Abstract
This study investigates the effects of soybean meal fermented by Enterococcus faecium as a replacement for soybean meal on growth performance, apparent total tract digestibility, blood profile and gut microbiota of weaned pigs. Eighty piglets (weaned at 21 days) [(Landrace × Yorkshire) × Duroc] with average body weight of 6.52 ± 0.59 kg) were selected and assigned to 4 treatments/4 replicate pens (3 barrows and 2 gilts). The four diets (SBM, 3, 6 and 9% FSBM) were formulated using fermented soybean meal to replace 0, 3, 6 and 9% of soybean meal, respectively. The trial lasted for 42 days phase 1, 2 and 3. Result showed that supplemental FSBM increased (P < 0.05) the body weight gain (BWG) of piglets at day 7, 21 and 42 and ADG at days 1–7, 8–21, 22–42 and 1–42, and ADFI at days 8–21, 22–42 and 1–42 and G: F at days 1–7, 8–21 and 1–42, and crude protein, dry matter, and gross energy digestibility at day 42, and lowered (P < 0.05) diarrhea at days 1–21 and 22–42. The concentration of glucose levels, WBC, RBC, and lymphocytes were increased while, concentration of BUN level in the serum was lowered in the FSBM treatment compared to the SBM group (P < 0.05). Microbiota sequencing found that FSBM supplementation increased the microbial Shannon, Simpsons and Chao indexs, (P < 0.05) and the abundances of the phylum Firmicutes, and genera prevotella, Lactobacillus, Lachnospiraceae and Lachnoclostridium (P < 0.05), lower in the abundances of the phylum bacteroidetes, Proteobacteria, genera Escherichia-Shigella, Clostridium sensu stricto1, Bacteroides and Parabacteroides (P < 0.05). Overall, FSBM replacing SBM improved the growth performance, apparent total tract digestibility, and blood profiles; perhaps via altering the faecal microbiota and its metabolites in weaned pigs. The present study provides theoretical support for applying FSBM at 6–9% to promote immune characteristics and regulate intestinal health in weaning piglets.
Similar content being viewed by others
Introduction
In the swine husbandry, weaning produce gut system dysfunctions and results from inconstant impairment of the gut barrier function, oxidative stress and absorption as a result of poor growth, diarrhoea and other diseases1,2. Weaning pigs are immediately required to undergo a change from high digestible sow’s milk to solid diets as well as complex protein3,4. Therefore, it is important to explore potential protein sources that should be added to diets to alleviate the weaning stress of piglets. The high cost, finite supply, and unstable variation of sources animal protein had become major reasons for limiting its supplement in diets on weaning pigs. Soybean meal (SBM) is an important protein feed ingredient in livestock diet, but variety of antinutritional factors (ANF) as well as β-conglycinin, glycinin and trypsin inhibitor, which would lead to immune responses, digestive disorders and negative effects on animal health3,5.
Probiotics are alternatives to in-feed antibiotics because they are successful in improving livestock production, efficiency and welfare6. Enterococcus faecium is a gram-positive gamma-hemolytic or non-hemolytic bacterium in the genus Enterococcus is a kind of facultative anaerobic lactobacillus that can colonize the gastrointestinal tract of humans and animals7. Enterococcus faecium bacterium feeds have shown positive effects in piglets intestinal microbiota, increasing immunoreactivity while reducing diarrhea8. The administration of living microbial preparations is one part of probiotic treatments. While, the another effective pathway is microbial fermentation of feed. Cheng et al.9 and Li et al.10 reported that bacterial fermentation could reduce content by ANFs and improving on nutritional quality and nutrient bioavailability. Fermented soybean meal (FSBM), a manufactured product mixed with solid SBM, liquid phases and the vaccinating the mixture with E. faecium11 could improve protein quality and reduce on ANFs levels of SBM with solid state fermentation12. It has been reported that FSBM has partial or total replacement of SBM to improve the growth performance and apparent tract total digestibility (ATTD) of crude protein and gross energy immune and antioxidant capacity in weaning pigs2. Jeong et al.13 found that compared with SBM, FSBM had improve the growth performance and the apparent ileal digestibility of crude protein, dry matter, and gross energy and amino acids in weaning piglets. Moreover, compared with SBM, FSBM showed greater concentration of crude protein and amino acid and reduce trypsin inhibitor and ANFs14.
The ideal situation would be to select feed probiotics that would improve soybean meal quality and enhance resistance to weaning stress by boosting the gut microbiota. Using Enterococcus faecium (E. faecium) toproduce a fermented soybean meal (FSBM) in weaning pigs has not been stated earlier and a probiotic-fermented SBM might show some different effects on piglets. The objective of the current study was to compare the effects of dietary SBM and FSBM on growth performance, ATTD, blood profile and gut microbiota of weaning pigs.
Materials and methods
Animal care
The animal care and experimental procedures described in this experiment were conducted according to the Animal Welfare Committee guidelines and had the approval of the Ethics committee of Animal Resource and Science College of Dankook University (DK-2-1936, Cheonan, South Korea). And the experiments were performed in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (https://arriveguidelines.org).
Preparation of fermented soybean meal (FSBM)
The Enterococcus faecium SLB130 were grown in de Man, Rogosa and Sharpe (MRS) medium at 37 °C. Fermentation was initiated by soaking SBM in distilled water to a achieve 30% moisture content. Water-soaked SBM and inoculated with a 10% of Enterococcus faecium SLB130 to achieve 106 cfu/g in SBM. SBM mixtures were anaerobically solid-state fermented at 37 °C for 48 h using a previously published protocol15. Finally, the fermented SBM was dried at 50–60 °C to a moisture concentration of 10% and then ground in a hammer mill. Final fermented SBM consisted of 5.8 × 107/g. Crude protein, KOH protein solubility of FSBM was determined by official methods of analysis16. Glycinin, β-conglycinin and trypsin inhibitor in FSBM were tested using a commercial kit (Feed Up Co., Ltd., Republic of Korea) (Table 1).
