Abstract
Residual ST-segment elevation after ST-segment elevation myocardial infarction (STEMI) has traditionally been considered a predictor of left ventricular (LV) dysfunction and ventricular aneurism. However, the implications in terms of long-term prognosis and cardiac magnetic resonance (CMR)-derived structural consequences are unclear. A total of 488 reperfused STEMI patients were prospectively included. The number of Q wave leads with residual ST-segment elevation > 1 mm (Q-STE) at pre-discharge ECG was assessed. LV ejection fraction (LVEF, %) and infarct size (IS, % of LV mass) were quantified in 319 patients at 6-month CMR. Major adverse cardiac events (MACE) were defined as all-cause death and/or re-admission for acute heart failure (HF), whichever occurred first. During a mean follow-up of 6.1 years, 92 MACE (18.9%), 39 deaths and 53 HF were recorded. After adjustment for baseline characteristics, Q-STE (per lead with > 1 mm) was independently associated with a higher risk of long-term MACE (HR 1.24 [1.07–1.44] per lead, p = 0.004), reduced (< 40%) LVEF (HR 1.36 [1.02–1.82] per lead, p = 0.04) and large (> 30% of LV mass) IS (HR 1.43 [1.11–1.85] per lead, p = 0.006) at 6-month CMR. Patients with Q-STE ≥ 2 leads (n = 172, 35.2%) displayed lower MACE-free survival, more depressed LVEF, and larger IS at 6-month CMR (p < 0.001 for all comparisons). Residual ST-segment elevation after STEMI represents a universally available tool that predicts worse long-term clinical and CMR-derived structural outcomes.
Similar content being viewed by others
Introduction
Despite the spectacular improvement in prognosis of patients with ST-segment elevation myocardial infarction (STEMI) during recent decades1, the short- and long-term risk of adverse outcome after the acute event is considerable2 and early risk stratification is recommended3,4. Systematic echocardiography performed before discharge should be recommended given the valuable prognostic information yielded by left ventricular (LV) ejection fraction (LVEF)5,6.
Electrocardiogram (ECG) is the paradigmatic non-invasive, inexpensive and universally available tool for risk stratification after STEMI. ST-segment resolution is a well-established predictor of coronary reperfusion and prognosis7,8. Residual ST-segment elevation after STEMI has conventionally been interpreted as predicting LV dysfunction and aneurysm9,10. More recently it has also been associated with more severe structural consequences in cardiac magnetic resonance (CMR) performed soon after STEMI11,12. Presence of Q waves on the presenting ECG or its development after STEMI have also been associated with worse clinical outcomes13,14,15 and more adverse early CMR-derived structural parameters16,17.
However, the contribution of pre-discharge ECG to predict long-term adverse events and the structural consequences in CMR performed late after STEMI is unclear. The aim of this study was to evaluate the relationship of combined Q wave and residual ST-segment elevation (Q-STE) with long-term cardiovascular events and CMR-derived LVEF and infarct size (IS).
Methods
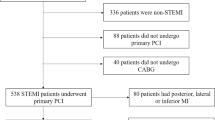
From 2012 to 2017, discharged STEMI patients treated with percutaneous coronary intervention were referred to a specific STEMI outpatient clinic, managed following current recommendations3 and scheduled to undergo CMR 6 months after infarction. Out of 488 prospectively recruited patients, 6-month CMR was performed in 319. The exclusion criteria were death (n = 9) or severe clinical instability (n = 7) before 6-month CMR. Unavailable or incomplete CMR studies (n = 49) or any contraindications to CMR (n = 17) were also criteria for exclusion. Other reasons for non-performance of 6-month CMR were medical decision (n = 58) or patient rejection (n = 29). The final study group therefore comprised 488 patients for evaluating the relationship between residual ST-segment elevation and long-term cardiovascular events, and 319 to evaluate the association of residual ST-segment elevation with LVEF and IS at 6-month CMR. The patient flowchart can be consulted in Supplementary Fig. S1.
This study is part of an ongoing STEMI registry of which several analyses have been previously reported6,10,11,18,19. The study protocol conforms to the principles of the Declaration of Helsinki and was approved by the local Human Research Ethics Committee. Patient informed consent was obtained.
Patient characteristics including Killip class at admission, peak creatine kinase MB mass, Thrombolysis in Myocardial Infarction (TIMI) flow grade in the culprit artery (before and after reperfusion) and the Global Registry of Acute Coronary Events (GRACE) and TIMI scores were recorded.
