Abstract
Mental rotation (mR) bases on imagination of actual movements. It remains unclear whether there is a specific pattern of mR impairment in focal dystonia. We aimed to investigate mR in patients with cervical dystonia (CD) and blepharospasm (BS) and to assess potential confounders. 23 CD patients and 23 healthy controls (HC) as well as 21 BS and 19 hemifacial spasm (HS) patients were matched for sex, age, and education level. Handedness, finger dexterity, general reaction time, and cognitive status were assessed. Disease severity was evaluated by clinical scales. During mR, photographs of body parts (head, hand, or foot) and a non-corporal object (car) were displayed at different angles rotated within their plane. Subjects were asked to judge laterality of the presented image by keystroke. Both speed and correctness were evaluated. Compared to HC, CD and HS patients performed worse in mR of hands, whereas BS group showed comparable performance. There was a significant association of prolonged mR reaction time (RT) with reduced MoCA scores and with increased RT in an unspecific reaction speed task. After exclusion of cognitively impaired patients, increased RT in the mR of hands was confined to CD group, but not HS. While the question of whether specific patterns of mR impairment reliably define a dystonic endophenotype remains elusive, our findings point to mR as a useful tool, when used carefully with control measures and tasks, which may be capable of identifying specific deficits that distinguish between subtypes of dystonia.
Similar content being viewed by others
Introduction
Dystonia is a hyperkinetic movement disorder syndrome which is still considered a rare disease1. The key feature of dystonia is involuntary muscle contraction causing abnormal and partly bizarre postures of different body parts. According to body distribution, focal, segmental, multifocal, and generalized forms of dystonia have been defined2. Non-motor symptoms like depression, pain and sleep disorders are quite common and, in addition to the motor symptoms, may severely affect patients’ quality of life3.
Despite being first described by Oppenheim as early as 1911, over a hundred years ago, the pathophysiology of dystonia remains poorly understood. Three potential pathophysiological mechanisms are frequently discussed (for a review:4): loss of cortical inhibition (e. g.5,6), synaptic malplasticity (e. g.7,8) and altered sensorimotor integration (e. g.9,10).
Mental rotation (mR) tests the capacity to imagine rotational movements of objects11 and is proposed to serve as a measure of sensorimotor integration12,13,14. Brain areas responsible for planning and execution of movements have been found to be activated during mental rotation suggesting the engagement of higher order motor processes for this task12,15. Mental rotation of pictures of body parts and non-corporal objects has already been assessed in focal dystonias, yet this research revealed partly inconclusive outcomes. First, Fiorio et al. were able to show mR impairment for the rotation of hands in focal hand dystonia16. The same group demonstrated reduced mR performance for body parts but not for non-corporal objects in cervical dystonia (CD)17. Other authors published negative18,19 or partly conflicting results20, like an impaired mR of capital letters, but not of schematic illustrations of full bodies.
Therefore, an impairment of mR could plausibly serve as a correlate of disturbed sensorimotor integration in dystonia. However, the inconclusive findings published so far necessitate further effort to question their reliability and specificity for a particular dystonic condition. As mR evidently interferes with other conditions but dystonia, aspects like sex21, age22, and cognitive fitness23 also need to be considered when interpreting collected data sets.
To test the hypothesis of mR defining a robust endophenotype of focal dystonia we assessed the task in a cohort of patients suffering from the two most frequent forms of focal dystonia: CD and blepharospasm (BS). Additionally, we aimed at registering potential modulators and/or confounders like cognitive state, dystonic symptom topology and severity, finger dexterity, and working speed systematically.
Methods
The protocol conformed to the principles of the declaration of Helsinki and was approved by the Ethics Committee of the Medical Faculty at the University of Würzburg. All participants gave their written informed consent for participation in the study.
Participants
Sixty-three patients were recruited from our outpatient clinic for movement disorders. Patients had been diagnosed with CD (n = 23), BS (n = 21), or hemifacial spasm (HS, n = 19) by a movement disorder specialist. Demographic data are summarized in Tables 1 and 2.
All patients were treated with botulinum neurotoxin A injections on a regular basis, while only few received concomitant oral antidystonic therapy or other CNS-effective drugs (for details, see Supplementary Table 1). The experiment was scheduled at least 10 weeks after the last injection date, when no or minor treatment effects remained, as judged both by the experimenter and the patient. Patients with clinically relevant psychiatric or neurological comorbidities were excluded from the study.
Additionally, a control group of 23 healthy volunteers (HC) was recruited. The HC group was matched to the CD group with regard to age, sex, and education level (Table 1). The same matching applied for BS and HS group.
Clinical assessment
Severity of CD symptoms was assessed via Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS)24 by an experienced rater. BS severity was scored on the Blepharospasm Severity Scale25, Blepharospasm Disability Index26 and Jankovic Rating Scale27.