Experimental design, animals and feeding method
Eighty [(Landrace × Yorkshire) × Duroc] crossed weaned piglets (21 of age; 6.52 ± 0.59 kg) were randomly selected and allocated into 4 diets with 4 pens replicates according to the average initial body weight and sex, and each pen has 5 piglets (3 barrows and 2 gilts). Four diets were formulated using FSBM to replace 0, 3, 6 and 9% of SBM, respectively. The trial lasted for 42 days (phase 1 (days 1–7), phase 2 (8–21) and phase 3 (days 22–42). Basal diets were formulated to meet the NRC (2012) requirements (Tables 2, 3 and 4). Throughout the trial, all pigs had free access to feed and water, and the room temperature was maintained at 24–26 °C with 60–70% humidity, respectively.
Growth performance
Each piglet was weighed on days 0, 7, 21 and 42 and feed consumption was also recorded on a pen basis to determine average daily gain (ADG), average daily feed intake (ADFI) and gain to feed ratio (G:F = ADG/ADFI).
Diarrhea score
The piglets’ anuses were checked one by one at 09:00 and 17:00 daily during the experiment to observe and recorded any fecal contamination and redness method by Ma et al.17. The number of piglets with diarrhea per treatment was counted at the days 1–21 and 22–42 and the diarrhea rate was calculated with the following formulation:
Apparent total tract digestibility
To determine dry matter, crude protein, and gross energy digestibility, chromium oxide was added to the diet as an indigestible marker at 2 g/kg of the diet for7 day prior to fecal collection. Fecal samples were collected from 8 pigs randomly selected per treatment via rectal massage, and the sample was stored in a freezer at − 20 °C and was dried in a 65 °C for 72 h and the feed and fecal samples were grounded to passed through 1-mm sieve for the measurement of dry matter, crude protein, and gross energy of FSBM, SBM, diets and feces samples were determined following to the Association of Official Analytical Chemists18 procedures.
Blood profile
At 06:00 on days 21 and 42, two pigs for each replicate was randomly selected to collect blood from the jugular vein and subsequently centrifuged at 3,000 × g for 15 min at 4 °C to obtain the serum sample and was kept at − 80 °C until analysis by Muniyappan et al.19 The concentrations of lymphocyte counts, Red blood cells (RBC), White blood cell (WBC), Blood urea nitrogen (BUN), and glucose in serum were measured with an automatic biochemical analyzer (Model 7020, Hitachi, Tokyo, Japan). The serum creatinine concentration was determined using anAstra-8 analyzer (Beckman Instruments, Inc., Brea, CA, US).
Characteristics of microbial population in feces
On day 42, fresh fecal samples were collected from six pigs for microbiota analysis. Genomic DNA of fecal samples was extracted by using a DNA Kit (Omega Bio-tek, Norcross, GA, USA), according to manufacturer’s instructions. The quantity and quality of extracted genomic DNA were checked using a UV spectrophotometer (Mecasys, Daejeon, Korea). Amplification and sequencing of the V5–V6 hypervariable region of the 16S rRNA gene was performed using an Illumina MiSeq platform (Illumina, San Diego, CA, USA). We performed alpha-diversity and taxonomic analyses of the raw paired-end sequences using EZBioCloud pipeline20. Then the samples were grouped into microbiome taxonomic profile sets for further analyses. Relative abundance cut-offs at the phylum and genus levels were set to 0.1%. Charts depicting the results from the alpha-diversity and taxonomic analyses were generated using GraphPad Prism software version 9.0 (GraphPad, San Diego, California, USA).
Statistical analysis
All results are presented as mean ± standard deviation (SD). GraphPad 9.0 was used for figures. Statistical analyses were performed using IBM SPSS 20.0 (SPSS Inc., Chicago, IL, USA) and the differences between treatments were compared with one-way ANOVA followed by Dunnett’s multiple comparison procedure. For all tests, P < 0.05 was considered as significant.
Results
Growth performance
As shown the Fig. 1, the BW of piglets fed dietary FSBM was higher than SBM. The 3% and 6% FSBM (P < 0.05) during days 7, 21 and 42, 9% FSBM (P < 0.05) during days 7 and 22, (P < 0.01) during day 42 all showed significant increases. Compared with SBM treatments, piglets fed 3, 6 and 9% FSBM (P < 0.05) on the higher ADG, ADFI and G:F during days 1–7, 8–21, 22–42 and 1–42. Importantly, the diarrhea rate lower in FSBM treatments (1–21, 22–42). (P < 0.05).
Diarrhea score
Piglets fed FSBM diets had significantly lower (P < 0.05) the diarrhea score on days 1–21 and 22–42 (Fig. 2).
Apparent total tract digestibility
Dietary supplementation with 3, 6 and 9% FSBM increased (P < 0.05) the ATTD of dry matter, crude protein, and gross energy compared to the SBM (Fig. 3). Higher (P < 0.05) dry matter was found in the piglets dietary supplemented with 6% FSBM than 3 and 9% FSBM. However, 9% FSBM diet showed elevated crude protein levels than piglets fed 3 and 6% FSBM.