ECG analysis
Standard 12-lead electrocardiogram (ECG) was recorded in all patients at admission, at 90 min, 6, 24, 48, and 96 h after primary percutaneous coronary intervention (pPCI), and at pre-discharge. We used a standard calibration of the ECG (10 mm/mV amplification and paper speed of 25 mm/s). ECG data was retrospectively evaluated by an independent observer blinded to clinical and angiographic data.
ST-segment elevation (STE) was measured manually in every lead 20 ms after the J-point, in accordance with previous studies11,12. Maximum and minimum sum of ST-segment elevation (sum-STE) before and after pPCI and at any time were quantified in mm and calculated in V1 to V6, I and aVL leads for anterior infarction and II, III, aVF, V5 and V6 for non-anterior infarction. ST-segment resolution (STR) was defined as the percentage reduction in the sum-STE from baseline (ECG on admission) to 90 min after pPCI. Finally, the number of leads with Q wave and residual STE > 1 mm (Q-STE) at pre-discharge ECG were registered.
In the present study, we focused our analyses on the impact of residual ST-segment elevation (as derived from the number of leads with Q-STE at pre-discharge ECG). The rationale underpinning this assessment was as follows: (1) Residual ST-segment elevation in Q wave leads has been previously used and validated by our group as a marker of short-term prognostic and structural outcomes after STEMI10,11. (2) In the hierarchical multivariable model, Q-STE emerged as the ECG index most potently associated with the pre-defined clinical and CMR-derived endpoints.
Q-STE ≥ 2 leads was used for dichotomic analyses. This approach uses the best Youden index-derived cut-off point applied to the receiver operating characteristic analysis of Q-STE to predict clinical and structural endpoints. This same definition has been previously used by our group to predict the short-term consequences of STEMI10,11.
Echocardiography
All patients were studied with pre-discharge (5 ± 2 days post-STEMI) echocardiography, carried out by local cardiologists who quantified parameters and prospectively included the data in the database. LVEF (%), LV end-diastolic volume (mL) and LV end-systolic volume (mL) were assessed using the biplane method of disks (modified Simpson’s rule). Tricuspid annular plane systolic excursion (mm), as a proxy of right ventricle function, was measured in the apical 4-chamber view by means of M-mode. A wave velocity (m/s), E wave velocity (m/s), and left atrium diameter (mm) were also recorded.
Cardiac magnetic resonance imaging
Six-month CMR (197 [181–232] days) was performed in a subgroup of 319 patients, following the exclusion criteria depicted in Supplementary Fig. S1.
Patients were examined with a 1.5 T system (Sonata Magnetom, Siemens, Erlangen, Germany) according to a previously described standard protocol6,18. Images were acquired by phased-array body surface coil during breath-holds and were ECG triggered. Studies were interpreted by local cardiologists specialized in CMR imaging with > 15 years of experience.
Cine images were acquired in two-, three-, and four-chamber views, and in short-axis views using a steady-state free precession sequence (repetition time/echo time: 2.8/1.2 ms; flip angle: 58 degrees; matrix: 256 × 300; field of view: 320 × 270 mm; slice thickness: 7 mm).
LV end-diastolic volume index (LVEDVI, mL/m2), end-systolic volume index (LVESVI, mL/m2) and LVEF (%) were quantified by manual planimetry of endocardial and epicardial borders in short-axis view cine images.
Late gadolinium enhancement imaging was performed 10 min after administering gadolinium-based contrast in the same locations as in the cine images using a segmented inversion recovery steady-state free precession sequence (repetition time/echo time: 750/1.26 ms; flip angle: 45 degrees; matrix: 256 × 184; field of view: 340 × 235 mm; slice thickness: 7 mm). Inversion time was adjusted to nullify normal myocardium. IS was measured by manual planimetry and defined as percentage of LV mass showing late gadolinium enhancement.
Clinical endpoint
The primary endpoint of the study was MACE (major adverse cardiovascular events), defined as all-cause mortality or re-admission for acute heart failure, whichever occurred first. Current criteria for acute heart failure were used20. Clinical cardiologists prospectively adjudicated events by periodic electronic regional health system registry review.