Handedness was defined by means of the Edinburgh Handedness Inventory28. The Montreal Cognitive Assessment (MoCA) was used to screen for cognitive deficits29, and a MoCA score below 26 points led to exclusion from the HC group. To be aware of unspecific and potentially confounding factors of mR performance, we implemented screening tools for global reaction time in terms of a red light/green light test (here referred to as Online Reaction Time Test [ORTT], University of Washington, https://faculty.washington.edu/chudler/java/stopl.html) and finger dexterity (Nine-Hole Peg Test [9HPT]30, average time of right and left hand).
Mental rotation task
The mR task was performed in accordance with the procedures described by Fiorio et al.16,17. The free software Open Sesame31 was used to present photographs of body parts (hand, foot and head) and a picture of a car on an ordinary 19 inch computer screen. There were pictures of right and left hands, and feet. As to the images of the head, either the right or left eye was marked with a black spot. The car bore a black mark on either the right or left headlight. The images were presented at six different angles rotated within their plane. Each angle (0°, 60°, 120°, 180°, 240°, and 300°) of each laterality (right and left) was presented four times in a randomized order, but in separate trials for each object (1st run: 48 hands, 2nd run: 48 feet, 3rd run: 48 heads, 4th run: 48 cars; see Fig. 1). Subjects were asked to judge laterality of the presented image by pressing the right and left CTRL keys for right and left responses respectively. Speed and accuracy of responses were recorded. RT for left and right sided stimuli were pooled. Additionally, RT for 60° and 300°, and 120° and 240° were pooled as they are the same angle of rotation in the clockwise and anti-clockwise direction, respectively. To rule out the effect of individual reaction time differences, the difference in RT compared to 0° (no rotation) were used for further analyses.
Statistical analyses
To evaluate differences in demographic and clinical measures between each group, one-way independent samples analyses of variances (ANOVA), with Tukey post hoc comparisons, were performed with group as the between-subjects variable. Levene’s test was performed to evaluate homogeneity of variances, and when the data violated the assumption of homogeneity of variances, a Welch ANOVA and Games-Howell post hoc test was performed. Data were also tested for normality using the Shapiro–Wilk test, and when the data violated the assumption of normality, the non-parametric one-way ANOVA, i.e. Kruskal–Wallis test was performed.
To evaluate group differences in the mental rotation of the different stimuli, a mixed model ANOVA was performed for each type of stimuli, with group (CD, HC, BS and HS) as the between-subjects variable, and angle of rotation (60°, 120°, and 180°) as the within-subjects variable. We felt justified to conduct analysis of pooled data as paired t-tests revealed no significant differences between clockwise and anticlockwise rotations (60° vs. 300°; 120° vs. 240°) for all stimuli except feet, where rotation performance only at 240° was slightly quicker than 120° (T(84) = 3.77, p < 0.001). Concerning equality of laterality of stimuli we ran a mixed model ANOVA as paired t-tests seemed to suggest group differences for hand and car images. After Tukey post-hoc test only one significant finding persisted: mR RT in 180° rotation solely of the right hand stimulus was slower in CD in comparison to HC (F(3, 82) = 2.98, p = 0.036). Therefore and for reasons of clarity, we decided to present our data in a pooled form for right and left stimuli as has previously been done19,20.
The assumption of normality and sphericity was tested using the Shapiro–Wilk and Mauchly tests, respectively. When the assumption of sphericity was violated, a Greenhouse–Geisser correction was applied. The non-parametric repeated measures ANOVA, the Friedman’s test was used when the data were not normally distributed. Tukey post-hoc comparisons were performed.
One-tailed Spearman’s correlations were performed to assess the relationships between symptom severity as measured by TWSTRS, sum scores of Blepharospasm Severity Scale, Blepharospasm Disability Index, and Jankovic Rating Scale, and response times on the mental rotation task. Spearman’s correlations were also performed to evaluate relationships between ORTT and MoCA with reaction times on the rotation of the different objects.
Ethical standard
The study was approved by the Local Committee and was conducted in accordance with the ethical standards of the Helsinki Declaration. Informed consent was obtained from all participants after the nature of the study was explained to them.
Results
Demographic data
Demographic data of patients and controls are summarized in Table 1, and detailed demographic data of the patients and controls are given in Tables 2 and 3. The groups did not significantly differ in age and sex. However, HC had higher education levels than CD (p < 0.010), and HS (p < 0.001). They also had higher MoCA scores than CD (p < 0.10) and HS (p < 0.001).
Clinical assessment
Clinical assessments of the patients and controls are summarized in Table 1, and detailed clinical assessment measures are given in Table 4. The groups did not significantly differ on the 9HPT and ORTT.
Mental rotation task
Cervical Dystonia and Hemifacial Spasm patients are impaired on the mental rotation of hands
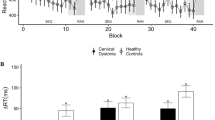
A mixed-model ANOVA revealed significant RT differences between groups for hands (F(3, 82) = 5.04, p = 0.003) but not for feet, head and cars (p > 0.027; Fig. 2A-D). As differences to the 0° condition are reported here, negative values are possible. Games-Howell post-hoc comparisons confirmed significantly higher RT in CD and HS patients compared to HC only for an angle of rotation of 180° (Fig. 2A).