Blood profile
As illustrated in Fig. 4, piglets diets FSBM diets had increased (P < 0.05) the glucose levels, WBC, RBC, and lymphocytes and lowered (P < 0.05) the BUN level on days day 21 and 42. High (P < 0.05) glucose, RBC, WBC, Lymphocyte, and lower BUN compared with SBM was observed (Fig. 3) in serum gathered from days 21 and 42 piglets supplemented with the 6% FSBM. BUN was lower in the latter two group than in 3 and 6% FSBM. The Creatinine was not influenced in the serum by feeding 3, 6 and 9%FSBM.
Effects of FSBM on fecal microbial composition
The OTUs Venn analysis identified 21, 28, 46 and 69 unique OTUs among FSBM and SBM treatments, respectively (Fig. 5A). Figure 4B illustrates the results of alpha diversity analysis of which the Shannon, Simpsons and Chao indices were improved (P < 0.05) on FSBM compared to the SBM (Fig. 5B).
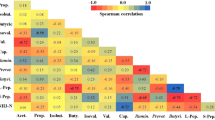
As shown in Fig. 6, the phylum levels analysis illustrated that the dietary supplementation of FSBM higher in the abundance of Firmicutes (P < 0.05) and lower the abundance of Bacteroidetes and Proteobacteria (P < 0.05). At the genus level, FSBM group increased Lactobacillus, prevotella, Lachnospiraceae and Lachnoclostridium (P < 0.05) in fecal microbiota (P < 0.05). Compared with SBM treatment, FSBM treatments lower in the abundance of Escherichia-Shigella, Clostridium sensu stricto1, Bacteroides and Parabacteroides (P < 0.05) (Fig. 7).
Discussion
Growth performance and diarrhea score
Sources of plant protein like FSBM have been extensively used in the diets of weaning pigs to enhance growth and immune status2,4. Earlier studies, illustrated that dietary FSBM supplementation increased the growth performance on weaning pigs21,22,23 and broilers9. Zhu et al.24 reported a significantly in the increased growth performance in weaning piglets due to dietary FSBM supplementation. In current study, the BW, ADG, and ADFI were improved in weaning pigs supplemented with FSBM treatments. Trypsin inhibitor content and antigenic protein content was reduced to 0.33 and 20%, respectively. Stachyose and raffinose were completely absent in FSBM. Pigs fed with FSBM could improve nutrient digestibility that may explain an enhanced ADG in weaning pigs.
Diarrhea score
The positive effect of SBM substitution by FSBM on diarrhea reduction may be attributed to the degradation of the main antigenic soybean proteins such as α and α′ subunits of β-conglycinin and acidic subunits of glycinin and some antinutritional factors such as trypsin inhibitors25,26. These proteins are partially digested by proteases secreted by microorganisms responsible for the fermentation process27,28. Similarly, Jeong et al.11 showed that degradation of most antigenic proteins (glycinin and β-conglycinin) and protease inhibitors in SBM fermented by Enterococcus Faecium improved intestinal morphology and digestive enzyme activities in weaned pigs. Fermentation of soybeans also degrades its proteins and carbohydrates to the extent of low molecular weight and water-soluble compounds that facilitate their digestibility29. The combination of a better nutritional status and a reduced immunological challenge when pigs are fed FSBM, or FSB may help to prevent diarrhea after weaning. Previous study, we demonstrated that dietary FSBM inclusion reduce the diarrhea in weaning pigs24,30. In the current study, piglets fed FSBM decreased the diarrhea in weaning pigs. In addition, it has been proposed that some microorganisms present in fermented products can inhibit intestinal colonization of pathogens that causes diarrhea in pigs31. The reduction of diarrhea is of great importance in pig production as it decreases the predisposition of these animals to Escherichia coli infection and improves feed efficiency, especially in weaning pigs32.
Apparent total tract digestibility
In the current study, supplementation of FSBM significantly increased digestibility of crude protein, dry matter, and gross energy which was consistent with Yan and Kim33 and Muniyappan et al.15 who reported that FSBM inclusion increased apparent total tract of crude protein, dry matter, and gross energy in weaning pigs. The beneficial effects of FSBM on nutrient digestibility may be attributed to the effects of FSBM on gastrointestinal development and the concomitant increase in digestive enzyme secretion4. Similarly, Min et al.34 found that dietary FSBM did not have significant effect on the digestibility of dry matter, crude protein, and gross energy in weaning pigs. In contrast, the negative effects of crude protein, dry matter, and gross energy on nutrient digestibility were also observed in previous studies13,35. Similarly, Hossain et al.36 and Lan and Kim37 found that dietary inclusion of FSMB did not have a significant effect on the digestibility of crude protein, dry matter, and gross energy in weaning pigs. The inconsistent results may be related to many factors such as FSBM source, additional levels, and composition of the basal diet, with effects being more pronounced when moderate amounts of FSBM are added to SBM basal diets21,34.
Blood profile
The BUN concentration and producing are affected by way of protein catabolism, and its concentrating is negatively associated with digestibility of proteins and amino acids38. Creatinine exists a natural waste result arises from the muscles and is eliminated from the body through kidney. Pigs fed FSBM showed decreased diarrhea score and the glucose levels improved which was in accordance with earlier studies24,39. Intriguingly, serum blood urea nitrogen reduced in FSBM treatments compared with the control. This shown that the fermenting procedure change nitrogen distribution inside the feed40. The levels of white blood cells, lymphocytes, were also increased, in the current studies. Lymphocyte growth exists as a major phase during the immune reaction in an animal and a proliferative reaction is a specific antigen41. Gizzarelli et al.42 and Wang et al.43 reported that weaning pig immunity is lower on β-conglycinin is not adequate deactivated during fermentation. Our results found a higher immune resistance on piglets this directly corresponded with a decrease in glycinin (80.29%) and β-conglycinin (69.43%) on FSBM. Therefore, overall growth performance and blood profiles were in accordance with each other.