Structural endpoints
We measured two adverse structural endpoints at 6-month CMR: LVEF < 40% was used to define patients with reduced LV systolic function at chronic phase, while IS > 30% of LV mass was used to select patients with large myocardial infarction. The deleterious prognostic effects of these indexes and the respective cut-off points applied have already been validated by our study group in the same patient series6,18,21.
Statistical analysis
The one-sample Kolmogorov–Smirnov test was used to test normal data distribution. For continuous parametric variables, data are expressed as mean ± standard deviation and analysed by Student’s t test. Continuous non-parametric variables are shown as median plus interquartile range and compared with Mann–Whitney U test. Qualitative variables are presented as percentages and compared by chi-square test or Fisher’s exact test.
Univariate analyses were performed to identify baseline, echocardiographic and ECG variables associated with clinical (MACE) and structural (LVEF < 40% and IS > 30% at 6-month CMR) endpoints.
The association of baseline, echocardiographic and ECG variables before discharge with time to first MACE was assessed by means of multivariable Cox proportional hazard regression models. Results are presented as hazard ratio (HR) and 95% confidence interval (CI). Variables with p value < 0.1 in univariate analysis were included as cofactors in multivariate analysis. Hierarchical models were used to avoid overfitting of variables. Model 1 included only baseline characteristics. In Model 2, variables of Model 1 plus echocardiographic indices were used. In Model 3 (final model), variables of Model 2 plus ECG indices were tested.
A multivariate binary logistic regression model using the same methodology was performed to assess the association of variables with 6-month CMR structural (LVEF < 40% and IS > 30% at 6-month CMR) endpoints.
The SPSS statistical package version 20.0 (SPSS Inc., Chicago, Illinois) and STATA version 9.0 (StataCorp, College Station, Texas) were used for statistical analysis. Statistical significance was set at p < 0.05.
Ethics declaration
The study protocol was approved by the Drug Research Ethics Committee of the Hospital Clínico Universitario of Valencia. Patient informed consent was obtained.
Results
Characteristics of the cohort
We included 488 patients in our registry. Baseline characteristics are displayed in Table 1. Mean age was 59 ± 12 years, 80.3% were male, and smoking was the most prevalent cardiovascular risk factor. Multivessel disease was present in 25.4%, the most frequent infarct localization was anterior (50.4%) and final TIMI 3 flow grade after reperfusion was restored in 91.2% of patients. Median time to revascularization was 190 min.
Pre-discharge echocardiographic and ECG variables are shown in Table 2. Mean LVEF was 54.9 ± 10.9%. Q-STE ≥ 2 leads was detected in 172 patients (35.2%).
Association of ECG variables with MACE
During a mean 6.1-year follow-up, we registered 92 (18.9%) cases of MACE (39 all-cause deaths and 53 re-admissions for acute heart failure). In hierarchical multivariable analysis, the number of leads with Q-STE > 1 mm at pre-discharge ECG was the most potent ECG predictor of MACE (HR 1.24 [1.07–1.44] per lead, p = 0.004). Hypertension, previous CAD, GRACE, and echocardiographic LVEF were also independently associated with MACE (Table 3). After adjustment, other ECG variables such as maximum and minimum sum-STE were not independently associated with MACE in the multivariable model.
Out of 172 patients with Q-STE ≥ 2 leads, 45 (26.2%) experienced MACE during follow-up, compared to 47 out of 316 (14.9%) patients with Q-STE in 0–1 leads. The adjusted survival curves are displayed in Fig. 1.
MACE-free survival by Q-STE category. Survival curves adjusted for predictors of MACE other than Q-STE (hypertension, previous CAD, GRACE risk score and echocardiography-derived LVEF). Abbreviations: CAD = coronary artery disease. GRACE = Global Registry of Acute Coronary Events. HR = hazard ratio. LVEF = Left ventricular ejection fraction. MACE = major adverse cardiovascular events. Q-STE = Q wave and residual STE > 1 mm.
Association of ECG variables with 6-month CMR parameters
In hierarchical multivariable analysis, the number of leads with Q-STE > 1 mm at pre-discharge ECG was the most potent ECG predictor of reduced (< 40%) LVEF and large (> 30% of LV mass) IS. After adjustment for baseline and ECG characteristics, the number of leads with Q-STE was independently and positively associated with risk of reduced (< 40%) LVEF (HR 1.36 [1.02–1.82], p = 0.04) and large (> 30% of LV mass) IS (HR 1.43 [1.11–1.85], p = 0.006) at 6-month CMR (Supplementary Tables S1, S2 and S3). Along with Q-STE, pre-discharge echocardiography LVEF was also independently associated with reduced LVEF (HR 0.86 [0.82–0.91], p < 0.001) and large IS (HR 0.88 [0.84–0.92], p < 0.001) at 6-month CMR.
