency of the identified sncRNA types and that the R. prolixus sncRNA collection is enriched with miRNAs. Analysis of read abundance within each sample demonstrated a similar expression pattern in all tissues (Fig. 6). Although miRNAs represented a minor proportion of the sncRNA diversity analysis (Supplementary Fig. S4), conserved miRNAs are the most abundant type of sncRNA in G (84.21%), H (64.7%), and SG (91.36%) tissues (Fig. 6). R. prolixus novel miRNAs constitute 6.63% (G), 7% (H), and 4.01% (SG). The remaining fraction of reads corresponds to non-miRNA types, representing a minor proportion of the total sncRNAs.
Small RNA abundance of Rhodnius prolixus samples. The bar graphs summarize reads abundance of genome-mapped reads aligned against a collection of sncRNAs from Triatominae downloaded from the RNAcentral database (https://rnacentral.org) and the extracted R. prolixus miRNAs sequences (conserved and novel). The horizontal axis represents the abundance (%) of aligned reads (log2 of the enrichment ratio).
In silico prediction of miRNA:mRNA (Rhodnius prolixus miRNA:human blood) target site interactions suggests a cross-species gene expression regulation
Triatomines have evolved salivary molecules that specifically target critical immune and hemostatic pathways in the host to enhance the blood-feeding process. Considering that the primary role of miRNAs is to bind to the 3′ UTR of target mRNAs, we employed an in silico approach to assess miRNA:mRNA interactions between vector and host molecules. We focused on the top eight most highly expressed miRNAs in R. prolixus SGs, those with AI > 70 (namely miR-283, miR-12a, miR-281, bantam, miR-8, miR-375, miR-276b, and miR-306). mRNA transcripts from human blood were used for target analysis prediction. The overall results revealed that out of 4514 blood mRNA transcripts, 322 were identified as potential targets for the top eight expressed miRNAs from R. prolixus SGs, including 72 being targeted by at least two different miRNAs. Notably, multiple targets were observed when a single miRNA targeted the 3′UTRs of different genes. A comprehensive list of the mRNAs targeted by SG miRNAs and the 1128 putative extracted interactions can be observed in Supplementary Table S3. Supplementary Fig. S5 provides the number of interactions.
To gain biological insight from the generated data, we performed an enrichment analysis using Reactome Pathways and Biological Process from Gene Ontology on the set of the target mRNA80. The analysis highlighted biological pathways involved in host responses. Meaningful results within the TOP 20 enriched pathways are presented in Fig. 7A. These include Hemostasis, Factors involved in megakaryocyte development and platelet production, Signaling by VEGF, Signaling by receptor tyrosine kinases, Platelet activation, signaling and aggregation, VEGFA-VEGFR2 Pathway, and Complement cascade. Additionally, the enriched set of terms from Gene Ontology related to the targeted genes revealed biological processes involved in hemostasis, coagulation, and vasculature (Fig. 7B). Previous studies on A. aegypti showed that five salivary miRNAs (miR-184, miR-375, miR-2490, miR-12 and miR-100) play potential roles in the transmission of Chikungunya virus (CHIKV) and in the establishment of infection during blood feeding on the host60. Furthermore, the analysis of salivary miRNAs from Ixodes ricinus tick suggests they modulate vertebrate host homeostasis81. More recently, a study on the malaria vector Anopheles coluzzii revealed the presence of salivary miRNAs that share similarity with human miRNAs, indicating their potential role in regulating host mRNAs associated with immune and inflammatory responses39.
Enriched pathways and Gene ontology Biological Process terms related to genes targeted by the top eight most highly expressed miRNAs from Rhodnius prolixus salivary glands. (A) Enriched pathways. The horizontal axis represents the Log2 of the enrichment ratio. The vertical axis represents the − Log10 value of the FDR rate. The size and color of the dots are proportional to the size of the category. The most significant pathways are towards the top. Especially meaningful categories for anti-hemostatic salivary function within the TOP 20 functional categories with FDR < = 0.05 are labeled. (B) Enriched set of terms from Geneontology Biological Process. The horizontal axis represents the Log2 of the enrichment ratio. The vertical axis represents the − Log10 value of the FDR rate. The size and color of the dots are proportional to the size of the category. The most significant pathways are towards the top. The TOP 20 terms with FDR < = 0.05 are labeled.