Effects of FSBM on fecal microbial composition
Diets offer available substrates inclusive of protein carbohydrates for the gut microbiota and affect microbial structure and metabolism might encourage the performance and gut health of weaned pigs2. Generally, dietary nutrients are absorbed and digested in the foregut, later undigested food residues and endogenous are fermented by gut microbiota in the hindgut44,45. Fermentation of carbohydrates is enhanced to colonic cells due to the production of SCFA2,45, during fermentation of indigestible protein manufacture potentially metabolite toxicity inclusive of Ammonia, amines, phenols, and indoles that have harmful effects on intestinal health44. Alpha diversity can be utilized as an indicator of the functional resilience of ecological diversity of the intestinal microbiota, including species richness indices (Observed species, Chao, and Ace), and species diversity (Shannon and Simpson)46. In the current study, dietary inclusion with FSBM significantly increased the Shannon, Simpson, and Chao1 richness, which was in accordance with our previous findings in pigs that dietary inclusion of FSBM significant difference the Shannon, Simpson47. Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, and Tenericutes were the most pre-dominant bacteria phylum in the piglets48. In the current study, FSBM group shapes gut microbiota in weaning pigs, including lower in the abundances of the phylum Bacteroidetes, and Proteobacteria and the genera Escherichia-Shigella, Clostridium sensu stricto1, Bacteroides and Parabacteroides, and a higher in the abundances of the phylum Firmicutes and the genera Lactobacillus, prevotella, Lachnospiraceae and Lachnoclostridium. Bacteroidetes, Proteobacteria and Firmicutes as three major communities, are essential to growth performance and energy metabolism homeostasis49,50. Shin et al.51 found a lower in the abundance of the phylum Proteobacteria in the gut of healthy humans. Therefore, Litvak et al.52 reported that increase in Proteobacteria abundance have been associated with in humans with gut inflammation, colorectal cancer, irritable bowel syndrome and metabolic syndrome and could be bacterial signature of gut dysbiosis51. The abundance of the phylum Proteobacteria contains many possible opportunistic pathogens, as well as Campylobacter spp, Klebsiella spp,Escherichia, and Salmonella and its rise could be shown as a potential indicator of gut diseases. The Firmicutes abundance have been evidence to be positive relationship with energy and active transport, facilitated diffusion, endocytosis and passive diffusion, whereas improve in fecal Proteobacteria and Bacteroidetes is associated with inferior nutrient digestibility2. Therefore, higher abundances of the phylum Firmicutes along with lower abundances of the phylum Proteobacteria and Bacteroidetes might promote nutrient digestibility in weaning pigs. Eren et al.53 reported that the herbivores have an increase abundance of Lachnospiraceae than carnivores in animal. Vacca et al.54 reported that reduce abundance of Lachnospiraceae, while multiple sclerosis and ulcerative colitis patients. All participants of Lachnospiraceae are anaerobic, hemoorganotrophic and fermentative and could degrade non-starch polysaccharides and butyrate and acetic acid. Butyrate provide the major energy source for intestinal epithelial cell growth, increased intestinal protection mediated epithelial cells and favoring the suppression of inhibits inflammatory responses55,56. Stanley et al.57 showed that Lachnospiraceae is associated with improve growth performance in animals. The abundances of the genera Lachnoclostridium—butyric-acid-producing microbes that have been associated in the mitigation on intestinal inflammation- were better in the FSBM groups10. The Lactobacillus as a possibility probiotic, possess the opposition to pathogen, anti– inflammatory, antioxidant capacity, and capability to higher of fecal microbiota58,59. Zhu et al.48 reported an improved Lactobacilli and totality anaerobic bacteria counts in the gut microbiota weaning piglets due to dietary FSBM supplementation. In the current study, inclusion of FSBM treatments an increased in the abundances of the genera Lactobacillus were significantly increased RBC, WBC, Lymphocyte, glucose levels and decreased BUN. Lactobacillus is familiar to have a positive influence on the GIT, growth performance, and nutrient digestibility in pigs and regularly used as probiotics in livestock production60,61. Yan et al.62 reported that Lactobacillus could commonly increase growth performance and the GIT of animals by defense the intestine from pathogens and encourage efficient nutrient and energy extraction by the host. The genus Prevotella is saccharolytic and produce succinic and acetic acids as ending fermentation products63. Prevotella specialized in degrading fiber diet, which had also been correspondent with could improve intestinal immune and decrease diarrhea64. Wu et al.65 reported evidence indicated a close relationship among Prevotella and long period of time carbohydrates diets or carbohydrates from fiber-rich diets. It was also reported that a higher abundance of Prevotellaceae dominated in fecal microbiota of healthy piglets when compared to post-weaning diarrheic piglets 64. Feng et al.47 and Forsyth et al.66 reported a higher Prevotella abundance, the higher the mucin composite content, but bacterial toxins would reduce gut penetrability and the sensitivity of the region-specific gut to systemic exposure. Accordingly, higher in the Prevotella abundances in FSBM it could be helpful in improve on growth performance in weaning piglets. The relative abundance of Clostridium sensu stricto1 and bacteoides showed a positively correlated with the frequency of diarrhea. Clostridium is the main cause of diarrhoea in humans and is responsible for community-acquired out breaks67. Harlow et al.68 reported that Clostridium perfringens, Clostridium difficile and Salmonella spp. are the most common microbes associated with diarrhea. In our study, lower in the abundances of the genera Clostridium sensu stricto1 in the FSBM group, and was positively correlated with decreased diarrhoea. Another result of FSBM groups were a decrease Escherichia-Shigella, and lower Escherichia-Shigella was main participant of the decreased in Proteobacteria abundance compared to the SBM treatments. The genera Escherichia–Shigella comprises an opportunistically pathogenic bacterium. Sousa et al.69 and Gong et al.70 reported the genera Escherichia–Shigella could destroy the gut structure and must be pro-inflammatory actions through multiple pathways and like the secretion of virulence factors, consequent in the improved danger of infection and diarrhea in the host. Parabacteroides and Bacteroides, which occur at the initial phase of the lifetime, have been informed to generate gamma amino butyric acid, closely related to growth71,72. A higher abundance of the genera Bacteroides is commonly used in the events of colorectal cancer, functional gastrointestinal disorders and ulcerative colitis73,74,75. Therefore, the biological results found that appropriate inclusion of FSBM in diets can inhibit the gut pathogens (such as Escherichia–Shigella, Bacteroides and Parabacteroides) and enhance beneficial bacteria (such as Prevotella and Lactobacillus) and further enhance the immunity and health status of weaning piglets.