Furthermore, ANOVA revealed stimulus orientation to be a significant within-subject factor (p < 0.001), with increasing RTs with increasing angles of disparity.
There were no significant correlations between RT in the mR task and symptom severity of CD as assessed by the TWSTRS total score (ref. Table 5). The same applied to each of its subscales.
Similarly, there were no significant correlations between RT and clinical measures of BS (sum scores of Blepharospasm Severity Scale, Blepharospasm Disability Index, and Jankovic Rating Scale). Coefficients and corresponding p-values are summarized in Table 5.
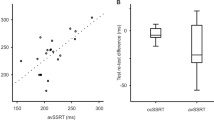
In order to estimate the degree of specificity of RT differences in the mR task, their correlation with global RT (ORTT) was computed. This revealed a moderate positive association for all stimulus objects, which may indicate a contribution of both global RT and mR-specific aspects (hand: r = 0.388, p < 0.001; foot: r = 0.473; p < 0.001, head: r = 0.423, p < 0.001; cars: r = 0.451, p < 0.001; see Fig. 3A).
Aside from reaction time, performance in the MR task may be described by the accuracy (ACC) of right/left choices. Accordingly, a mixed-model ANOVA with identical factors (“group”, “stimulus object”, and “stimulus orientation”) was run for ACC as the dependent variable. While there were no group differences in ACC with respect to mR of corporal objects (F(1, 44) = 1.91, p = 0.174), HC achieved higher accuracies than CD that were marginally significant (F(1, 44) = 4.134, p = 0.048).
Cognition as a potential confounder
Comparison of MoCA scores had indicated lower cognitive performance in CD and HS patients compared to HC. Accordingly, significant negative correlations were found between MoCA scores and RT in the mR task across all stimulus objects and all subject groups (hand: rs(DF) = − 0.434, p < 0.001; foot: rs(DF) = − 0.419; p < 0.001; head: rs(DF) = − 0.363, p = 0.001; cars: rs(DF) = − 0.425, p < 0.001, see Fig. 3B). Probing single subdivisions of MoCA (visuospatial, executive, naming, memory, attention, language, abstraction, delayed recall, and orientation) separately for correlations with mR RT, Spearman’s correlations remain significant except for two conditions (attention item with hand stimuli (p = 0.145) and delayed recall item with head stimuli (p = 0.072)).
A comparison of RTs on the mR of hands in patients with and without mild cognitive impairment, defined as a MoCA score lower than 26, revealed that patients with mild cognitive impairment took longer to respond to hand stimuli rotated at an angle of 180° (see Fig. 4).
Thus, in order to exclude cognitive disparities as a confounder in the mR of hands, the mixed-model ANOVA was repeated excluding all patients with MoCA scores < 26 points. In this group of patients and HC without cognitive deficits, there were no significant differences in ORTT (F(3, 63) = 2.63, p = 0.111) and 9HPT (F(3, 63) = 2.06, p = 0.114), while a group difference concerning lower MoCA scores in CD (new mean/SD 27.9 ± 1.7) persisted after the removal (H = 8.52, p = 0.036).
For this subgroup, mixed-model ANOVA showed a significant difference between groups on the mental rotation of hands (F(3, 63) = 4.34, p = 0.008). Post hoc comparisons revealed that CD patients needed longer response times than HC and BS (p < 0.001).
Discussion
The present study intended to further probe the concept of mR as an endophenotype of focal dystonia. Hereby, we were able to confirm increased RT in CD and HS compared to HC only on hand stimuli. In contrast, mR RT in patients with BS, another type of focal dystonia, did not differ from healthy controls. Furthermore, assessing potential confounders of mR performance, we detected significant correlations of prolonged mR RT both with reduced MoCA scores and with increased RT in an unspecific reaction speed test. After removing patients with mild cognitive impairment from the analyses, CD continued to show slowed response times to hand stimuli compared to HC while the performance of HS became comparable to HC.
Mental rotation and cervical dystonia
While performance on mR for HC were well within the range of previously described RTs (between 0.5 and 2.5 s) in healthy subjects, the increased RTs in our CD patients confirm an impairment of mR specifically on hand stimuli in CD. In line with findings by Fiorio et al., we show no differences in performance on mR of non-corporeal objects in our CD patients. Fiorio et al. suggested two main aspects which may contribute to reduced mR speed in dystonia17: On the one hand, the dystonic motor syndrome itself might cause delays in mR processing; on the other hand, alterations of the egocentric coordinate system in dystonia are suspected to cause an mR impairment. This interpretation was not only informed by their study in CD patients, but also by a former publication of this group which had shown increased RT in a cohort of patients with writer’s cramp (WC) exclusively during mR of hands while mR of a non-dystonic body region (lower limbs) remained unaffected32. The latter was interpreted as an indicator of altered sensorimotor integration due to an aberrant cognitive representation of hand movements in focal hand dystonia32. The now replicated observation that mR of body parts is particularly impaired in CD—under the precondition of matched MoCA scores—might be interpreted as to confirm this view. Evidence derived from mR studies in other diseases additionally underpins the assumption that mR comprises basic aspects of sensorimotor integration33,34,35,36. For instance, in complex regional pain syndrome, mR of hands was prolonged and accompanied by a decrease of fMRI activity in brain networks suspected to be essential for sensorimotor integration33.