These findings support that a dynamic cross-species gene expression regulation mediated by miRNAs from insect vectors may occur within the vector-host and vector-pathogen-host scenarios during blood feeding. Our results indicate that miRNAs from R. prolixus saliva can also target host responses, such as the human hemostatic response. Moreover, host exposure to vector bites leads to physiological changes that may be attributed to miRNA's vector molecules, highlighting the potential of circulating miRNAs as biomarkers of vector bites. Incorporating such information provides valuable insight into a new paradigm of salivary molecule functions in hematophagous arthropods. It is important to note that the identification and characterization of individual miRNAs from hematophagous insect vectors and their potential targets have not been fully elucidated to date.
Conclusion
Here we present the first comprehensive miRNome profiles of R. prolixus tissues using high-throughput sequencing. Our findings successfully identified conserved as well as predicted novel miRNAs. The results highlight the ubiquitous presence of conserved miRNAs in R. prolixus tissues, with tissue-specific enrichment. Gene expression control is a complex process influenced by multiple regulators. Regarding miRNAs from G and H, they may have a role in blood digestion, metabolism regulation, and immunity. Additionally, our in silico analysis indicates that miRNAs expressed in the SGs could potentially exert a cross-species gene expression regulation when delivered in the vertebrate host at the bite site, specifically targeting the host’s hemostatic responses. It is plausible that these miRNAs may be packaged in exosomes, providing protection against degradation until they reach the host. Although the precise biological functions of R. prolixus miRNAs remain a major challenge, their identification provides new insights into the hematophagous vector molecules, offering pieces of knowledge that could be used for the development of novel approaches to the control of Chagas disease and improving our understanding on the intricate vector-parasite-host interactions.
Materials and methods
Sample collection and total RNA extraction
Rhodnius prolixus triatomines were reared in standard insectary conditions at the University of Brasilia, Brazil (27 ± 1 °C, 70–75% relative humidity, and 12 h/12 h light/dark cycle). The blood source of these insects was Gallus gallus domesticus. Adult insects were dissected under a stereomicroscope at 4× magnification in cold sterile phosphate-buffered saline to obtain their SG and G. To collect the H, the end of insect legs was sectioned with scissors, and the fluid was collected using a microtiter pipette. SGs and H samples were collected from 30 individuals at 10-, 20- and 30-days post-blood feeding. The Gs from 20 individuals were collected 15 days post-blood feeding. Tissues were dissected, pooled in separate microtubes containing cold TRIzol® reagent (Invitrogen, Carlsbad, CA, USA), and stored at − 80°°C until total RNA extraction following the manufacturer’s protocol. Following the extraction, samples were treated with turbo-DNase (Ambion, Austin, TX, USA) and quantified using Qubit 2.0 (Invitrogen, USA). RNA integrity was determined by lab-on-chip analysis using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and the three samples were transferred to RNAstable® microtubes (Biomatrica, San Diego, USA).
Small RNA-seq and bioinformatic analysis
Small RNA extraction, library construction, and sequencing were performed by Macrogen, Inc. (Seoul, South Korea). Individual adapter sequences were added to each tissue pool sample and three cDNA libraries were constructed using the TruSeq Small RNA Library Prep Kit (Illumina Inc., San Diego, CA). Sample libraries were sequenced on an Illumina HiSeq 2500 system (Illumina, San Diego, CA, USA) following a single-end 51 nt strategy. More than 200 million reads were generated across the three libraries. After sequencing, raw reads were checked for data quality using FastQC v0.11.9 (Babraham Institute, Cambridge, UK)82. Reads were then trimmed or discarded depending on their quality and size using Cutadapt v3.383. Only processed reads with a minimum of 18 nt and a maximum of 50 nt were maintained. This process yielded high-quality and reliable data. In-house Python scripts were designed to shape the input data along the pipeline as well as R scripts for plots and command lines, which are available at https://github.com/waldeyr/Rhodnius_prolixus_miRNA_project.