Conclusions
In conclusion, FSBM replacing SBM improved the growth performance, apparent total tract digestibility, blood profiles, possibly via altering gut microbiota profile in weaned piglets. The present study provides theoretical support for applying FSBM at 6 to 9% promote blood profiles and regulate intestinal health in weaning piglets.
Data availability
All data generated in this study are included in the published article. The datasets generated during the current study are available from the corresponding author on demand upon reasonable request.
References
Hu, R. et al. Dietary protocatechuic acid ameliorates inflammation and up-regulates intestinal tight junction proteins by modulating gut microbiota in LPS-challenged piglets. J. Anim. Sci. Biotechnol. 11, 1–12. https://doi.org/10.1186/s40104-020-00492-9 (2020).
Zhang, L. & Piao, X. Different dietary protein sources influence growth performance, antioxidant capacity, immunity, fecal microbiota and metabolites in weaned piglets. Anim. Nutr. 8, 71–81. https://doi.org/10.1016/j.aninu.2021.06.013 (2022).
Ma, X. K., Shang, Q. H., Wang, Q. Q., Hu, J. X. & Piao, X. S. Comparative effects of enzymolytic soybean meal and antibiotics in diets on growth performance, antioxidant capacity, immunity, and intestinal barrier function in weaned pigs. Anim. Feed Sci. Technol. 248, 47–58. https://doi.org/10.1016/j.anifeedsci.2018.12.003 (2019).
Ma, X. et al. Effects of replacing soybean meal, soy protein concentrate, fermented soybean meal or fish meal with enzyme-treated soybean meal on growth performance, nutrient digestibility, antioxidant capacity, immunity and intestinal morphology in weaned pigs. Livest. Sci. 225, 39–46 (2019).
Gu, X. et al. Fermented cottonseed meal as a partial replacement for soybean meal could improve the growth performance, immunity and antioxidant properties, and nutrient digestibility by altering the gut microbiota profile of weaned piglets. Front. Microbiol. 12, 100. https://doi.org/10.3389/fmicb.2021.734389 (2021).
Wang, Y. et al. Effects of probiotic Bacillus as a substitute for antibiotics on antioxidant capacity and intestinal autophagy of piglets. AMB Express 7, 1–11. https://doi.org/10.1186/s13568-017-0353-x (2017).
Sureshkumar, S., Park, J. H. & Kim, I. H. Effects of Enterococcus faecium SLB 130 probiotic on the performance of weaning pigs. Vet. Med. 67, 562–568 (2022).
Scharek-Tedin, L. et al. Probiotic treatment decreases the number of CD14-expressing cells in porcine milk which correlates with several intestinal immune parameters in the piglets. Front. Immunol. 6, 108. https://doi.org/10.3389/fimmu.2015.00108 (2015).
Cheng, Y. H. et al. Mixed fermentation of soybean meal by protease and probiotics and its effects on the growth performance and immune response in broilers. J. Appl. Anim. Res. 47, 339–348. https://doi.org/10.1080/09712119.2019.1637344 (2019).
Li, Y. et al. Effects of fermented soybean meal supplementation on the growth performance and cecal microbiota community of broiler chickens. Animals 10, 1098. https://doi.org/10.3390/ani10061098 (2020).
Jeong, J. S. & Kim, I. H. Comparative efficacy of up to 50% partial fish meal replacement with fermented soybean meal or enzymatically prepared soybean meal on growth performance, nutrient digestibility and fecal microflora in weaned pigs. Anim. Sci. J. 86, 624–633. https://doi.org/10.1111/asj.12335 (2015).
Song, Y. S., Pérez, V. G., Pettigrew, J. E., Martinez-Villaluenga, C. & de Mejia, E. G. Fermentation of soybean meal and its inclusion in diets for newly weaned pigs reduced diarrhea and measures of immunoreactivity in the plasma. Anim. Feed Sci. Technol. 159, 41–49. https://doi.org/10.1016/j.anifeedsci.2010.04.011 (2010).
Jeong, J. S., Park, J. W., Lee, S. I. & Kim, I. H. Apparent ileal digestibility of nutrients and amino acids in soybean meal, fish meal, spray-dried plasma protein and fermented soybean meal to weaned pigs. Anim. Sci. J. 87, 697–702. https://doi.org/10.1111/asj.12483 (2016).
