Abstract
The aim of this study was to investigate the impact of jackfruit inner skin fibre (JS) incorporated with whey protein isolate (WPI) and soybean oil (SO) as a wall material for probiotic encapsulation to improve probiotic stability against freeze-drying and gastrointestinal (GI) tract conditions. Bifidobacterium bifidum TISTR2129, Bifidobacterium breve TISTR2130, and Lactobacillus acidophilus TISTR1338 were studied in terms of SCFA production and the antibiotic-resistant profile and in an antagonistic assay to select suitable strains for preparing a probiotic cocktail, which was then encapsulated. The results revealed that B. breve and L. acidophilus can be used effectively as core materials. JS showed the most influential effect on protecting probiotics from freeze-drying. WPI:SO:JS at a ratio of 3.9:2.4:3.7 was the optimized wall material, which provided an ideal formulation with 83.1 ± 6.1% encapsulation efficiency. This formulation presented > 50% probiotic survival after exposure to gastro-intestinal tract conditions. Up to 77.8 ± 0.1% of the encapsulated probiotics survived after 8 weeks of storage at refrigeration temperature. This study highlights a process and formulation to encapsulate probiotics for use as food supplements that could provide benefits to human health as well as an alternative approach to reduce agricultural waste by increasing the value of jackfruit inner skin.
Similar content being viewed by others
Introduction
Encapsulation is an effective technique to protect living microorganisms from harsh conditions1. Polysaccharide-based materials such as alginate, pectin, and carrageenan2,3,4 have been reported to be potential wall materials for encapsulating microorganisms. An increase in the value of agricultural waste is an alternative approach to reduce the carbon footprint caused by climate change5. Jackfruit (Artocarpus heterophyllus) is widely cultivated in several countries in Southeast Asia, including Thailand. Approximately 65–80% of the total weight of jackfruit is waste6. It has been reported that the inner skin of jackfruit is a natural fibre containing a large amount of non-digestible polysaccharides and a major source of prebiotics7. Moreover, this agricultural waste contains a large amount of cellulose and pectin8, which are excellent wall materials for microorganism encapsulation9, 10. Based on its functional properties, jackfruit inner skin fibre might protect beneficial microorganisms from the human gastrointestinal (GI) tract as well as provide health benefits. When encapsulating probiotics, a dry powder is the most convenient form with which extend shelf life, as it allows easy storage and transport11. However, the transformation of probiotics into a dry powder is the major cause of the loss of living microorganisms10, 12, which is the pain point of probiotic materials. Protein- and lipid-based materials have been reported to be effective wall materials to protect microorganisms from drying conditions. Previous evidence13, 14 has indicated that a combination of protein, lipid, and natural fibre materials from jackfruit inner skin could be of interest for the development of a potential wall material for probiotic encapsulation.
Bifidobacterium and Lactobacillus have been reported as the most widely used probiotic bacteria included in many functional foods and dietary supplements15 and are associated with a low health risk after human consumption16. Several research studies have demonstrated that these probiotics affect the composition of the gut microbiota, which results in a loss of total body weight17 because of their ability to produce short-chain fatty acids (SCFAs; includes acetic acid, butyric acid, and propionic acid).
This study aimed to evaluate the potential of jackfruit inner skin fibre (JS) as wall material incorporating with commercial materials (whey protein isolate (WPI) and soybean oil (SO)) on probiotic protection ability against harsh conditions (freeze-drying, gastro-intestinal tract). B. bifidum TISTR2129, B. breve TISTR2130, and L. acidophilus TISTR1338 were screened and selected for preparing a probiotic cocktail as core material based on their ability to produce SCFAs, antibiotic-resistant profiles and their relationship by antagonistic assay. The morphology of encapsulated probiotics was characterized by using a scanning electron microscope (SEM), while the storage stability of encapsulated probiotics was also evaluated at ambient and refrigeration temperatures for 8 weeks.
Materials and methodology
Chemicals and reagents
All antibiotics used in the antibiotic sensitivity test and the reagents for GI tract simulation (pepsin, bile salt, and pancreatin) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). WPI (Davisco Foods International, USA), SO (purchased from a supermarket), and JS (purchased from a local market in Khon Kaen, Thailand) were used as wall materials for encapsulation.