The processed reads were mapped to the RprolixusV48 reference genome obtained from the VectorBase (https://vectorbase.org)84. For this task, miRDeep2.0.1.275 employed Bowtie v1.2.385 through the mapper.pl script. Only perfect alignments were retained (full length, 100% identity). Using the mapped reads and a file (downloaded from miRBase database, release 22.156) containing 8763 known miRNA sequences from Ecdysozoa taxa, R. prolixus miRNAs were annotated, and novel miRNAs were predicted using miRDeep2.pl script with default options. The miRDeep2 core algorithm evaluated the presence of typical miRNA hairpin structures. If the reads fit in the hairpin as would be expected from Dicer processing, then the potential precursor was assigned as a genuine miRNA. As a constraint to add more reliability to the results, the reads should align to mature (guide strand) and star sequences (defined as the passenger strand base pairing to the potential mature sequence). Complementarily, the quantifier module from the miRDeep2 tool summed up miRNA read counts. The extracted known miRNAs were named according to their most similar miRNAs in the miRBase database. Results were manually curated and integrated into a Microsoft® Excel spreadsheet that contains detailed information about the miRNAs extracted by the miRDeep2 package in the sequencing datasets (Supplementary Table S4). Mature and novel miRNAs were considered high-confidence and selected as valid when filling parameters Significant randfold p-value: Yes; miRDeep2 score: > = 1; Mature read count: > 10; and Star read count: > = 1.
We assumed the rRNA population is present proportionally to total RNA in tissues and applied a normalization strategy that considers rRNA read counts as a biological internal standard86,87. This internal control was used to manage between-sample biases enabling a comparison of miRNA expression levels. The term “abundance index” (AI) was employed to compare the relative expression of miRNAs, which here is defined as the number of reads mapped to total rRNA counts divided by the number of reads mapped to a single miRNA (both known and novel). AI > 70 was set as the enrichment cut-off (miRNAs were considered highly expressed with AI values until tenfold less expressed than miR-283 from SG tissue, AI = 718, the most expressed miRNA across the three tissues). χ2 test and p-value were calculated to detect the significance level of changes. During the text, the discussed abundance levels are based on this outlined strategy.
In parallel, to classify the sncRNA types in the samples, such as ribosomal RNA (rRNA), Piwi-interacting RNA (piRNA), transfer RNA (tRNA), small nuclear RNA (snRNA), and small nucleolar RNA (snoRNA); the reads that mapped to the R. prolixus genome were also aligned against Triatominae non-coding RNAs downloaded from the RNAcentral database release 10.088 using Blast89. The graphical representations of miRNAs secondary structures were obtained by using RNAfold with default settings90. The two most abundant miRNAs of each tissue were selected for this analysis. The best structure for each sequence was selected based on minimum free energy values.
In-silico mapping of Rhodnius prolixus miRNAs to human blood transcripts
miRNA target prediction was conducted using the miRNAconsTarget tool from sRNAtoolbox (https://arn.ugr.es/srnatoolbox)91, which employs four miRNA target prediction programs: TargetSpy, miRanda, PITA, and Seed2. Only miRNA:mRNA interactions predicted by the four programs were considered. A target is only considered a consensus if all four methods predict an overlapping interaction in the same region inside the targeted sequence. The prediction was done using the eight most abundant miRNAs expressed in the SGs and a set of 3′UTRs (≥ 30 nt) of mRNAs from human blood downloaded from Human Protein Atlas as input files92. mRNA blood transcripts were downloaded from Ensembl, using the BioMart tool93. An over-representation analysis (ORA) method available in the WebGestalt tool94 was used to perform enrichment analysis of the set of the predicted targeted genes to gain insight into enriched metabolic pathways in Reactome95 and biological processes in Gene Ontology80,96. The parameters used were as follows: option “Top 20” on “Significance Level”, meaning the categories are first ranked based on the FDR threshold, and then the 20 most significant categories are selected; 0.05 on FDR threshold; and BH on Multiple Test Adjustment.