Cervantes-Pahm, S. K. & Stein, H. H. Ileal digestibility of amino acids in conventional, fermented, and enzyme-treated soybean meal and in soy protein isolate, fish meal, and casein fed to weanling pigs. J. Anim. Sci. 88, 2674–2683. https://doi.org/10.2527/jas.2009-2677 (2010).
Muniyappan, M., Lee, Y. & Kim, I. H. Comparative efficacy of soybean meal vs fermented soybean meal on ileal digestibility and urine contents in weaned pigs. Livest. Sci. 263, 105016. https://doi.org/10.1016/j.livsci.2022.105016 (2022).
Weiner, M. L. et al. An infant formula toxicity and toxicokinetic feeding study on carrageenan in preweaning piglets with special attention to the immune system and gastrointestinal tract. Food Chem. Toxicol. 77, 120–131. https://doi.org/10.1016/j.fct.2014.12.022 (2015).
Ma, J., Long, S., Wang, J., Gao, J. & Piao, X. Microencapsulated essential oils combined with organic acids improves immune antioxidant capacity and intestinal barrier function as well as modulates the hindgut microbial community in piglets. J. Anim. Sci. Biotechnol. 13, 1–17. https://doi.org/10.1186/s40104-021-00670-3 (2022).
AOAC International. Official Methods of Analysis of AOAC. International, 16th edn. Association of Official Analytical Chemists, Washington, DC, USA (2000).
Muniyappan, M., Palanisamy, T. & Kim, I. H. Effect of microencapsulated organic acids on growth performance, nutrient digestibility, blood profile, fecal gas emission, fecal microbial, and meat-carcass grade quality of growing-finishing pigs. Livest. Sci. 252, 104658. https://doi.org/10.1016/j.livsci.2021.104658 (2021).
Yoon, S. H. et al. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 67(5), 1613. https://doi.org/10.1099/ijsem.0.001755 (2017).
Kim, S. W., Van Heugten, E., Ji, F., Lee, C. H. & Mateo, R. D. Fermented soybean meal as a vegetable protein source for nursery pigs: I. Effects on growth performance of nursery pigs. J. Anim. Sci. 88, 214–224. https://doi.org/10.2527/jas.2009-1993 (2010).
Jones, C. K. et al. Effects of fermented soybean meal and specialty animal protein sources on nursery pig performance. J. Anim. Sci. 88, 1725–1732. https://doi.org/10.2527/jas.2009-2110 (2010).
Zhang, H. Y. et al. The metabolizable energy value, standardized ileal digestibility of amino acids in soybean meal, soy protein concentrate and fermented soybean meal, and the application of these products in early-weaned piglets. Asian Australas. J. Anim. Sci. 26, 691. https://doi.org/10.5713/ajas.2012.12429 (2013).
Zhu, J. et al. Effects of soybean meal fermented by L. plantarum, B. subtilis and S. cerevisieae on growth, immune function and intestinal morphology in weaned piglets. Microb. Cell Factories 16, 1–10. https://doi.org/10.1186/s12934-017-0809-3 (2017).
Hong, K. J., Lee, C. H. & Kim, S. W. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J. Med. Food 7, 430–435. https://doi.org/10.1089/jmf.2004.7.430 (2004).
Min, B. J. et al. The effect of feeding processed soy protein on the growth performance and apparent ileal digestibility in weanling pigs. Asian Australas. J. Anim. Sci. 17, 1271–1276. https://doi.org/10.5713/ajas.2004.1271 (2004).
Frias, J., Song, Y. S., Martínez-Villaluenga, C., De Mejia, E. G. & Vidal-Valverde, C. Immunoreactivity and amino acid content of fermented soybean products. J. Agric. Food Chem. 56, 99–105. https://doi.org/10.1021/jf072177j (2008).
Feng, J., Liu, X., Xu, Z. R., Lu, Y. P. & Liu, Y. Y. The effect of Aspergillus oryzae fermented soybean meal on growth performance, digestibility of dietary components and activities of intestinal enzymes in weaned piglets. Anim. Feed Sci. Technol. 134, 295–303. https://doi.org/10.1016/j.anifeedsci.2006.10.004 (2007).
Kiers, J. L., Rombouts, F. M. & Nout, M. J. R. In vitro digestibility of Bacillus fermented soya bean. Int. J. Food Microbiol. 60, 163–169. https://doi.org/10.1016/S0168-1605(00)00308-1 (2000).
Kim, Y. G., Lohakare, J. D., Yun, J. H., Heo, S. & Chae, B. J. Effect of feeding levels of microbial fermented soy protein on the growth performance, nutrient digestibility and intestinal morphology in weaned piglets. Asian Australas. J. Anim. Sci. 20, 399–404. https://doi.org/10.5713/ajas.2007.399 (2007).
Kiers, J. L. et al. Effect of fermented soya beans on diarrhoea and feed efficiency in weaned piglets. J. Appl. Microbiol. 95, 545–552. https://doi.org/10.1046/j.1365-2672.2003.02011.x (2003).
Pluske, J. R., Pethick, D. W., Hopwood, D. E. & Hampson, D. J. Nutritional influences on some major enteric bacterial diseases of pig. Nutr. Res. Rev. 15, 333–371. https://doi.org/10.1079/NRR200242 (2002).
Yan, L. & Kim, I. H. Effect of probiotics supplementation in diets with different nutrient densities on growth performance, nutrient digestibility, blood characteristics, faecal microbial population and faecal noxious gas content in growing pigs. J. Appl. Anim. Res. 41, 23–28. https://doi.org/10.1080/09712119.2012.739092 (2013).