In contrast, a study by Conson et al.20 who performed an mR task of capital letters (and their mirror-reversed forms) and schematic human figures (showing the whole body with hands marked in black to define laterality) in a group of 21 CD patients demonstrated a significant group difference concerning accuracy for letters only, but not for bodies, probably suggesting that CD predominantly affects spatial, but not egocentric object transformation20. In support of this, evidence for an impairment of spatial processing in CD is emerging37,38,39. For example, Filip et al. were able to demonstrate elevated fMRI activation in the cerebellum, the parietal cortex and other regions during a visuospatial task as well as reduced connectivity of the cerebellum with basal ganglia structures39. This network of cerebellar, thalamic and basal ganglia structures is meanwhile commonly accepted to play a crucial role in the pathophysiology of dystonia40,41,42,43,44.
In the present study, we were unable to find any differences in RTs between our CD and HC groups on the mR of foot and head stimuli, nor on non-corporeal objects. One explanation for this is that the differences observed in the RTs of foot and head stimuli, as well as non-corporeal objects were largely driven by differences in reaction times between CD and HC. Indeed, our preliminary analyses revealed longer RTs in CD compared to HC for all stimuli, and at nearly all angles of rotation, as well as in the no rotation (0°) condition. By using only the RT differences between rotation and no rotation in our analyses, we were able to account for the effect of individual reaction time differences. Therefore, in an experimental setting, RT differences are also driven by inter-individual differences in reaction times and these differences must be accounted for in order to identify true differences in mR capabilities.
A second possible confound are the cognitive abilities of the patients in our sample compared to the sample in the study by Fiorio and colleagues. While we evaluated their MoCA scores and reanalysed our data in the subset of patients without cognitive impairment, such measures were not reported by Fiorio and colleagues, while Conson and colleagues included patients with MoCA scores as low as 16. Hence, their sample of CD patients may have included a larger proportion of patients with cognitive impairment driving their poorer performance on mR. Nevertheless, we demonstrated that the impairment in CD on the mR of hand stimuli goes beyond mild cognitive impairment. This suggests that an altered body representation is likely to underlie their impairment on this task. Several different processes contribute to the ability to mentally rotate, namely covert motor rotation, higher order visuospatial thinking and visual object recognition. Removing cognition from possible factors influencing their performance, it is possible that an altered body representation, confined to upper limbs, may hinder their ability to mentally rotate these particular objects. WC patients have also been found to be impaired on the mental rotation of hand30. While covert motor processes have been found to share neural correlates with basic movement, processes distinct from basic movements must also underlie these covert operations since CD patients show no differences in dexterity compared to HC, as would be observed in focal hand dystonia, but remain impaired on these covert motor operations.
A cognitive role in mental rotation
While assessing cognition systematically as a potential confounder of mR in a dystonia cohort, we found a strong relationship between cognitive capability and mR performance. Longer RTs in the mR task were associated with lower MoCA scores. Indeed, mental rotation is a complex task relying on multiple processes including but not exclusive to cognitive processes. Therefore, cognitive abilities must be addressed when evaluating mR capacity. It might be suspected that some cognitive subdomains might contribute more to mR than others, in particular visuospatial, executive, and attentional function could be assumed to be crucial. However, we found significant correlations of nearly all MoCA test subdomains with mR performance. While this precludes conclusions concerning a specific interrelationship between particular cognitive domains and mR performance, it comes as little surprise given the simple design of the MoCA test as a rapid screening tool. Of note, education levels were also correlated with mR performance, but even stronger with MoCA scores (in line with45), indicating a kind of circular relationship of these measures. Additionally, cognitive impairment itself may be viewed as a characteristic non-motor feature of dystonia46.
Reduced mR capacity has already been identified in several forms of dementia including Alzheimer’s Disease47, dementia with Lewy bodies48, and ischemic vascular dementias49. Some studies even suggest mR as a screening tool for mild cognitive impairment23,50. Interestingly, also healthy ageing is associated with altered mR performance51; an age-dependent transformation of strategies concerning the rotation procedure is discussed to be the cause22,52. Additionally, older persons seem to focus more on accuracy at the expense of longer RT53. The latter observation might point to the process of decision-making itself and may also affect mR duration. In CD, this particular phenomenon has already been postulated as the rationale for a reduced capacity in temporal discrimination tasks54. Similarly, uncertainty in the context of decision-making during mR could make an important contribution to prolonged RT. As non-motor symptoms including anxiety and depression are common comorbid conditions in CD46, and as these mental disorders severely affect decision-making55, this additional potential confounder of mR should be kept in mind when planning upcoming trials.