Small RNA extraction and RNASeq results validation by RT-qPCR amplification
To establish a correlation between RT-qPCR Ct values and read abundance from small RNA-seq, it was necessary to choose identified miRNAs that covered a range of expression levels, including both high and low abundance39. Five highly-abundant salivary miRNAs (miR-283, miR-12a, miR-281, bantam, miR-8; AI ≥ 500) and two lowly-abundant miRNAs (miR-315, miR-92b; AI < 7) were selected. The validation of this subset was performed using the Stem-loop Reverse-Transcription Polymerase Chain Reaction (slRT-PCR) strategy74. For this purpose, 90 SGs pairs were collected in 500 µL of TRIzol™ Reagent (Invitrogen®) as described above, in Sample Collection Methods. The small RNAs (< 50 nt) were isolated from the tissue using RNeasy Mini Kit (QIAGEN®) according to the manufacturer’s guidelines. First-strand cDNA synthesis was generated in a 20 μL reaction volume from 4 µL of small RNA using the GoScript™ Reverse Transcription System (Promega®) according to the manufacturer's instructions and the specific stem-loop primers (1 μM).
Reactions of RT-qPCR amplification were performed in a final volume of 12 μL, comprising 2× GoTaq® qPCR Master Mix (Promega®), 0.12 µL of Supplemental CXR Reference Dye, the specific forward and universal reverse primers (0.5 μM each) and 0.25 μL of the specific first strand cDNA reactions. The reactions were conducted using the StepOnePlus Real-Time PCR System (Applied Biosystem®). Amplification was performed as follows: polymerase activation stage of 2 min at 95 °C followed by 45 cycles (15 s at 95 °C, 1 min at 60 °C). Melting curves were obtained for each miRNA to verify the absence of unspecific amplification products with detection steps every 0.8% temperature increment. All reactions were performed in triplicate. The degree of linear association between read counts and Ct values was measured by calculating the Pearson coefficient correlation using GraphPad Software®, San Diego California USA. To estimate the strength of the relationship between data, p-value (derived from t-test) was used. A list of primer sequences, including the specific stem-loop and reverse primers, as well as the universal primer can be accessed in Supplementary Table S5.
Data availability
Small RNA-seq raw datasets were deposited in the National Center for Biotechnology Information (NCBI) in the Sequence Read Archive (SRA) under BioProject ID: PRJNA925536, Biosamples SAMN32800154 (G), SAMN32800155 (H) and SAMN32800156 (SG); and Sequence Read Archive (SRA) accessions SRR23130199, SRR23130198, and SRR23130197.
References
Lidani, K. C. F. et al. Chagas disease: From discovery to a worldwide health problem. Front. Public Health 7, 166 (2019).
Antinori, S. et al. Chagas disease in Europe: A review for the internist in the globalized world. Eur. J. Intern. Med. 43, 6–15 (2017).
WHO. Chagas disease key facts (2020, accessed 30 Mar 2020); https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis).
Andrade, D. V., Gollob, K. J. & Dutra, W. O. Acute chagas disease: New global challenges for an old neglected disease. PLoS Negl. Trop. Dis. 8(7), e3010 (2014).
Stanaway, J. D. & Roth, G. The burden of Chagas disease: Estimates and challenges. Glob. Heart 10(3), 139–144 (2015).
Browne, A. J. et al. The contemporary distribution of Trypanosoma cruzi infection in humans, alternative hosts and vectors. Sci. Data 4, 170050 (2017).
Flores-Ferrer, A. et al. Evolutionary ecology of Chagas disease; what do we know and what do we need?. Evol. Appl. 11(4), 470–487 (2018).
WHO. Chagas disease (also known as American trypanosomiasis) (2022, accessed 10 Oct 2022); https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis.