Min, B. J. et al. Effects of fermented soy protein on growth performance and blood protein contents in nursery pigs. Asian Australas. J. Anim. Sci. 22(7), 1038–1042. https://doi.org/10.5713/ajas.2009.80240 (2009).
Ding, Z., Chang, K. H. & Kim, I. Effects of fermented soybean meal on growth performance, nutrients digestibility, blood profile and fecal microflora in weaning pigs. Korean J. Agric. Sci. 47(1), 1–10. https://doi.org/10.7744/kjoas.20190062 (2020).
Hossain, M. M. et al. Apparent total tract digestibility and ileal digestibility of dry matter, nitrogen, energy and amino acids in conventional, Bacillus subtilis-fermented and enzyme-treated soybean meal fed to weanling pigs. Vet. Med. 61(12), 669–680. https://doi.org/10.17221/389/2014-VETMED (2016).
Lan, R. & Kim, I. Enterococcus faecium supplementation in sows during gestation and lactation improves the performance of sucking piglets. Vet. Med. Sci. 6, 92–99. https://doi.org/10.1002/vms3.215 (2020).
Cai Y. Impact of nutritional and environmental factors on plasma urea and amino acid concentrations in pigs. Doctoral dissertation, Iowa State University (1992).
Shi, C. et al. Effects of Aspergillus niger fermented rapeseed meal on nutrient digestibility, growth performance and serum parameters in growing pigs. Anim. Sci. J. 8, 557–563. https://doi.org/10.1111/asj.12457 (2016).
Cho, J. H. et al. Evaluation of FSP (fermented soy protein) to replace soybean meal in weaned pigs: Growth performance, blood urea nitrogen and total protein concentrations in serum and nutrient digestibility. Asian Australas. J. Anim. Sci. 20, 1874–1879. https://doi.org/10.5713/ajas.2007.1874 (2007).
Sadeghi, G. & Pourreza, J. Serum proteins and some biochemical parameters in broiler chickens fed with raw and treated bitter vetch (Vicia ervilia) seeds. Pak. J. Biol. Sci. 10, 977–981. https://doi.org/10.3923/pjbs.2007.977.981 (2007).
Gizzarelli, F. et al. Evaluation of allergenicity of genetically modified soybean protein extract in a murine model of oral allergen-specific sensitization. Clin. Exp. Allergy 36, 238–248. https://doi.org/10.1111/j.1365-2222.2005.02415.x (2006).
Wang, X. et al. Effects of β-conglycinin on growth performance, immunoglobulins and intestinal mucosal morphology in piglets. Arch. Anim. Nutr. 68, 186–195. https://doi.org/10.1080/1745039X.2014.919733 (2014).
Li, R. et al. Colonic microbiota and metabolites response to different dietary protein sources in a piglet model. Front. Nutr. 6, 151. https://doi.org/10.3389/fnut.2019.00151 (2019).
Li, R. et al. Different dietary protein sources in low protein diets regulate colonic microbiota and barrier function in a piglet model. Food. Funct. 10, 6417–6428. https://doi.org/10.1039/C9FO01154D (2019).
Ijaz, M. U. et al. Beef, casein, and soy proteins differentially affect lipid metabolism, triglycerides accumulation and gut microbiota of high-fat diet-fed C57BL/6J mice. Front. Microbiol. 9, 2200. https://doi.org/10.3389/fmicb.2018.02200 (2018).
Feng, H. et al. Effect of fermented soybean meal supplementation on some growth performance, blood chemical parameters, and fecal microflora of finishing pigs. R. Bras. Zootec. https://doi.org/10.37496/rbz4920190096 (2020).
Zhu, J. J. et al. Changes in bacterial diversity and composition in the faeces and colon of weaned piglets after feeding fermented soybean meal. J. Med. Microbiol. 67, 1181–1190. https://doi.org/10.1099/jmm.0.000766 (2018).
Singh, K. M. et al. High through put 16S rRNA gene-based pyrosequencing analysis of the fecal microbiota of high FCR and low FCR broiler growers. Mol. Biol. Rep. 39, 10595–10602. https://doi.org/10.1007/s11033-012-1947-7 (2012).
Bai, M. et al. Dietary Moutan cortex Radicis improves serum antioxidant capacity and intestinal immunity and alters colonic microbiota in weaned piglets. Front. Nutr. 8, 266. https://doi.org/10.3389/fnut.2021.679129 (2021).
Shin, N. R., Whon, T. W. & Bae, J. W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33, 496–503 (2015).
Litvak, Y., Byndloss, M. X., Tsolis, R. M. & Bäumler, A. J. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 39, 1–6. https://doi.org/10.1016/j.mib.2017.07.003 (2017).
Eren, A. M. et al. A single genus in the gut microbiome reflects host preference and specificity. ISME J 9, 90–100 (2015).
Vacca, M. et al. The controversial role of human gut lachnospiraceae. Microorganisms 8, 573. https://doi.org/10.3390/microorganisms8040573 (2020).
Yang, P. & Zhao, J. Variations on gut health and energy metabolism in pigs and humans by intake of different dietary fibers. Food Sci. Nutr. 9, 4639–4654. https://doi.org/10.1002/fsn3.2421 (2021).
Gelmez, E., Jeron, A. & Bruder, D. Negative elongation factor: A key factor in the maintenance of intestinal epithelial barrier integrity. Cell Mol. Immunol. https://doi.org/10.1038/s41423-021-00817-2 (2022).