Screening and preparation of the probiotic cocktail for the encapsulation study
Bacterial strain and growth conditions
Probiotic strains, including B. bifidum TISTR2129, B. breve TISTR2130, and L. acidophilus TISTR1338 (Table 1), were purchased from the TISTR Culture Collection, Thailand. The probiotic strains were stored as working stocks at − 30 °C. The cultivation of all probiotic strains was described by Chang et al.18. Bifidobacterium were cultured in De Man, Rogosa and Sharpe (MRS) (HiMedia Laboratories Pvt. Ltd., India) supplemented with 0.05% l-cysteine hydrochloride (Sigma‒Aldrich, St. Louis, Mo, USA). The Lactobacillus cultures were grown in MRS medium without supplementation. All cultures were incubated at 37 °C for 36–60 h under anaerobic conditions.
Short-chain fatty acid production study
All three tested probiotic strains were cultured as described above for 5 days. Supernatant samples of the probiotics cultured for 1, 3, and 5 days were filtered with a nylon syringe filter with a pore size diameter of 0.45 µm. The method for the determination of acetic acid, butyric acid, and propionic acid followed Chang et al. with modifications18. The filtrates (2 µL) were injected into a 7890A series gas chromatography system (Agilent Technologies, Santa Clara, CA, USA) equipped with a DB-FFAP capillary column (30 m × 0.25 mm, 0.25 µm) and a flame ionization detector (FID) at a split ratio of 1:5. The analytical conditions were an injection port temperature of 250 °C and detector temperature of 250 °C. The oven temperature was programmed to increase from 100 to 180 °C at a rate of 10 °C/min (no hold) and from 180 to 250 °C at a rate of 30 °C/min (hold for 3 min). The flow rate of helium gas was 1.5 mL/min. The chromatographic peaks of the samples were identified from the retention times of the standards and computed with Agilent OpenLAB CDS ChemStation GC Drivers A.01.03 software. Each sample was analysed with two replicates.
Antibiotic sensitivity test
To study the antibiotic-resistant profiles of all tested probiotic strains, the broth dilution method was performed as described by Petsong et al.19 The experiment was conducted with three biological replicates. To prepare antibiotic stock solutions, ampicillin (256 µg/mL), gentamicin (4096 µg/mL), streptomycin (4096 µg/mL), and vancomycin (256 µg/mL) were dissolved in water. Erythromycin (256 g/mL) and tetracycline (1024 µg/mL) were dissolved in absolute ethanol. The sensitivity of the probiotic strains (105 CFU/mL) to each antibiotic was evaluated using 96-well microtiter plates containing twofold serial dilutions of each antibiotic (ampicillin, gentamicin, erythromycin, streptomycin, and tetracycline). Each mixture was incubated at 37 °C for 24 h under anaerobic conditions. The growth of the probiotic strains was observed at 600 nm using a microplate reader (Spectramax-M3, USA).
Antagonistic assay
An antagonistic assay was performed using the disk inhibition method following a previous study20 with modifications. B. bifidum, B. breve, and L. acidophilus were grown in suitable medium as described above. The inoculum was incubated at 37 °C under anaerobic conditions to obtain approximately 108 CFU/mL. Each potential sensitive strain was streaked on MRS agar plates using a sterile cotton swab. Paper disks (Whatman no. 4; Sigma–Aldrich, 5 mm) containing 20 µL of a possible producer of inhibitory substances was placed on prepared agar plates. The growth of all tested strains was determined after incubation for up to 72 h. The presence of clear zones around the inoculated disks was recorded as an inhibitory phenomenon by the bacteria contained in the disks against bacteria streaked on the agar plates. Paper disks containing incubated medium without any culture (20 µL) were used as the control. The experiment was conducted with three biological replicates. Probiotic strains that showed no negative relationship with the others were selected to study encapsulation.
Preparation of a probiotic cocktail
Bifidobacterium breve and L. acidophilus were selected to develop the core material to be encapsulated, since no antagonism was observed between these strains. Probiotic strains were cultured as described above. Cultures in the log phase were centrifuged, and the cells were washed with 0.85% NaCl three times before use. The ratio of B. breve to L. acidophilus was 1:1 (approximately 109 CFU/mL), and the mixture was suspended in 0.85% NaCl (10 mL) to prepare a probiotic suspension to study encapsulation.