In HS patients without cognitive impairment, and after controlling for simple interindividual reaction time differences, which should account for differences in time required for decision-making, poorer mR performance was no longer observed. This suggests that the impairment of HS on mR is driven purely by cognitive abilities, and not by other symptoms of the disorder. In CD patients without cognitive impairment on the other hand, the impairment for mR of hands remained. This suggests that a deficit other than cognitive impairment and slowed decision making underlies this impairment. Carefully controlling for confounders such as cognitive abilities and interindividual response time differences will allow us to correctly identify which deficit among the multiple processes crucial for mental rotation drives the impairment in our specific population. Only then can we better understand the disorder.
Differential networks of BS and CD?
To further evaluate the hypothesis of body part-related mR impairment as a general trait marker of focal dystonia, the identical mR paradigm was applied to patients with BS. In contrast to the distinctive finding in CD, BS patients performed non-inferior to a control cohort of HS patients.
It is important to note that we purposely decided to select a clinical rather than a healthy control group. While BS, as a form of focal dystonia, is commonly labeled as central network disorder56, HS arises from ectopic or ephaptic excitation due to compression or demyelination of the facial nerve57 and is therefore a peripheral neurologic disorder. Apart from these differential pathophysiological roots, the clinical phenotypes of both disorders show a remarkable overlap in terms of frequent involuntary contractions of the orbicularis oculi muscle. Consecutive eye blinks are most likely to interfere with neuropsychological tasks requiring fast recognition of pictures and execution of a simple motor feedback (keystroke). Here, we assume that the extent of this interference does not necessarily depend on whether repeated eye blinks occur uni- (HS) or bilaterally (BS). Indirect support of this assumption might be derived from our finding that symptom severity of BS is not correlated with RT in mR.
By contrast with our observation of an unimpaired mR performance in BS, there is virtually no literature on diverging behavior in CD and BS with respect to other behavioral measures of sensorimotor integration. For example, latencies in a spatial discrimination task were similarly increased in BS and CD58. In the same vein, temporal discrimination tasks for visual/tactile59 and somatosensory60 stimuli revealed significant impairment in CD and BS patients compared to HC, but no differences between the two focal dystonia groups. Beyond that, visuomotor and visuospatial ability is supposed to be disturbed in BS as well as in CD61,62. Therefore, despite the clearly diverging phenotypes of focal dystonia, neuropsychological performance seems to be similar in CD and BS, suggesting those findings as a common endophenotype. Moreover, imaging studies suggest that BS and CD pathophysiology may share the same brain network, which essentially contains cortical, basal ganglia, thalamic, and cerebellar structures63,64,65, and so far, there is no convincing evidence of fundamentally differential networks involved in BS and CD pathophysiology.
A dystonic endophenotype?
Taking these aspects and previous findings into account, there is no simple answer to the question of whether increased RTs in mR are a specific endophenotype of focal dystonia and, further, whether there is a particular relationship between clinically affected body regions and topological patterns of mR impairment, including the divergence between corporal and non-corporal objects.
We were able to replicate the finding of an mR impairment for hands in CD patients with normal MoCA scores, thereby reflecting the particular endophenotypic pattern proposed by Fioro et al. at least partly17. However, in the light of literature with opposing results18,19,20 and potential limitations of our approach and Fiorio’s study mentioned above, the specificity of this pattern remains insecure. Notably, Fiorio et al. were able to demonstrate reduced mR performance in patients with CD for the rotation of all body parts and not solely of hands17. In contrast, we only found slowed mR in CD patients in a non-dystonic body part, which comprises a topical incongruence of clinical phenotype and neuropsychological endophenotype. Additionally, BS patients did not show comparable alterations of mR, and symptom severity of dystonia did not correlate with the level of mR impairment in the CD group. Altogether, these aspects clearly limit the interpretation of mR defining a distinct dystonic endophenotype. Apart from that, the contribution of unspecific factors, like cognitive state and global reaction speed, appears substantial. While the strong correlation of global reaction speed with mR performance does not exclude an accentuation of speed reduction for the rotation of body parts, the impairment in various speed dependent tasks61 may lead to the speculation that a dystonic endophenotype is rather characterized by global deceleration of neurocognitive processes than by selective impairment of the ability to mentally rotate body parts or objects.