Champagne, D. E. Antihemostatic strategies of blood-feeding arthropods. Curr. Drug. Targets Cardiovasc. Haematol. Disord. 4(4), 375–396 (2004).
Champagne, D. E. Antihemostatic molecules from saliva of blood-feeding arthropods. Pathophysiol. Haemost. Thromb. 34(4–5), 221–227 (2005).
de Araújo, C. N. et al. Interactome: Smart hematophagous triatomine salivary gland molecules counteract human hemostasis during meal acquisition. J. Proteom. 75(13), 3829–3841 (2012).
Santiago, P. B. et al. The pharmacopea within triatomine salivary glands. Trends Parasitol. 36(3), 250–265 (2020).
Garcia, E. S. et al. Interactions between intestinal compounds of triatomines and Trypanosoma cruzi. Trends Parasitol. 26(10), 499–505 (2010).
Kanost, M. R. Chapter 117—hemolymph. In Encyclopedia of Insects (Second Edition) (eds. Resh, V. H. & Cardé, R. T.) 446–449 (Academic Press, 2009).
Wojda, I. et al. Insect defense proteins and peptides. Subcell Biochem. 94, 81–121 (2020).
Lucas, K. & Raikhel, A. S. Insect microRNAs: Biogenesis, expression profiling and biological functions. Insect Biochem. Mol. Biol. 43(1), 24–38 (2013).
Kim, K., Hung, R. J. & Perrimon, N. miR-263a regulates ENaC to maintain osmotic and intestinal stem cell homeostasis in drosophila. Dev. Cell 40(1), 23–36 (2017).
Mukherjee, S., Paricio, N. & Sokol, N. S. A stress-responsive miRNA regulates BMP signaling to maintain tissue homeostasis. Proc. Natl. Acad. Sci. U. S. A. 118, 21 (2021).
Varghese, J., Lim, S. F. & Cohen, S. M. Drosophila miR-14 regulates insulin production and metabolism through its target, sugarbabe. Genes Dev. 24(24), 2748–2753 (2010).
Ge, W. et al. Overlapping functions of microRNAs in control of apoptosis during Drosophila embryogenesis. Cell Death Differ. 19(5), 839–846 (2012).
Hilgers, V., Bushati, N. & Cohen, S. M. Drosophila microRNAs 263a/b confer robustness during development by protecting nascent sense organs from apoptosis. PLoS Biol. 8(6), e1000396 (2010).
Li, Y. & Padgett, R. W. bantam is required for optic lobe development and glial cell proliferation. PLoS ONE 7(3), e32910 (2012).
Liu, S. et al. MicroRNA expression profiling during the life cycle of the silkworm (Bombyx mori). BMC Genom. 10, 455 (2009).
Fullaondo, A. & Lee, S. Y. Identification of putative miRNA involved in Drosophila melanogaster immune response. Dev. Comp. Immunol. 36(2), 267–273 (2012).
Zhang, X. et al. Identification of conserved and novel microRNAs in Manduca sexta and their possible roles in the expression regulation of immunity-related genes. Insect Biochem. Mol. Biol. 47, 12–22 (2014).
Kim, V. N., Han, J. & Siomi, M. C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell. Biol. 10(2), 126–139 (2009).
Belles, X. et al. 2-Insect microRNAs: From molecular mechanisms to biological roles. In Insect Molecular Biology and Biochemistry (ed. Gilbert, L. I.) 30–56 (Academic Press, 2012).
Bartel, D. P. MicroRNAs: Target recognition and regulatory functions. Cell 136(2), 215–233 (2009).
Ørom, U. A., Nielsen, F. C. & Lund, A. H. MicroRNA-10a binds the 5’UTR of ribosomal protein mRNAs and enhances their translation. Mol. Cell 30(4), 460–471 (2008).
Vasudevan, S., Tong, Y. & Steitz, J. A. Switching from repression to activation: MicroRNAs can up-regulate translation. Science 318(5858), 1931–1934 (2007).
Place, R. F. et al. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc. Natl. Acad. Sci. U. S. A. 105(5), 1608–1613 (2008).