Development of a wall material for probiotic encapsulation with enhanced protection provided by jackfruit inner skin fibre
To obtain an effective formulation of encapsulated probiotics that could survive under harsh conditions (freezing and GI tract conditions), this study was designed to evaluate the effect of wall materials (WPI, SO, and JS) on the survivability of the tested probiotics. The experiment followed the procedure of Petsong et al.21 with minor modifications. Various ratios of WPI, SO, and JS were used to obtain 10 g of each mixture (for the 13 formulations representing 10 different combinations, see Table 2) designed using Design-Expert version 13 (Stat-Ease Inc., Minneapolis, USA). The optimal formulation was designed based on the positive and negative effects of the individual wall materials and their combinations on the survivability of the probiotics after freeze-drying. JS was prepared by washing with tap water to eliminate the grime before being finely chopped. The moisture in JS was evaporated by using a hot air oven (60 °C, 48–72 h) before blending to obtain JS as a dry powder. All materials used for encapsulation were autoclaved (121 °C, 15 min) to eliminate the initial contaminating microorganisms. To unfold the protein structure, WPI was rehydrated in sterilized distilled water (80 mL) and incubated at 4 °C overnight. The prepared probiotic suspension and SO were added to the prepared WPI (heating to 80 °C for 30 min and cooling at room temperature for 45 min). The desired amount of JS was then added to the mixture. Each mixing step was conducted after 10 min of agitation at 2400 rpm using a homogenizer (Nihon Seiki Kassha, Ltd, Japan). Each mixture was kept at − 60 °C before being tempered at − 50 °C using a laboratory-scale freeze-dryer (Gamma 2-16LSC, Christ, Germany) for 48–72 h. The dry powders of each encapsulated probiotic formulation were prepared in three separate batches.
Survivability of encapsulated probiotics after freeze-drying
The method to enumerate the number of encapsulated probiotics after freeze-drying was carried out according to a previous study10 with minor modifications. Probiotic powder (1 g) was mixed with 30 mL of 1 M sodium phosphate buffer (pH 7.5) supplemented with 0.75% Tween® 20. The mixture was agitated at 200 rpm for 60 min at room temperature. A spread plate technique was performed on MRS agar, and the samples were incubated at 37 °C under anaerobic conditions for 48–72 h. The survivability of the encapsulated probiotics after freeze-drying (encapsulation efficiency; EE%) was calculated as follows. The number of probiotics recovered from the powder (RP) was divided by the initial number of probiotics added for encapsulation (IP) and then multiplied by 100:
Characterization and stability studies of the encapsulated probiotics
Morphology and surface characterization of the encapsulated probiotics
Morphology and surface characterization of the encapsulated probiotics (including the optimal formulation, WPI-SO formulation, and JS-SO formulation) were examined using field-emission scanning electron microscopy (FE-SEM) (TESCAN: MIRA, Czech Republic). The samples were fixed on aluminium stubs with double adhesive tape and vacuum coated with a layer of gold before being visualized under a magnification of 2000×. Observations were carried out at acceleration voltages of 5 kV and 10 kV.
Stability of the encapsulated probiotics under gastro-intestinal (GI) tract conditions
This study investigated the survivability of the developed encapsulated probiotics in the GI tract as a continuous system. The method was developed using previously reported protocols22, 23. First, the dry powder of encapsulated probiotics (3 g) was mixed with 20 mL of saline solution (140 mmol/L KCl and 5 mmol/L NaCl) and agitated (95 rpm) at 37 °C for 10 min. The mixture was adjusted to pH 2 before 1 mL of gastric fluid (0.04 g/mL pepsin in 0.1 M HCl, pH 2.0) was added, and then the mixture was incubated under the previously mentioned conditions for 1 h. To simulate duodenal digestion, the mixture was then adjusted to pH 5.3 using 0.9 M sodium bicarbonate, 200 µL of bile salt (3 mg/L) and 100 µL of porcine pancreatin (80 mg/mL) were continuously added. The pH was adjusted to 7.4 with 1 M NaOH, and the mixture was continuously agitated for another 2 h. A sample (1 mL) was taken after incubation at the stages of gastric and intestinal digestion to determine the number of living probiotics. The survivability (%) of the probiotics after GI tract digestion was calculated using the following equation:
where NP is the number of probiotics recovered from each stage of digestion (gastric or intestinal) and IP is the initial number of probiotics. The survivability of the free cell probiotics under GI tract conditions was also determined as the control to study the effect of the encapsulation formulation. A total of three replicates of this experiment were performed.
Stability of the encapsulated probiotics during storage
To study the stability of the developed encapsulated probiotics, aluminium-laminated foil was selected as the effective packaging material to protect the encapsulated probiotics from the storage environment24. This evaluation was conducted at ambient temperature (28 ± 2 °C) and refrigeration temperature (6 ± 2 °C) over 8 weeks. The method by which the survivability of the encapsulated probiotics was determined was performed as mentioned in probiotic survivability assay after freeze-drying.