Ultimately, the question of whether mR impairment mainly arises from altered sensorimotor integration or from altered spatial processing remains unanswered, yet might be the missing link to understanding the nature of the putative dystonic endophenotype. What seems clear from our study and from several others is that mental rotation is a complex task requiring several crucial processes, and that different performances of the different subtypes of dystonia on specific types of mR tasks may be driven by different processes. When carefully used with several control measures and tasks, mental rotation possibly serves as a useful tool, which could help us understand the neural mechanisms underlying the different dystonia subtypes. Although presenting with similar phenotypes, these subtypes of dystonia may have a different pathophysiology. For example, while WC patients are impaired on rotating hand stimuli both of the affected and unaffected sides, musician’s dystonia patients remain unimpaired on this task16,18. Furthermore, WC patients tend to develop a similar dystonia in the unaffected hand when retraining to use the unaffected hand. This suggests that the preexisting abnormalities in WC may be more severe and may bring on dystonia on practice of relatively simple and low-skilled actions. Similarly, the CD patients in our study are impaired on rotating hands. This suggests that while one type of focal dystonia seems largely task specific, musician’s dystonia in this case, there may be more severe underlying abnormalities in the sensorimotor network predating the presentation of the disorder that drives the impairment of CD and WC patients on mR.
Finally, we need to admit that the pathophysiology of the network disorder dystonia still raises plenty of questions to the scientific community. While it appears challenging to get to the heart of the spatial discordance of (clinical) phenotype and (subclinical) endophenotype, we are well aware of the fact that one and the same genotype may be associated with fundamentally different dystonic phenotypes—and vice versa. For example, DYT1 dystonia is well-known to present not only as generalized form but also as multifocal, segmental and even pure focal dystonia66. The brain network, which is held to be impaired due to a genetic predisposition, may obviously come along with dystonic symptoms in either one or few body regions or may virtually affect the whole body. This might fuel speculations whether the topology of endophenotype and clinical symptomatology may show similar variability in dystonia.
Further elucidation of the neural underpinnings of mR, for instance by means of functional imaging and TMS inference, is required and under-way67. Prior to this, mR performance of patients with (focal) dystonia needs to be interpreted with caution, bearing in mind potential confounding parameters like cognition and speed.
Conclusion
The question of whether specific patterns of mR impairment reliably define an endophenotype of (focal) dystonia remains partly elusive. However, our findings highlight mR as a powerful tool, when used carefully with control measures and tasks, which may be capable of identifying specific deficits that differentiate different subtypes of dystonia.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA:
-
Analyses of variances
- BS:
-
Blepharospasm
- CD:
-
Cervical dystonia
- HC:
-
Healthy controls
- HS:
-
Hemifacial spasm
- MoCA:
-
Montreal Cognitive Assessment
- mR:
-
Mental rotation
- ORRT:
-
Online Reaction Time Test
- RT:
-
Reaction time(s)
- SD:
-
Standard deviation
- sec.:
-
Seconds
- TWSTRS:
-
Toronto Western Spasmodic Torticollis Rating Scale
- WC:
-
Writer’s cramp
- 9HPT:
-
9-Hole-Peg-Test
References
Defazio, G. & Berardelli, A. Is adult-onset dystonia a rare disease? Time for population-based studies. Mov. Disord. 36(5), 1119–1124 (2021).
Albanese, A. et al. Phenomenology and classification of dystonia: A consensus update. Mov. Disord. 28(7), 863–873 (2013).
Liang, Y. et al. Health-related quality of life in cervical dystonia using EQ-5D-5L: A large cross-sectional study in China. Front. Neurol. 13, 895272 (2022).
Quartarone, A. & Hallett, M. Emerging concepts in the physiological basis of dystonia. Mov. Disord. 28(7), 958–967 (2013).
Cohen, L. G. & Hallett, M. Hand cramps: Clinical features and electromyographic patterns in a focal dystonia. Neurology 38(7), 1005–1012 (1988).
Odorfer, T. M. et al. Increased finger-tapping related cerebellar activation in cervical dystonia, enhanced by transcranial stimulation: An indicator of compensation?. Front Neurol 10, 231 (2019).
Quartarone, A., Siebner, H. R. & Rothwell, J. C. Task-specific hand dystonia: Can too much plasticity be bad for you?. Trends Neurosci. 29(4), 192–199 (2006).
Weise, D. et al. The two sides of associative plasticity in writer’s cramp. Brain 129(Pt 10), 2709–2721 (2006).
Tinazzi, M. et al. Sensory functions in dystonia: Insights from behavioral studies. Mov Disord 24(10), 1427–1436 (2009).
Zittel, S. et al. Normalization of sensorimotor integration by repetitive transcranial magnetic stimulation in cervical dystonia. J Neurol 262(8), 1883–1889 (2015).
Shepard, R. N. & Metzler, J. Mental rotation of three-dimensional objects. Science 171(3972), 701–703 (1971).
Kosslyn, S. M. et al. Mental rotation of objects versus hands: Neural mechanisms revealed by positron emission tomography. Psychophysiology 35(2), 151–161 (1998).
Bonda, E. et al. Neural correlates of mental transformations of the body-in-space. Proc Natl Acad Sci U S A 92(24), 11180–11184 (1995).