Li, S. et al. Direct sequencing and expression analysis of a large number of miRNAs in Aedes aegypti and a multi-species survey of novel mosquito miRNAs. BMC Genom. 10, 581 (2009).
Gu, J. et al. miRNA genes of an invasive vector mosquito, Aedes albopictus. PLoS ONE 8(7), e67638 (2013).
Mead, E. A. & Tu, Z. Cloning, characterization, and expression of microRNAs from the Asian malaria mosquito, Anopheles stephensi. BMC Genom. 9, 244 (2008).
Liu, W. et al. Identification and characterization of the expression profile of microRNAs in Anopheles anthropophagus. Parasit. Vectors 7, 159 (2014).
Biryukova, I., Ye, T. & Levashina, E. Transcriptome-wide analysis of microRNA expression in the malaria mosquito Anopheles gambiae. BMC Genom. 15, 557 (2014).
Skalsky, R. L. et al. Identification of microRNAs expressed in two mosquito vectors, Aedes albopictus and Culex quinquefasciatus. BMC Genom. 11, 119 (2010).
Hussain, M. et al. Blood meal induced microRNA regulates development and immune associated genes in the Dengue mosquito vector, Aedes aegypti. Insect. Biochem. Mol. Biol. 43(2), 146–152 (2013).
Arcà, B. et al. MicroRNAs from saliva of anopheline mosquitoes mimic human endogenous miRNAs and may contribute to vector-host-pathogen interactions. Sci. Rep. 9(1), 2955 (2019).
Hussain, M. et al. Wolbachia uses host microRNAs to manipulate host gene expression and facilitate colonization of the dengue vector Aedes aegypti. Proc. Natl. Acad. Sci. U. S. A. 108(22), 9250–9255 (2011).
Osei-Amo, S. et al. Wolbachia-induced aae-miR-12 miRNA negatively regulates the expression of MCT1 and MCM6 genes in Wolbachia-infected mosquito cell line. PLoS ONE 7(11), e50049 (2012).
Zhang, G. et al. Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti. Proc. Natl. Acad. Sci. U. S. A. 110(25), 10276–10281 (2013).
Slonchak, A. et al. Expression of mosquito microRNA Aae-miR-2940-5p is downregulated in response to West Nile virus infection to restrict viral replication. J. Virol. 88(15), 8457–8467 (2014).
Turchinovich, A., Tonevitsky, A. G. & Burwinkel, B. Extracellular miRNA: A collision of two paradigms. Trends Biochem. Sci. 41(10), 883–892 (2016).
Makarova, J. A. et al. Intracellular and extracellular microRNA: An update on localization and biological role. Prog. Histochem. Cytochem. 51(3–4), 33–49 (2016).
Gallo, A. et al. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE 7(3), e30679 (2012).
Leung, A. K., Calabrese, J. M. & Sharp, P. A. Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc. Natl. Acad. Sci. U. S. A. 103(48), 18125–18130 (2006).
O’Brien, J. et al. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. (Lausanne) 9, 402 (2018).
Zhu, Y. et al. A human-blood-derived microRNA facilitates flavivirus infection in fed mosquitoes. Cell Rep. 37(11), 110091 (2021).
Amin, N., McGrath, A. & Chen, Y.-P.P. Evaluation of deep learning in non-coding RNA classification. Nat. Mach. Intell. 1(5), 246–256 (2019).
Palazzo, A. F. & Lee, E. S. Non-coding RNA: What is functional and what is junk?. Front. Genet. 6, 2 (2015).
Luteijn, M. J. & Ketting, R. F. PIWI-interacting RNAs: From generation to transgenerational epigenetics. Nat. Rev. Genet. 14(8), 523–534 (2013).
Malik, M. I. et al. A microRNA profile of saliva and role of miR-375 in Haemaphysalis longicornis (Ixodida: Ixodidae). Parasit. Vectors 12(1), 68 (2019).