Statistical analysis
Significant differences among the means of the results of short-chain fatty acid production by the tested bacterial strains at different times (1, 3, and 5 days) and the stability of various formulations of probiotics in the GI tract were calculated using Duncan’s multiple range tests at a 95% confidence level by IBM SPSS Statistics 28. The encapsulation of probiotics experiments were run in triplicate using simplex lattice mixture design (SLMD)21 to evaluate the effect of the ratio of wall material component (WPI, SO, and JS) on the probiotic recovery in dry powder form. EE (%) was considered as a response in this design.
Results
Selection and preparation of a probiotic cocktail
Ability of the probiotic strains to produce short-chain fatty acids (SCFAs)
Acetic acid, butyric acid, and propionic acid have been reported to be beneficial SCFAs produced by beneficial bacteria in the gut. These SCFAs play an important role in several processes, especially lipid metabolism, resulting in human weight loss25. From our preliminary study on the production of acetic acid, butyric acid, and propionic acid, acetic acid was the only short-chain fatty acid detected, regardless of the strain used and incubation time (data not shown). The production of acetic acid from B. bifidum, B. breve, and L. acidophilus after incubation for 1, 3, and 5 days at 37 °C was then evaluated as shown in Fig. 1. After 5 days of incubation, B. bifidum and L. acidophilus showed significant increase of acetic acid production (P < 0.05), while there was no difference, comparing between those observed from day 1 and day 3 (P > 0.05). On the other hand, the similar acetic acid production was found from B. breve for every incubation time tested (P > 0.05).
Antibiotic-resistant profiles of the probiotic strains
Antibiotic resistance has become a major human health issue. Although using probiotics is a natural approach to improve human health, these beneficial bacteria have been reported to be reservoirs of antibiotic resistance determinants, especially in dietary supplements that contain multiple strains of probiotics26. The aim of this study was to develop an encapsulated probiotic formulation to improve dietary supplements in a further study. Thus, information on the antibiotic-resistant profiles of the tested probiotic strains is needed. In this study, a total of five antibiotics (ampicillin, gentamicin, erythromycin, streptomycin, and tetracycline) were used to investigate the antibiotic-resistant profiles of B. bifidum, B. breve and L. acidophilus. The antibiotic susceptibility assay conducted by the broth dilution method (Table 3) revealed that B. bifidum, B. breve and L. acidophilus were resistant to ampicillin (32 µg/L), streptomycin (512 µg/L) and gentamicin (32 µg/L), respectively.
Relationships among the probiotic strains
Combining effective bacterial strains has been recognized as a potential approach for a specific purpose. However, antagonism among bacterial cocktails needs to be identified. Bacteria can produce secondary metabolites that can act as inhibitory antibiotics or growth factors to support the growth of other bacteria in the same environment27. In this study, an aim was the development of multiple probiotic strains as a cocktail. Thus, the negative relationships between selected probiotics were determined. Antagonism among the tested probiotic strains was determined by the disk inhibition method (Table 4). The results showed an inhibition zone (approximately 0.2 mm) around the disks consisting of B. breve and L. acidophilus on B. bifidum lawns. This suggested that B. breve and L. acidophilus might produce metabolites that could inhibit the growth of B. bifidum. However, there were no inhibition zones around the B. bifidum disks on B. breve and L. acidophilus lawns. In addition, there was no evidence to indicate that B. breve and L. acidophilus inhibit each other. These results showed that B. bifidum should be excluded from the probiotic cocktail if it is to be used as the core material to be encapsulated.
Development of a wall material made of jackfruit inner skin fibre, whey protein isolate and soybean oil for probiotic encapsulation
Each wall material, WPI, SO and JS, was evaluated individually for its effect on the survivability of the probiotic cocktail (B. breve and L. acidophilus) under freeze-drying conditions. ANOVA revealed that the effect of the wall materials on probiotic survivability was as follows: % survivability = + 7.9521WPI + 8.3847SO + 8.5732JS + 0.4171WPI*SO − 0.3894WPI*JS + 0.1637SO*JS (data not shown). This equation indicated that JS had the greatest positive effect on the survivability of the probiotics after freeze-drying, followed by SO and WPI. In addition, combinations of these 3 different wall materials were also studied, and the efficiencies of 13 formulations with 10 different ratios of WPI, SO, and JS were evaluated (Table 2). The encapsulation efficiency (EE %) ranged from 74.65 ± 3.97 to 93.77 ± 4.06 (Fig. 2). Moreover, the results showed that the combinations WPI-SO and SO-JS also produced positive effects. However, the combination of WPI and JS showed a negative effect that decreased the likelihood of the probiotics surviving under freeze-drying conditions. Although WPI-SO at an equal ratio showed the highest EE among the 10 tested formulations (93.77 ± 4.06%; data not shown), in this study, it was noted that JS was the source of prebiotics and SO and WPI maintained the encapsulation efficiency and transformed the encapsulated probiotics into dry powder (Fig. 3). Thus, the optimal formulation should contain all of the tested wall materials. The optimal formulation of the encapsulated probiotics was designed based on its limitations. Thus, a formula with WPI, SO, and JS ratios of 3.9, 2.4, and 3.7, respectively, was designed. This formulation was then developed and showed an encapsulation efficiency of 83.1 ± 6.1%. The optimal formulation was further characterized in terms of its physical morphology to investigate its efficiency to protect probiotics during GI tract digestion. Finally the stability of encapsulated probiotics during storage was evaluated under different temperatures.