Gentilucci, M., Daprati, E. & Gangitano, M. Right-handers and left-handers have different representations of their own hand. Brain Res. Cogn. Brain Res. 6(3), 185–192 (1998).
Vingerhoets, G. et al. Motor imagery in mental rotation: An fMRI study. Neuroimage 17(3), 1623–1633 (2002).
Fiorio, M., Tinazzi, M. & Aglioti, S. M. Selective impairment of hand mental rotation in patients with focal hand dystonia. Brain 128(Pt 6), 1471 (2005).
Fiorio, M. et al. Mental rotation of body parts and non-corporeal objects in patients with idiopathic cervical dystonia. Neuropsychologia 45(10), 2346–2354 (2007).
Erro, R. et al. Mental rotation and working memory in musicians’ dystonia. Brain Cogn. 109, 124–129 (2016).
Katschnig, P. et al. Mental rotation of body parts and sensory temporal discrimination in fixed dystonia. Mov. Disord. 25(8), 1061–1067 (2010).
Conson, M. et al. Spatial and egocentric mental rotation in patients with cervical dystonia. J. Neurol. 267(8), 2281–2287 (2020).
Long, H. et al. Sex-related difference in mental rotation performance is mediated by the special functional connectivity between the default mode and salience networks. Neuroscience 478, 65–74 (2021).
Nagashima, I. et al. Age-related differences in strategy in the hand mental rotation task. Front. Hum. Neurosci. 15, 615584 (2021).
Suzuki, A. et al. Establishing a new screening system for mild cognitive impairment and Alzheimer’s disease with mental rotation tasks that evaluate visuospatial function. J. Alzheimers Dis. 61(4), 1653–1665 (2018).
Consky, E. et al. The Toronto Western Spasmodic Torticollis Rating Scale (TWSRTS): Assessment of validity and inter-rater reliability. Neurology 40, 445 (1990).
Defazio, G. et al. Development and validation of a clinical scale for rating the severity of blepharospasm. Mov. Disord. 30(4), 525–530 (2015).
Lindeboom, R. et al. The blepharospasm disability scale: An instrument for the assessment of functional health in blepharospasm. Mov Disord 10(4), 444–449 (1995).
Jankovic, J. & Orman, J. Botulinum A toxin for cranial-cervical dystonia: A double-blind, placebo-controlled study. Neurology 37(4), 616–623 (1987).
Oldfield, R. C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9(1), 97–113 (1971).
Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53(4), 695–699 (2005).
Mathiowetz, V. et al. Grip and pinch strength: Normative data for adults. Arch. Phys. Med. Rehabil. 66(2), 69–74 (1985).
Mathôt, S., Schreij, D. & Theeuwes, J. OpenSesame: an open-source, graphical experiment builder for the social sciences. Behav Res. Methods 44(2), 314–324 (2012).
Fiorio, M., Tinazzi, M. & Aglioti, S. M. Selective impairment of hand mental rotation in patients with focal hand dystonia. Brain 129(Pt 1), 47–54 (2006).
Kohler, M. et al. Differences in neuronal representation of mental rotation in patients with complex regional pain syndrome and healthy controls. J. Pain. 20(8), 898–907 (2019).
Vastano, R. & Widerstrom-Noga, E. Event-related potentials during mental rotation of body-related stimuli in spinal cord injury population. Neuropsychologia 179, 108447 (2023).
Boccia, M. et al. Neural modifications in lower limb amputation: An fMRI study on action and non-action oriented body representations. Brain Imaging Behav. 14(2), 416–425 (2020).
Cona, G. et al. The role of motor system in mental rotation: New insights from myotonic dystrophy type 1. J. Int. Neuropsychol. Soc. 26(5), 492–502 (2020).
Chillemi, G. et al. Biased visuospatial attention in cervical dystonia. J. Int. Neuropsychol. Soc. 24(1), 22–32 (2018).
Chillemi, G. et al. Spatial and temporal high processing of visual and auditory stimuli in cervical dystonia. Front. Neurol. 8, 66 (2017).
Filip, P. et al. Disruption in cerebellar and basal ganglia networks during a visuospatial task in cervical dystonia. Mov. Disord. 32(5), 757–768 (2017).
Tewari, A., Fremont, R. & Khodakhah, K. It’s not just the basal ganglia: Cerebellum as a target for dystonia therapeutics. Mov Disord 32(11), 1537–1545 (2017).
Kaji, R., Bhatia, K. & Graybiel, A. M. Pathogenesis of dystonia: Is it of cerebellar or basal ganglia origin?. J. Neurol. Neurosurg. Psychiatry 89(5), 488–492 (2018).
Schirinzi, T. et al. Dystonia as a network disorder: A concept in evolution. Curr. Opin. Neurol. 31(4), 498–503 (2018).
Prudente, C. N., Hess, E. J. & Jinnah, H. A. Dystonia as a network disorder: What is the role of the cerebellum?. Neuroscience 260, 23–35 (2014).