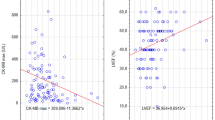
Patients with Q-STE ≥ 2 leads displayed more reduced LVEF (50.5 ± 13.6% vs. 58.6 ± 12.7%) and more extensive IS (24.9 ± 11.6% vs. 14.4 ± 11.4%) at 6-month CMR (p < 0.001 for all comparisons, Figs. 2 and 3).
Structural changes at 6-month CMR according to Q-STE category. Variables are presented as mean ± standard deviation. * = p < 0.001. Abbreviations: CMR = cardiac magnetic resonance. IS = infarct size. LVEF = left ventricular ejection fraction. LVEDVI = left ventricular end-diastolic volume index. LVESVI = left ventricular end-systolic volume index. Q-STE = Q wave and residual STE > 1 mm.
Examples of Q-STE and structural changes at 6-month CMR. Pre-discharge ECG showing no Q-STE (A) and Q-STE in ≥ 2 leads (B). Short axis CMR in diastole (left) and systole (right) indicating preserved (C) and reduced (< 40%, D) LVEF at 6 months. Late gadolinium enhancement imaging depicting non-extensive (E) and extensive (> 30% of LV mass, F) IS. Abbreviations: CMR = cardiac magnetic resonance. IS = infarct size. LV = left ventricular. LVEF = left ventricular ejection fraction. Q-STE = Q wave and residual STE > 1 mm.
Among patients with Q-STE ≥ 2 leads, 22.7% (n = 27) displayed LVEF < 40% and 33.9% (n = 41) had IS > 30% of LV mass at 6-month CMR, compared to 9.1% (n = 18) and 9.1% (n = 18) in patients with Q-STE in 0–1 leads, respectively (p < 0.001 for all comparisons, Fig. 4). After adjustment, patients with Q-STE ≥ 2 leads had a 3.04 [1.27–7.29]-fold and 5.83 [2.68–12.67]-fold increased probability of reduced LVEF and extensive IS at follow-up CMR compared to patients with Q-STE 0–1 leads at pre-discharge ECG (p = 0.01 and < 0.001, respectively).
Pre-discharge Q-STE categories and structural changes at 6-month CMR. The number and percentage of patients with reduced (< 40%) LVEF and large (> 30% of LV mass) IS at 6-month CMR is shown in each Q-STE category (0–1 leads and ≥ 2 leads). Abbreviations: CMR = cardiac magnetic resonance. IS = infarct size. LV = left ventricular. LVEF = left ventricular ejection fraction. Q-STE = Q wave and residual STE > 1 mm.
Similar to the whole study group, in both anterior and non-anterior infarction cases, patients with Q-STE ≥ 2 leads displayed a tendency towards more altered CMR-derived structural parameters at follow-up CMR compared to those with Q-STE in 0–1 leads (Supplementary Fig. S2).
Discussion
The main finding of the present study is that residual ST-segment elevation after reperfused STEMI contributes valuable information towards identifying a subset of patients at increased risk of long-term MACE as well as of depressed LVEF and large IS at 6-month CMR. A summary is provided in Fig. 5.
Visual summary depicting the most relevant findings of the study. Abbreviations: CMR = cardiac magnetic resonance. HR = hazard ratio. IS = infarct size. LV = left ventricular. LVEF = left ventricular ejection fraction. STEMI = ST-segment elevation myocardial infarction. Q-STE = Q wave and residual STE > 1 mm.
Risk stratification after STEMI
Current management strategies in STEMI, including widespread primary percutaneous coronary intervention and optimized medical treatment, have delivered remarkable improvements in patient prognosis1,3. Nevertheless, the risk of MACE during the following months and years (5–20%) is non-negligible in recent registries3, which highlights a need for early risk stratification of STEMI patients. Several combined risk scores such as the GRACE and TIMI scores have been developed for this purpose3. Age is a relevant predictor of prognosis since older patients are more likely to experience all-cause death or other adverse outcomes. Nonetheless, validation of easy-to-obtain and broadly available indexes for accurate prediction of long-term clinical and structural outcomes after reperfused STEMI is still of utmost importance.
