Abstract
Middle Eastern immigrants constitute a growing proportion of the European population and compared to native Swedes are more insulin resistant, which can contribute to atherosclerosis. Quantitative first pass perfusion (qFPP) using cardiovascular magnetic resonance (CMR) can detect early signs of cardiovascular disease (CVD). The aim was to study if myocardial perfusion differs between healthy male Middle Eastern immigrants and native male Swedes. Eighteen Iraqi- and twelve Swedish born controls, all males, never smokers with no CVD risk factors were included. Global myocardial perfusion at rest and stress was assessed using qFPP and by phase-contrast CMR imaging of coronary sinus flow. Quantitative first pass perfusion analysis (mean ± SD) demonstrated no difference at rest between Iraqi and Swedish males (0.8 ± 0.2 vs 1.0 ± 0.4 ml/min/g, P = 0.38) but lower perfusion during adenosine in Iraqi males (2.9 ± 0.7 vs 3.5 ± 0.7 ml/min/g, P = 0.02). Myocardial perfusion assessed by coronary sinus flow demonstrated similar results with no difference in resting perfusion between groups (0.7 ± 0.2 vs 0.8 ± 0.2 ml/min/g, P = 0.21) but a lower perfusion during adenosine in the Iraqi group (3.0 ± 0.2 vs 3.7 ± 0.6 ml/min/g, P = 0.01. Myocardial perfusion during adenosine stress was lower in healthy Iraqi immigrants compared to Swedish controls suggesting impaired microvascular function and risk of underestimating CVD risk in healthy individuals of Middle Eastern origin.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is the most common cause of death in Europe and one of the strongest risk factors for CVD is type 2 diabetes1. Middle Eastern immigrants constitute a growing proportion of the European population and are at high risk for type 2 diabetes, obesity and hyperlipidemia2, translating to an increased risk for CVD3. In Sweden, one of the largest immigrant populations are from Iraq4. The Iraqi immigrant group in southern Sweden has been studied within the population based MEDIM (impact of Migration and Ethnicity on Diabetes in Malmö) study. The studies from MEDIM have previously demonstrated that Iraqi immigrants irrespective of diabetes related risk factors, are more insulin resistant compared to native Swedes, which can contribute to increased risk of atherosclerosis5. In a recent cohort study including new onset type 2 diabetes patients (the ANDIS study), it was shown that first generation Middle Eastern immigrants had a more insulin‐deficient diabetes phenotype and genotype than native Swedes, as well as a higher risk of macrovascular diabetic complications such as coronary events6. However, the pathophysiological basis for the increased risk of CVD in Middle Eastern immigrants is still not clear.
Coronary microvascular function, using positron emission tomography (PET), has been shown to be impaired in patients with the metabolic syndrome as well as in individuals at high risk for CVD without the metabolic syndrome7. Single-photon emission computed tomography (SPECT) can also be used for quantitative estimates of myocardial perfusion but there are inherent limitations as low tracer uptake at high flow rates8,9. Cardiovascular magnetic resonance (CMR) imaging conducted without radioactive tracers, offers superior morphological and functional characterization of the myocardium compared to SPECT and PET and the possibility to study both microvascular function by quantitative (ml/min/g) first pass perfusion (qFPP)10 and extracellular volume mapping11,12. Quantitative first pass perfusion has been integrated inline in the clinical work flow and validated against PET in patients10,13.
In the current study we hypothesized that healthy Middle Eastern immigrants from Iraq living in Sweden without traditional CVD risk factors have altered myocardial microvascular function compared to native Swedes. Since there are sex differences in cardiometabolic risk, and males are at higher CVD risk than females, the current study was performed on males only. Therefore, the aim of the current study was to investigate if quantitative myocardial perfusion using CMR differs between male Iraqi immigrants, without cardiometabolic disease and native male Swedish controls.