Van den Brande, S. et al. Identification and profiling of stable microRNAs in hemolymph of young and old Locusta migratoria fifth instars. Curr. Res. Insect Sci. 2, 100041 (2022).
Zhang, Q. et al. Genome-wide analysis of microRNAs in relation to pupariation in oriental fruit fly. Front. Physiol. 10, 301 (2019).
Kozomara, A., Birgaoanu, M. & Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 47(D1), D155–D162 (2019).
Ylla, G. et al. The microRNA toolkit of insects. Sci. Rep. 6, 37736 (2016).
Ibáñez-Ventoso, C. et al. Modulated microRNA expression during adult lifespan in Caenorhabditis elegans. Aging Cell 5(3), 235–246 (2006).
Moné, Y. et al. Characterization and expression profiling of microRNAs in response to plant feeding in two host-plant strains of the lepidopteran pest Spodoptera frugiperda. BMC Genom. 19(1), 804 (2018).
Maharaj, P. D. et al. Discovery of mosquito saliva microRNAs during CHIKV infection. PLoS Negl. Trop. Dis. 9(1), e0003386 (2015).
Fiorillo, C. et al. MicroRNAs and other small RNAs in Aedes aegypti saliva and salivary glands following chikungunya virus infection. Sci. Rep. 12(1), 9536 (2022).
Lee, G. J., Jun, J. W. & Hyun, S. MicroRNA miR-8 regulates multiple growth factor hormones produced from Drosophila fat cells. Insect. Mol. Biol. 24(3), 311–318 (2015).
Hyun, S. et al. Conserved MicroRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell 139(6), 1096–1108 (2009).
Loya, C. M. et al. miR-8 controls synapse structure by repression of the actin regulator enabled. Development 141(9), 1864–1874 (2014).
Karres, J. S. et al. The conserved microRNA miR-8 tunes atrophin levels to prevent neurodegeneration in Drosophila. Cell 131(1), 136–145 (2007).
Chen, J. et al. Conserved microRNAs miR-8-5p and miR-2a-3p modulate chitin biosynthesis in response to 20-hydroxyecdysone signaling in the brown planthopper, Nilaparvata lugens. Insect. Biochem. Mol. Biol. 43(9), 839–848 (2013).
Boulan, L., Martín, D. & Milán, M. bantam miRNA promotes systemic growth by connecting insulin signaling and ecdysone production. Curr. Biol. 23(6), 473–478 (2013).
Lam, V. et al. bantam miRNA is important for Drosophila blood cell homeostasis and a regulator of proliferation in the hematopoietic progenitor niche. Biochem. Biophys. Res. Commun 453(3), 467–472 (2014).
Brennecke, J. et al. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113(1), 25–36 (2003).
Lerner, I. et al. Clk post-transcriptional control denoises circadian transcription both temporally and spatially. Nat. Commun. 6, 7056 (2015).
Iovino, N., Pane, A. & Gaul, U. miR-184 has multiple roles in Drosophila female germline development. Dev. Cell 17(1), 123–133 (2009).
Li, W. et al. MicroRNA-276a functions in ellipsoid body and mushroom body neurons for naive and conditioned olfactory avoidance in Drosophila. J. Neurosci. 33(13), 5821–5833 (2013).
Su, J. et al. Screening for differentially expressed miRNAs in Aedes albopictus (Diptera: Culicidae) exposed to DENV-2 and their effect on replication of DENV-2 in C6/36 cells. Parasit. Vectors 12(1), 44 (2019).
Kramer, M. F. Stem-loop RT-qPCR for miRNAs. Curr. Protoc. Mol. Biol. 15, 10 (2011).
Friedländer, M. R. et al. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 40(1), 37–52 (2012).
Berezikov, E. Evolution of microRNA diversity and regulation in animals. Nat. Rev. Genet. 12(12), 846–860 (2011).
Dozmorov, M. G. et al. Systematic classification of non-coding RNAs by epigenomic similarity. BMC Bioinform. 14(14), S2 (2013).
Liu, B. et al. Deciphering the tRNA-derived small RNAs: Origin, development, and future. Cell Death Dis. 13(1), 24 (2021).