LVEF by echocardiography is the cornerstone of non-invasive imaging risk stratification after STEMI, and lower pre-discharge values have been associated with an increased long-term risk of death and re-admission for heart failure5,22.
CMR has become the most reliable and reproducible method for a comprehensive evaluation of the structural consequences of STEMI23,24, permitting highly accurate and reproducible quantification of systolic function and IS in this setting. In recent years, we and other authors have demonstrated the solid prognostic value of CMR-derived LVEF and IS19,25,26. Unfortunately, restrictions deriving from availability, costs and need for expertise limit the generalized use of CMR.
Q-STE to predict long-term risk and CMR-derived IS and LVEF
Electrocardiography represents a non-invasive, lower cost, and universally attainable test that is sequentially performed in every STEMI patient. In the present study, we evaluated the long-term prognostic value of residual ST-segment elevation (as derived from the number of leads with Q-STE at pre-discharge) beyond traditional baseline and echocardiographic risk stratification. Additionally, we tested the value of this index to predict CMR-derived LVEF and IS in chronic phase after STEMI.
ST-segment resolution has been widely used as a proxy for successful coronary reperfusion7. Lower percentage post-reperfusion ST resolution8,27,28,29 and higher residual ST-segment elevation, either sum30 or residual single-lead ST-deviation29, have been associated with worse short-term clinical outcomes after reperfused STEMI31. Also, Q waves (either on the presenting ECG or developing after STEMI) have also been associated with worse clinical outcomes13,14,15 and more adverse early CMR-derived structural parameters16,17.
Our findings show that assessment of the number of Q wave leads with residual ST-segment elevation > 1 mm (Q-STE) at pre-discharge ECG permits immediate, robust long-term risk prediction above and beyond routine clinical and echocardiographic stratification. After adjustment, the extent of Q-STE (per number of leads) at pre-discharge appeared as an independent predictor of MACE over a 6.1-year mean follow-up. Moreover, Q-STE outperformed the rest of the collected ECG indexes for this purpose and the cut-off point proposed (Q-STE ≥ 2 leads) identified patients at high risk of MACE in a long-term perspective.
The deleterious effects of depressed LVEF and large IS are largely acknowledged, and as derived from CMR in chronic phase after STEMI, these indexes represent the cornerstone for evaluating the overall structural cardiac consequences of myocardial infarction18,21,32,33,34,35. Absence of ST-segment resolution has been associated with lower pre-discharge echocardiography-derived LVEF9. We and others have previously shown that residual ST-segment elevation associates with larger IS, more microvascular obstruction, less myocardial salvage, lower LVEF, and larger indexed LV volumes in CMR performed soon (1 week) after STEMI10,12 as well as more extensive IS by single-photon emission computed tomography31. However, the value of residual ST-segment elevation at pre-discharge ECG to predict CMR-derived LVEF and IS in chronic phase was unknown.
Our study is the first to explore in STEMI patients the association of pre-discharge ECG indexes with the long-term (6-month) CMR-derived structural consequences. Extent of Q-STE (per number of leads) was independently associated with reduced (< 40%) LVEF and large (> 30% of LV mass) IS, and the presence of Q-STE in ≥ 2 leads at pre-discharge ECG permitted good discrimination of patients at highest risk of severe structural deterioration at follow-up CMR.
Study limitations
As a primary limitation, referral bias cannot be excluded due to the observational nature of our study and the retrospective revision of several variables. Second, this is a single-centre study and patients referred for 6-month CMR may not be entirely representative of the whole STEMI population. Prospectively designed studies could overcome these limitations. Third, several biochemical and cardiac imaging parameters that could have shown additional prognostic value were not collected. Last, changes in treatment could have influenced clinical and/or structural outcomes, requiring an in-depth analysis of the effect of therapies that is beyond the scope of the present study.
Conclusions
Analysis of the number of Q wave leads with residual ST-segment elevation at pre-discharge ECG represents an easy-to-obtain and universally obtainable parameter which contributes rapid, valuable data on the risk of MACE, depressed LVEF and large IS at long-term follow-up. This justifies further exploration of the clinical implications of these findings, to guide more intensive follow-up, selective use of other non-invasive tests and improved therapeutic strategies.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Nabel, E. G. & Braunwald, E. A tale of coronary artery disease and myocardial infarction. N. Engl. J. Med. 366, 54–63 (2012).