Stanley, D., Hughes, R. J., Geier, M. S. & Moore, R. J. Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion: Challenges presented for the identification of performance enhancing probiotic bacteria. Front. Microbiol. 7, 187. https://doi.org/10.3389/fmicb.2016.00187 (2016).
He, T. et al. Lactobacillus johnsonii L531 reduces pathogen load and helps maintain short-chain fatty acid levels in the intestines of pigs challenged with Salmonella enterica Infantis. Vet. Microbiol. 230, 187–194. https://doi.org/10.1016/j.vetmic.2019.02.003 (2019).
Yang, A. et al. Degradation of major allergens and allergenicity reduction of soybean meal through solid-state fermentation with microorganisms. Food. Funct. 9, 1899–1909. https://doi.org/10.1039/C7FO01824J (2018).
Sato, Y. et al. Effects of dietary supplementation with enterococcus faecium and clostridium butyricum, either alone or in combination, on growth and fecal microbiota composition of post-weaning pigs at a commercial farm. Front. Vet. Sci. 6, 26. https://doi.org/10.3389/fvets.2019.00026 (2019).
He, T., Zheng, Y., Piao, X. & Long, S. Determination of the available energy, standardized ileal digestibility of amino acids of fermented corn germ meal replacing soybean meal in growing pig diets. Anim. Nutr. 9, 259–268. https://doi.org/10.1016/j.aninu.2021.11.007 (2022).
Yan, W., Sun, C., Yuan, J. & Yang, N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci. Rep. 7, 1–11 (2017).
Yu, T. et al. Low-molecular-weight chitosan supplementation increases the population of Prevotella in the cecal contents of weanling pigs. Front. Microbial. 8, 2182. https://doi.org/10.3389/fmicb.2017.02182 (2017).
Dou, S. et al. Characterisation of early-life fecal microbiota in susceptible and healthy pigs to post-weaning diarrhoea. PLoS ONE 12, e0169851. https://doi.org/10.1371/journal.pone.0169851 (2017).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108. https://doi.org/10.1126/science.1208344 (2011).
Forsyth, C. B. et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 6, e28032. https://doi.org/10.1371/journal.pone.0028032 (2011).
Alcalá, L. et al. Impact of clinical awareness and diagnostic tests on the underdiagnosis of Clostridium difficile infection. Eur. J. Clin. Microbiol. Infect. Dis. 34, 1515–1525. https://doi.org/10.1007/s10096-015-2380-3 (2015).
Harlow, B. E., Lawrence, L. M. & Flythe, M. D. Diarrhea-associated pathogens, lactobacilli and cellulolytic bacteria in equine feces: Responses to antibiotic challenge. Vet. Microbiol. 166, 225–232. https://doi.org/10.1016/j.vetmic.2013.05.003 (2013).
Sousa, M. A. B., Mendes, E. N., Apolônio, A. C. M., Farias, L. D. M. & Magalhaes, P. P. Bacteriocin production by Shigella sonnei isolated from faeces of children with acute diarrhoea. APMIS 118, 125–135. https://doi.org/10.1111/j.1600-0463.2009.02570.x (2010).
Gong, Y. et al. Early intervention with cecal fermentation broth regulates the colonization and development of gut microbiota in broiler chickens. Front. Microbiol. https://doi.org/10.3389/fmicb.2019.01422 (2019).
Yan, H. et al. Reduced feeding frequency improves feed efficiency associated with altered fecal microbiota and bile acid composition in pigs. Front. Microbiol. https://doi.org/10.3389/fmicb.2021.761210 (2021).
Strandwitz, P. et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 4, 396–403 (2019).
Hills, R. D. Jr. et al. Gut microbiome: Profound implications for diet and disease. Nutrients 11, 1613. https://doi.org/10.3390/nu11071613 (2019).
Zhai, H., Adeola, O. & Liu, J. Phosphorus nutrition of growing pigs. Anim. Nutr. 9, 127–137. https://doi.org/10.1016/j.aninu.2022.01.010 (2022).
Liu, J. B. et al. Effects of dietary phosphorus concentration and body weight on postileal phosphorus digestion in pigs. Anim. Feed Sci. Technol. 242, 86–94. https://doi.org/10.1016/j.anifeedsci.2018.06.003 (2018).
Acknowledgements
This research was supported by Basic Science Research Capacity Enhancement Project through Korea Basic Science Institute (National research Facilities and Equipment Center) grant funded by the Ministry of Education (Grant No. 2019R1A6C1010033). The Department of Animal Resource & Science was supported through the Research-Focused Department Promotion & Interdisciplinary Convergence Research Projects as a part of the University Innovation Support Program for Dankook University in 2022.
Author information
Authors and Affiliations
Contributions
M.M., S.S., J.H.P., K.H. and I.H.K.: Conceptualization and designed the trials. M.M.: writing – original draft preparation, performed the animal trials, M.M., S.S., I.H.K., J.H.P. and K.H.: Software, Methodology, Formal analysis, Writing – review and editing, I.H.K.: Supervision. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muniyappan, M., Shanmugam, S., Park, J.H. et al. Effects of fermented soybean meal supplementation on the growth performance and apparent total tract digestibility by modulating the gut microbiome of weaned piglets. Sci Rep 13, 3691 (2023). https://doi.org/10.1038/s41598-023-30698-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30698-6
This article is cited by
-
Dietary eubiotics of microbial muramidase and glycan improve intestinal villi, ileum microbiota composition and production trait of broiler
Journal of Animal Science and Biotechnology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.