Characterization and stability studies of the encapsulated probiotics
Morphology and surface characterization of the encapsulated probiotics
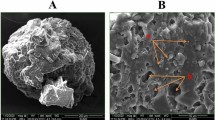
Morphological images of the probiotics encapsulated in the optimal formulation compared to probiotics encapsulated with the WPI-SO and JS-SO formulations are shown in Fig. 4. The probiotics encapsulated by the WPI-SO formulation showed a rough surface with pores of various sizes in the structure. The JS-SO formulation also showed a rough surface as well as the presence of cracks and a fibrous structure. Interestingly, the optimal formulation showed a uniform morphology with a smooth and dense matrix.
Scanning electron microscopic photographs at 2000× magnification of encapsulated probiotics. WPI-SO (A); encapsulated probiotics with whey protein isolate and soybean oil at 1:1 ratio, JS-SO (B); encapsulated probiotics with jackfruit inner skin fibre and soybean oil at 1:1 ratio, Optimal formulation (C); encapsulated probiotics with whey protein isolate, soybean oil, and jackfruit inner skin fibre at 3.9:2.4:3.7 ratio.
Stability of the encapsulated probiotics under stimulated GI tract conditions
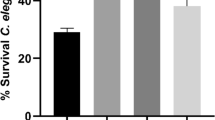
This study simulated the conditions of GI tract digestion as a continuous system. Probiotics encapsulated by the optimal formulation and the WPI-SO formulation and free-cell probiotics were studied to demonstrate the effect of JS as a potential wall material to protect the probiotics in the GI tract. The optimal formulation provided the probiotics with remarkable protection from harsh conditions (Fig. 5). After incubation in gastric fluid (pH 2) for 1 h, the reduction in probiotics released from the optimal formulation was 3.2 ± 0.4 log CFU/mL (62.3 ± 2.7% survivability). After intestinal digestion, the total reduction probiotics encapsulated by the optimal formulation lead to 4.2 ± 0.4 log CFU/mL (50.4 ± 2.3% survivability). The probiotics encapsulated by the WPI-SO formulation were studied in this assay because this formulation had the highest %EE. The reduction in probiotics after exposure to gastric fluid and intestinal digestion was 5.3 ± 0.2 log CFU/mL (43.8 ± 1.1% survivability) and 5.7 ± 0.3 log CFU/mL (39.1 ± 2.7% survivability), respectively. The survivability of free-cell probiotics under GI tract conditions showed a reduction in 7.2 ± 0.1 log CFU/mL (34.5 ± 1.1% survivability) after treatment with gastric fluid. Moreover, unencapsulated probiotics could not survive after intestinal tract digestion.
Survivability of encapsulated probiotics with optimal formulation under stimulated continuous gastro-intestinal tract in-vitro compared to WPI-SO formulation, and free cells. Bars represent the mean standard deviation. a,b,c indicated significant differences (P < 0.05) between the sample in gastric digestion condition. A,B,C indicated significant differences (P < 0.05) between the sample in intestinal digestion condition.
Stability of the encapsulated probiotics during storage
This study used aluminium-laminated foil bags as the packaging material for the developed encapsulated probiotics due to reports that suggest that this form of packaging can effectively protect encapsulated microorganisms from moisture, oxygen, and light24. Furthermore, this study evaluated the stability of the encapsulated probiotics at ambient and refrigeration temperatures for 8 weeks (Fig. 6). At an ambient temperature, an approximate 3 log reduction was observed after 1 week of storage, compared with that of 0 week of storage. Moreover, surviving probiotics were not detected after 4 weeks of storage. Remarkably, storage at refrigeration temperature showed a reduction in probiotic survival by approximately 2 log reduction (77.8 ± 0.1% of survivability) after 8 weeks. Thus, it is recommended that such developed products be stored at a low temperature to extend probiotic viability.