Jinnah, H. A. & Hess, E. J. A new twist on the anatomy of dystonia: The basal ganglia and the cerebellum?. Neurology 67(10), 1740–1741 (2006).
Wu, Y., et al. Influence of education level on MMSE and MoCA scores of elderly inpatients. Appl. Neuropsychol. Adult. 1–5 (2021).
Monaghan, R. et al. Non-motor features of cervical dystonia: Cognition, social cognition, psychological distress and quality of life. Clin. Park Relat. Disord. 4, 100084 (2021).
Adduri, C. A. & Marotta, J. J. Mental rotation of faces in healthy aging and Alzheimer’s disease. PLoS ONE 4(7), e6120 (2009).
Phillips, J. R. et al. An adaptive measure of visuospatial impairment in dementia with lewy bodies. Mov. Disord. Clin. Pract. 9(5), 619–627 (2022).
Armstrong, C. L. & Cloud, B. The emergence of spatial rotation deficits in dementia and normal aging. Neuropsychology 12(2), 208–217 (1998).
Bourrelier, J. et al. Mental rotation as an indicator of motor representation in patients with mild cognitive impairment. Front. Aging Neurosci. 7, 238 (2015).
Zhao, B.L., S. Della Sala, and E. Gherri, Age-Associated Delay in Mental Rotation. Psychology and Aging, 2019. 34(4): p. 502–511.
Dror, I. E., Schmitz-Williams, I. C. & Smith, W. Older adults use mental representations that reduce cognitive load: Mental rotation utilizes holistic representations and processing. Exp. Aging Res. 31(4), 409–420 (2005).
Hertzog, C., Vernon, M. C. & Rypma, B. Age-differences in mental rotation task-performance—the influence of speed accuracy tradeoffs. J. Gerontol. 48(3), P150–P156 (1993).
Sadnicka, A. et al. Mind the gap: temporal discrimination and dystonia. Eur. J. Neurol. 24(6), 796–806 (2017).
Kong, X. et al. State-independent and -dependent behavioral and neuroelectrophysiological characteristics during dynamic decision-making in patients with current and remitted depression. J. Affect. Disord. 309, 85–94 (2022).
Defazio, G. et al. Blepharospasm 40 years later. Mov. Disord. 32(4), 498–509 (2017).
Nielsen, V. K. Electrophysiology of the facial nerve in hemifacial spasm: ectopic/ephaptic excitation. Muscle Nerve 8(7), 545–555 (1985).
Molloy, F. M. et al. Abnormalities of spatial discrimination in focal and generalized dystonia. Brain 126(Pt 10), 2175–2182 (2003).
Bradley, D. et al. Temporal discrimination thresholds in adult-onset primary torsion dystonia: an analysis by task type and by dystonia phenotype. J. Neurol. 259(1), 77–82 (2012).
Scontrini, A. et al. Somatosensory temporal discrimination in patients with primary focal dystonia. J. Neurol Neurosurg. Psychiatry 80(12), 1315–1319 (2009).
Aita, S. L. et al. Neuropsychological functioning in primary dystonia: Updated and expanded multidomain meta-analysis. Mov. Disord. 37(7), 1483–1494 (2022).
Romano, R. et al. Impaired cognitive functions in adult-onset primary cranial cervical dystonia. Parkinsonism Relat. Disord. 20(2), 162–165 (2014).
Glickman, A. et al. Basal ganglia and cerebellar circuits have distinct roles in blepharospasm. Parkinsonism Relat. Disord. 78, 158–164 (2020).
Luo, B. et al. Frequency-dependent plasticity in the temporal association cortex originates from the primary auditory cortex, and is modified by the secondary auditory cortex and the medial geniculate body. J Neurosci 42(26), 5254–5267 (2022).
Huang, X. F. et al. Multiple neural networks malfunction in primary blepharospasm: An independent components analysis. Front. Hum. Neurosci. 11, 235 (2017).
Edwards, M., Wood, N. & Bhatia, K. Unusual phenotypes in DYT1 dystonia: A report of five cases and a review of the literature. Mov. Disord. 18(6), 706–711 (2003).
Hiew, S., Roothans, J., Eldebaky, H., Zeller, D. & Reich, M. TH181 Imaging the Spin: The network underlying mental rotation. Clin. Neurophysiol. 141, 138–139 (2022).
Funding
Open Access funding enabled and organized by Projekt DEAL. This publication was supported by the Open Access Publication Fund of the University of Wuerzburg. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
T.O.: study conceptualization, data analysis, project administration, writing/editing manuscript. M.Y.: data acquisition, data analysis, editing manuscript. S.H.: data analysis, writing/editing manuscript. J.V.: editing manuscript. D.Z.: study conceptualization, data analysis, project administration, editing manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Odorfer, T.M., Yabe, M., Hiew, S. et al. Topological differences and confounders of mental rotation in cervical dystonia and blepharospasm. Sci Rep 13, 6026 (2023). https://doi.org/10.1038/s41598-023-33262-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33262-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.