Pedersen, F. et al. Short- and long-term cause of death in patients treated with primary PCI for STEMI. J. Am. Coll. Cardiol. 64, 2101–2108 (2014).
Ibanez, B. et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 39, 119–177 (2018).
McLeod, P., Adamson, P. D. & Coffey, S. Do we need early risk stratification after ST-elevation myocardial infarction?. Heart https://doi.org/10.1136/heartjnl-2021-320030 (2021).
Mukherjee, J. T. et al. In-hospital measurement of left ventricular ejection fraction and one-year outcomes in acute coronary syndromes: Results from the IMMEDIATE Trial. Cardiovasc. Ultrasound 14 (2016).
Marcos-Garces, V. et al. Ejection fraction by echocardiography for a selective use of magnetic resonance after infarction. Circ. Cardiovasc. Imaging 13, e011491 (2020).
Fu, Y. et al. Time to treatment influences the impact of ST-segment resolution on one-year prognosis: Insights from the assessment of the safety and efficacy of a new thrombolytic (ASSENT-2) Trial. Circulation 104, 2653–2659 (2001).
Buller, C. E. et al. ST-segment recovery and outcome after primary percutaneous coronary intervention for ST-elevation myocardial infarction: Insights from the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial. Circulation 118, 1335–1346 (2008).
Matetzky, S. et al. The significance of persistent ST elevation versus early resolution of ST segment elevation after primary PTCA. J. Am. Coll. Cardiol. 34, 1932–1938 (1999).
Bodí, V. et al. ST-segment elevation on Q leads at rest and during exercise: Relation with myocardial viability and left ventricular remodeling within the first 6 months after infarction. Am. Heart J. 137, 1107–1115 (1999).
Husser, O. et al. The sum of ST-segment elevation is the best predictor of microvascular obstruction in patients treated successfully by primary percutaneous coronary intervention. Cardiovascular magnetic resonance study. Rev. Esp. Cardiol. 63, 1145–1154 (2010).
Rommel, K. P. et al. Prognostic significance and relationship of worst lead residual ST segment elevation with myocardial damage assessed by cardiovascular MRI in myocardial infarction. Heart 100, 1257–1263 (2014).
Goodman, S. G. et al. Development and prognosis of non-Q-wave myocardial infarction in the thrombolytic era. Am. Heart J. 144, 243–250 (2002).
Bao, M. H. et al. Prognostic implications of quantitative evaluation of baseline Q-wave width in ST-segment elevation myocardial infarction. J. Electrocardiol. 47, 465–471 (2014).
Kosmidou, I. et al. Prognostic implications of Q waves at presentation in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: An analysis of the HORIZONS-AMI study. Clin. Cardiol. 40, 982–987 (2017).
Topal, D. G. et al. Association between early Q waves and reperfusion success in patients with ST-segment–elevation myocardial infarction treated with primary percutaneous coronary intervention: A cardiac magnetic resonance imaging study. Circ. Cardiovasc. Interv. 10, e004467 (2017).
Topal, D. G. et al. Early Q-wave morphology in prediction of reperfusion success in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention—A cardiac magnetic resonance imaging study. J. Electrocardiol. 58, 135–142 (2020).
Gavara, J. et al. Magnetic resonance assessment of left ventricular ejection fraction at any time post-infarction for prediction of subsequent events in a large multicenter STEMI registry. J. Magn. Reson. Imaging 10, 11–12. https://doi.org/10.1002/jmri.27789 (2021).
Bonanad, C. et al. Prediction of long-term major events soon after a first ST-segment elevation myocardial infarction by cardiovascular magnetic resonance. Eur. J. Radiol. 85, 585–592 (2016).
McDonagh, T. A. et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. https://doi.org/10.1093/eurheartj/ehab368 (2021).
Bodi, V. et al. Prediction of reverse remodeling at cardiac MR imaging soon after first ST-segment-elevation myocardial infarction: Results of a large prospective registry. Radiology 278, 54–63 (2016).
Antoni, M. L. et al. Cardiovascular mortality and heart failure risk score for patients after ST-segment elevation acute myocardial infarction treated with primary percutaneous coronary intervention (Data from the Leiden MISSION! Infarct Registry). Am. J. Cardiol. 109, 187–194 (2012).