Discussion
The encapsulated probiotics in this study showed desirable properties that can be improved for use as food supplements. The production of acetic acid in this study was higher than that found in other studies. The amount of acetic acid produced by B. bifidum ATCC 29521 cultured with fructooligosaccharide at 37 °C for 48 h was 3.17 mg/mL28, and after L. acidophilus ATCC 11975 was cultured in MRS broth at 37 °C for 24 h, acetic acid production was 0.82 mg/mL29. Acetic acid can inhibit the growth of intestinal pathogens, provide energy to the liver and surrounding tissues, and it play a vital role in gluconeogenesis and lipogenesis30, 31. Therefore, this important short-chain fatty acid could play an important role in the reduction of metabolic regulation in humans. Information related to the antibiotic-resistant profiles of individual probiotic materials has remained scarce32. This study performed an in-vitro screening of the antibiotic-resistant profiles of certain bacteria to provide beneficial data on probiotic characteristic-related issues of concern. Although the probiotic strains in this study showed resistance to a few antibiotics, a previous study reported that probiotics that harbour resistance genes are themselves not harmful33. In addition, most probiotics, especially lactic acid bacteria (LAB), which were used in this study, have been considered to have generally recognized as safe (GRAS) status, which allows them to be used in food products33, 34. The results of this study agree with those a previous study that reported the importance of encapsulation to enhance the viability of probiotics under harsh conditions35. The probiotics used in this study were gram-positive bacteria that contained a thick peptidoglycan layer on the cell wall. The peptidoglycan layer comprises linear polysaccharide chains cross-linked with small peptides, which are covalently linked to teichoic acids36. This cross-linked network formed by H-bonding between the hydroxyl groups of polysaccharides (contained in the jackfruit inner skin fibre) might provide protection by entrapping probiotic cells in the porous structure of the polysaccharides. The cell walls of gram-positive bacteria contain small portions of proteins and lipids. An oil phase can interact with the hydrophobic portions of proteins and the nonpolar structures of the lipids on the bacterial cells. WPI has been reported to be an effective wall material that can protect microorganisms from potential protein‒protein interactions24. The results here indicated that WPI possibly covered the probiotic cells by interacting with the short peptides on the bacterial cell wall, thus protecting the probiotic cells from dry conditions. The protein molecules could have interacted with the polysaccharide as a gel complex, resulting in a decrease in the size of the pores in the network37. This phenomenon might be a barrier to probiotic cell entrapment, leading to probiotic cells being directly exposed to harsh conditions. Therefore, the protein structure may act as a buffer to protect living cells from the extreme pH of the gastric fluid38. The combination of a protein with the polysaccharide may have affected the release rate of the probiotics during simulated gastrointestinal digestion, leading to the strong protection of the encapsulated probiotics10. Encapsulated probiotics as hydrogel which contained protein based-materials (whey protein isolate and soy protein isolate) have been reported to be effective wall materials to protect probiotics from the GI tract39, 40. In addition, the synergistic effect of the combination of the protein, oil, and polysaccharide efficiently protected the probiotics from the drying process and GI tract10, 13. The storage stability evaluation results showed that the encapsulated probiotics in this study met the regulations for use in food, which state that foods containing probiotics must have no less than 106 CFU/g live probiotics throughout the shelf life of the product41. Overall, jackfruit inner skin fibre could be an effective wall material for probiotic encapsulation, particularly if it is incorporated with other commercial materials, such as whey protein isolate and soybean oil. Encapsulation formulation and process developed in this study was simple and provided high probability to scale-up. Moreover, the current formulation and process to develop encapsulated probiotics is of interest to improve as functional food for weigh control.
Conclusion
Jackfruit inner skin fibre (JS) successfully improved the efficiency of whey protein isolate and soybean oil as wall materials to protect probiotics from freeze-drying and GI tract conditions. The optimal formulation with WPI:SO:JS at 3.9: 2.4: 3.7 ratio showed high efficiency in protecting probiotics from freeze-drying conditions (83.1% survivability), gastric- and intestinal- digestion (62% and 50% survivability, respectively) as well as refrigeration storage (77.8% survivability). Therefore, JS could be a useful prebiotic source with a good protective effect as well as a wall material for the encapsulation of probiotics, particularly incorporation with other commercial materials as WPI and SO.
Data availability
The datasets generated in the current study are available from the corresponding author on reasonable request.
References
Maria, C., Zaskun, M. & Maria, V. Encapsulation technology to protect probiotic bacteria. In Probiotics (ed Everlon Cid Rigobelo) 501–540 (INTECH, 2012).