Chen, Z. et al. Two featured series of rRNA-derived RNA fragments (rRFs) constitute a novel class of small RNAs. PLoS ONE 12(4), e0176458 (2017).
Ashburner, M. et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25(1), 25–29 (2000).
Hackenberg, M. et al. In silico target network analysis of de novo-discovered, tick saliva-specific microRNAs reveals important combinatorial effects in their interference with vertebrate host physiology. RNA 23(8), 1259–1269 (2017).
Andrews, S. FastQC: A quality control tool for high throughput sequence data [Online]. (2010). http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17(1), 3 (2011).
Giraldo-Calderón, G. I. et al. VectorBase: An updated bioinformatics resource for invertebrate vectors and other organisms related with human diseases. Nucleic Acids Res. 43(Database issue), D707-13 (2015).
Langmead, B. Aligning short sequencing reads with Bowtie. Curr. Protoc. Bioinform. 11, 7 (2010).
Hori, Y., Engel, C. & Kobayashi, T. Regulation of ribosomal RNA gene copy number, transcription and nucleolus organization in eukaryotes. Nat. Rev. Mol. Cell Biol. 24, 414–429 (2023).
Long, E. O. & Dawid, I. B. Repeated genes in eukaryotes. Annu. Rev. Biochem. 49(1), 727–764 (1980).
The RNAcentral Consortium. RNAcentral: A hub of information for non-coding RNA sequences. Nucleic Acids Res. 47(D1), D1250–D1251 (2019).
Altschul, S. F. et al. Basic local alignment search tool. J. Mol. Biol. 215(3), 403–410 (1990).
Gruber, A. R. et al. The Vienna RNA websuite. Nucleic Acids Res. 36(1), W70–W74 (2008).
Aparicio-Puerta, E. et al. sRNAbench and sRNAtoolbox 2022 update: Accurate miRNA and sncRNA profiling for model and non-model organisms. Nucleic Acids Res. 50(W1), W710–W717 (2022).
Uhlén, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347(6220), 1260419 (2015).
Zerbino, D. R. et al. Ensembl 2018. Nucleic Acids Res. 46(D1), D754–D761 (2018).
Wang, J. et al. WebGestalt 2017: A more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 45(W1), W130–W137 (2017).
Gillespie, M. et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 50(D1), D687–D692 (2022).
Consortium, G.O. The Gene Ontology resource: Enriching a GOld mine. Nucleic Acids Res. 49(D1), D3 (2021).
Acknowledgements
This work was supported by grants awarded by the Fundação de Amparo à Pesquisa do Distrito Federal (FAP-DF, grants 0193.001802/2017, 00193-00000825/2021-19 and 00193-00002600/2022-70), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, grant 923/18 CAPES-COFECUB), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, INCT-MCTI/CNPq/CAPES/FAPs 16/2014), Financiadora de Estudos e Projetos (Finep, grant CT-Infra 0439/11). KLSB was the recipient of a Ph.D. grant supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We thank Renato Junio Ribeiro for triatomine picture assistance and Robert N. G. Miller for English language editing.
Author information
Authors and Affiliations
Contributions
P.B.S. and C.N.A.: conceived and designed the study. P.B.S., K.L.S.B., and Y.R.P.: sample and data collection. P.B.S., K.L.S.B., W.M.C.S., and S.O.C.: performed research, analyzed data. P.B.S., K.L.S.B., S.C. and C.N.A.: led the qPCR analysis. J.M.S., I.M.D.B., P.G., and M.A.S.S.F.: provided laboratory resources. C.N.A.: provided project oversight and funding. P.B.S. and K.L.S.B.: drafted the manuscript, which was critically revised by co-authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santiago, P.B., da Silva Bentes, K.L., da Silva, W.M.C. et al. Insights into the microRNA landscape of Rhodnius prolixus, a vector of Chagas disease. Sci Rep 13, 13120 (2023). https://doi.org/10.1038/s41598-023-40353-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40353-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.