Bodí, V. et al. Usefulness of a comprehensive cardiovascular magnetic resonance imaging assessment for predicting recovery of left ventricular wall motion in the setting of myocardial stunning. J. Am. Coll. Cardiol. 46, 1747–1752 (2005).
Bulluck, H., Dharmakumar, R., Arai, A. E., Berry, C. & Hausenloy, D. J. Cardiovascular magnetic resonance in acute ST-segment-elevation myocardial infarction: Recent advances, controversies, and future directions. Circulation 137, 1949–1964 (2018).
Eitel, I. et al. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol 64, 1217–1226 (2014).
Lonborg, J. et al. Final infarct size measured by cardiovascular magnetic resonance in patients with ST elevation myocardial infarction predicts long-term clinical outcome: An observational study. Eur. Heart J. Cardiovasc. Imaging 14, 387–395 (2013).
Spitaleri, G. et al. Role of ST-segment resolution in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention (from the 5-Year outcomes of the EXAMINATION [Evaluation of the Xience-V Stent in Acute Myocardial Infarction] Trial). Am. J. Cardiol. 121, 1039–1045 (2018).
Tomaszuk-Kazberuk, A. et al. Does lack of ST-segment resolution still have prognostic value 6 years after an acute myocardial infarction treated with coronary intervention?. Can. J. Cardiol. 27, 573–580 (2011).
De Luca, G. et al. Postprocedural single-lead ST-segment deviation and long-term mortality in patients with ST-segment elevation myocardial infarction treated by primary angioplasty. Heart 94, 44–47 (2008).
Verouden, N. J. W. et al. Prediction of 1-year mortality with different measures of ST-segment recovery in all-comers after primary percutaneous coronary intervention for acute myocardial infarction. Circ. Cardiovasc. Qual. Outcomes 3, 522–529 (2010).
Kuijt, W. J. et al. What is the best ST-segment recovery parameter to predict clinical outcome and myocardial infarct size? Amplitude, speed, and completeness of ST-segment recovery after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. J. Electrocardiol. 50, 952–959 (2017).
Bulluck, H. et al. Defining left ventricular remodeling following acute ST-segment elevation myocardial infarction using cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 19, 26 (2017).
van der Bijl, P. et al. Left ventricular post-infarct remodeling. JACC Heart Fail. 8, 131–140 (2020).
Rodriguez-Palomares, J. F. et al. Prognostic value of initial left ventricular remodeling in patients with reperfused STEMI. JACC Cardiovasc. Imaging 12, 2445–2456 (2019).
Gavara, J. et al. Prognostic value of strain by tissue tracking cardiac magnetic resonance after ST-segment elevation myocardial infarction. JACC Cardiovasc. Imaging 11, 1448–1457 (2018).
Funding
This work was supported by the Instituto de Salud Carlos III and co-funded by Fondo Europeo de Desarrollo Regional (FEDER) (Grants PI20/00637 and CIBERCV16/11/00486, postgraduate contract FI18/00320 to C.R.-N. and CM21/00175 to V.M.-G.), Generalitat Valenciana (Grant PROMETEO/2021/008), and by Sociedad Española de Cardiología (Grant SEC/FECINV-CLI 21/024). J. G. acknowledges financial support from the Agencia Estatal de Investigación (Grant FJC2020-043981-I/AEI/).
Author information
Authors and Affiliations
Contributions
H.M.G. and V.M.G. contributed equally to this work. H.M.G. and V.M.G. drafted the article and contributed to the conception and design of the work. H.M.G., V.M.G., J.G., A.P.T., M.P.L.L., J.V.M., N.P., C.R.N., E.D.D., A.G.P., P.R., C.B., F.J.C. and V.B. contributed to data collection. H.M.G., V.M.G., J.G. and V.B. contributed to data analysis and interpretation. H.M.G., V.M.G., J.G., A.P.T., M.P.L.L., J.V.M., N.P., C.R.N., E.D.D., A.G.P., P.R., C.B., F.J.C. and V.B. contributed critical revision of the article. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Merenciano-González, H., Marcos-Garcés, V., Gavara, J. et al. Residual ST-segment elevation to predict long-term clinical and CMR-derived outcomes in STEMI. Sci Rep 12, 21813 (2022). https://doi.org/10.1038/s41598-022-26082-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26082-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.