Zhang, Y., Lin, J. & Zhong, Q. S/O/W emulsions prepared with sugar beet pectin to enhance the viability of probiotic Lactobacillus salivarius NRRL B-30514. Food Hydrocoll. 52, 804–810 (2016).
Afzaal, M. et al. Survival and stability of free and encapsulated probiotic bacteria under simulated gastrointestinal and thermal conditions. Int. J. Food Prop. 23, 1899–1912 (2020).
Li, D., Wei, Z. & Xue, C. Alginate-based delivery systems for food bioactive ingredients: An overview of recent advances and future trends. Compr. Rev. Food Sci. Food Saf. 20, 5345–5369 (2021).
Food and Agricultural Organization of the United Nations. Food wastage footprint & climate change. https://www.fao.org/fileadmin/templates/nr/sustainability_pathways/docs/FWF_and_climate_change.pdf (2015).
Marzuki, M. N. A. et al. The effect of jackfruit skin powder and fiber bleaching treatment in PLA composites with incorporation of Thymol. Polymers (Basel) 12, 2622 (2020).
Wichienchot, S. et al. Extraction and analysis of prebiotics from selected plants from southern Thailand. Songklanakarin J. Sci. Technol. 33, 517–523 (2011).
Sundarraj, A. A. & Ranganathan, T. V. Physicochemical characterization of jackfruit (Artocarpus integer (Thumb.).) peel. RJPBCS 8, 2285–2295 (2017).
Afzaal, M. et al. Effect of cellulose–chitosan hybrid-based encapsulation on the viability and stability of probiotics under simulated gastric transit and in kefir. Biomimetics 7, 89 (2022).
Zhang, Y., Lin, J. & Zhong, Q. The increased viability of probiotic Lactobacillus salivarius NRRL B-30514 encapsulated in emulsions with multiple lipid-protein-pectin layers. Food Res. Int. 71, 9–15 (2015).
Wang, G., Chen, Y., Xia, Y., Song, X. & Ai, L. Characteristics of probiotic preparations and their applications. Foods 11, 2472 (2022).
Rajam, R. & Subramanian, P. Encapsulation of probiotics: Past, present and future. Beni-Suef Univ. J. Basic Appl. Sci. 11, 1 (2022).
Su, J. et al. Enhancing the viability of Lactobacillus plantarum as probiotics through encapsulation with high internal phase emulsions stabilized with whey protein isolate microgels. J. Agric. Food Chem. 66, 12335–12343 (2018).
Petsong, K., Benjakul, S. & Vongkamjan, K. Optimization of wall material for phage encapsulation via freezedrying and antimicrobial efficacy of microencapsulated phage against Salmonella. J. Food Sci. Technol. 58, 1937–1946 (2020).
Plaza-Diaz, J., Ruiz-Ojeda, F. J., Gil-Campos, M. & Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 1, S49–S66 (2019).
Wang, Y. et al. Whole-genome analysis of probiotic product isolates reveals the presence of genes related to antimicrobial resistance, virulence factors, and toxic metabolites, posing potential health risks. BMC Genom. 22, 210 (2021).
Kim, B., Choi, H.-N. & Yim, J.-E. Effect of diet on the gut microbiota associated with obesity. J. Obes. Metab. Syndr. 28, 216–224 (2019).
Chang, Y. H. et al. Quality characteristics of yogurts fermented with short-chain fatty acid-producing probiotics and their effects on mucin production and probiotic adhesion onto human colon epithelial cells. J. Dairy Sci. 104, 7415–7425 (2021).
Petsong, K., Uddin, M. J., Vongkamjan, K. & Ahn, J. Combined effect of bacteriophage and antibiotic on the inhibition of the development of antibiotic resistance in Salmonella Typhimurium. Food Sci. Biotechnol. 27, 1239–1244 (2018).
Kaur, J. et al. Pseudomonas aeruginosa inhibits the growth of Scedosporium aurantiacum, an opportunistic fungal pathogen isolated from the lungs of cystic fibrosis patients. Front. Microbiol. 6, 866 (2015).
Petsong, K., Benjakul, S. & Vongkamjan, K. Optimization of wall material for phage encapsulation via freezedrying and antimicrobial efficacy of microencapsulated phage against Salmonella. J. Food Sci. Technol. 58, 1937–1946 (2021).
Ryan, L., O’Connell, O., O’Sullivan, L., Aherne, S. A. & O’Brien, N. M. Micellarisation of carotenoids from raw and cooked vegetables. Plant Foods Hum. Nutr. 63, 127–133 (2008).
Buamard, N., Aluko, R. E. & Benjakul, S. Stability of tuna trypsin-loaded alginate-chitosan beads in acidic stomach fluid and the release of active enzyme in a simulated intestinal tract environment. J. Food Biochem. 44, e13455 (2020).
Petsong, K., Benjakul, S. & Vongkamjan, K. Evaluation of storage conditions and efficiency of a novel microencapsulated Salmonella phage cocktail for controlling S. Enteritidis and S. Typhimurium in-vitro and in fresh foods. Food Microbiol. 83, 167–174 (2019).
He, J. et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 21, 6356 (2020).
Wong, A., Ngu, D. Y. S., Dan, L. A., Ooi, A. & Lim, R. L. H. Detection of antibiotic resistance in probiotics of dietary supplements. Nutr. J. 14, 1 (2015).
Geesink, P. et al. Growth promotion and inhibition induced by interactions of groundwater bacteria. FEMS Microbiol. Ecol. 94, 11 (2018).
Kajiwara, S., Gandhi, H. & Ustunol, Z. Effect of honey on the growth of and acid production by human intestinal Bifidobacterium spp.: An in vitro comparison with commercial oligosaccharides and inulin. J Food Prot 65, 214–218 (2002).
Özcelik, S., Kuley, E. & Özogul, F. Formation of lactic, acetic, succinic, propionic, formic and butyric acid by lactic acid bacteria. LWT 73, 536–542 (2016).
Chen, L. et al. Digestibility of sulfated polysaccharide from the brown seaweed Ascophyllum nodosum and its effect on the human gut microbiota in vitro. Int. J. Biol. Macromol. 112, 1055–1061 (2018).
Di, T. et al. In vitro digestion by saliva, simulated gastric and small intestinal juices and fermentation by human fecal microbiota of sulfated polysaccharides from Gracilaria rubra. J. Funct. Foods 40, 18–27 (2018).
Zheng, M. et al. Assessing the risk of probiotic dietary supplements in the context of antibiotic resistance. Front. Microbiol. 8, 908 (2017).
Kaur, I. P., Chopra, K. & Saini, A. Probiotics: Potential pharmaceutical applications. Eur. J. Pharm. Sci. 51, 1–9 (2002).
Food and Drug Administration Thailand. Guideline of using probiotics in foods. http://food.fda.moph.go.th/law/data/announ_fda/61_Probiotic_Bacteria.pdf (2018).
Gu, Q. et al. Encapsulation of multiple probiotics, synbiotics, or nutrabiotics for improved health effects: A review. Adv. Colloid Interface Sci. 309, 102781 (2022).
Desvaux, M., Dumas, E., Chafsey, I. & Hébraud, M. Protein cell surface display in Gram-positive bacteria: From single protein to macromolecular protein structure. FEMS Microbiol. Lett. 256, 1–15 (2006).
Bealer, E. J. et al. Protein–polysaccharide composite materials: Fabrication and applications. Polymers 12, 464 (2020).
Othman, N. Z., Shadan, N. H., Ramli, S. & Sarmidia, M. R. Study on the effects of carbohydrate-protein-coconut oil on the viability of Lactobacillus bulgaricus during spray drying, simulated gastrointestinal conditions and unrefrigerated storage by simplex-lattice mixture design. CET. 63, 553–558 (2018).
Afzaal, M. et al. Probiotics encapsulated gastroprotective cross-linked microgels: Enhanced viability under stressed conditions with dried apple carrier. Food Sci. Nutr. 11, 817–827 (2023).
Zeashan, M. et al. Survival and behavior of free and encapsulated probiotic bacteria under simulated human gastrointestinal and technological conditions. Food Sci. Nutr. 8, 2419–2426 (2020).
Neffe-Skocińska, K., Rzepkowska, A., Szydłowska, A. & Kołożyn-Krajewska, D. Trends and possibilities of the use of probiotics in food production. In Alternative and replacement foods Vol. 17 (eds. Holban, A. M. & Grumezescu, A. M.) 65–94 (Academic Press, 2018).
Acknowledgements
This research was funded by the Young Research Development Project of Khon Kaen University Year 2022. Special thanks to Nattavong Faungpaiboon and Kanrawee Hunsakul for support and the recommendation to use the Design-Expert program.
Author information
Authors and Affiliations
Contributions
K.P. and P.T. designed the research framework. K.P. carried out the experiments with support from P.K., P.K., and S.K. K.P., K.N., S.K., and P.T. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Petsong, K., Kaewthong, P., Kingwascharapong, P. et al. Potential of jackfruit inner skin fibre for encapsulation of probiotics on their stability against adverse conditions. Sci Rep 13, 11158 (2023). https://doi.org/10.1038/s41598-023-38319-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38319-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.