Methods
Study population and study design
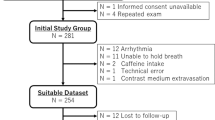
The study was approved by the Regional Ethics Committee at Lund University Sweden (Dnr 2015/507) and conformed to the Declaration of Helsinki. Written informed consent was given by all participants. A subset of male participants in the previous MEDIM cohort without any signs or established risk factors for CVD were invited to participate in the study. The cohort has been described in detail previously14. Briefly, the MEDIM cohort is a cross-sectional study conducted 2010–2012 including 2155 Iraqi or Swedish born residents of Malmö 30–75 years of age. In total 259 healthy, never smokers, non-obese males without CVD risk factors were identified from the baseline study and invited by mail to participate in the present study. A total of 18 Iraqi born and twelve native Swedish born males, of Caucasian origin and none had parents originating outside of Europe, fulfilled inclusion criteria and accepted to participate in this CMR sub-study, see Fig. 1A. Exclusion criteria included known CVD, diabetes, obesity, kidney disease, history of asthma, smoking or any active medication. Also, subjects with clear regional perfusion deficits on CMR were excluded. Subjects were recruited between 2017 and 2019 and examined at Lund University Hospital, Lund, Sweden. All patients underwent rest and adenosine stress CMR and adhered to a 24-h caffeine restriction prior to scanning.
Baseline characteristics and blood samples
Prior to the CMR exam, height, weight, waist circumference and blood pressure (in the supine position) were assessed. Fasting glucose, hemoglobin A1c, creatinine, cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein and apolipoprotein B/A1 in serum and urine albumin/creatinine were analyzed fasting as previously described5,14. Homeostasis model assessment (HOMA) was used to estimate both insulin resistance (HOMA-IR) and beta cell function (HOMA-β)5. An electrocardiogram was acquired prior to the CMR exam.
For objectively calculating the ten year risk of fatal CVD of study participants, the risk scoring systems “Framingham risk score for hard coronary heart disease”15 and the “systematic coronary risk evaluation (SCORE)”16 were used including the variables sex, age, systolic blood pressure, total cholesterol and smoking status.
CMR image acquisition
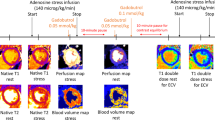
All images were acquired on a Magnetom Aera 1.5 T CMR system (Siemens Healthcare, Erlangen, Germany), see Fig. 1B for an overview of the CMR protocol.
Left ventricular volumes and function
Left ventricular volumes and function were assessed by cine imaging using a steady-state free precession sequence (SSFP) in breath hold both in short-axis and long-axis projections (2-, 3- and 4-chamber views). Typical imaging parameters included repetition time (TR) = 2.7 ms, echo time (TE) = 1.2 ms, flip angle 60°, spatial resolution 1.5 × 1.5 × 8 mm with no slice gap and field of view (FOV) 270 × 320 mm2.
Quantitative first pass perfusion
A basal, a mid-ventricular and an apical short-axis image were acquired at rest and during adenosine stress (Adenosin, Life Medical AB, Stockholm, Sweden, 140 µg/kg/min infusion) using qFPP imaging during administration of an intravenous bolus of contrast agent (0.05 mmol/kg, infusion rate 4 ml/s, Gadobutrol, Gadovist, Bayer AB, Solna, Sweden). Stress images were acquired first during 60 heart beats, starting three minutes after initiation of intravenous adenosine infusion and rest images were acquired approximately 15 min later. Measurements in low-resolution proton density images optimized for high gadolinium concentration in the LV blood pool were used to calculate the arterial input function10. After motion correction and conversion of signal intensities to gadolinium concentration, myocardial perfusion in ml/min/g was derived on a per-pixel basis10,13. Typical imaging parameters were: SSFP single shot readout, TE 1.0 ms, TR 2.5 ms, flip angle 50°, FOV 360 × 270 mm2, slice thickness 8.0 mm, parallel acquisition technique factor 3, acquisition time per single shot slice 142 ms and saturation delay 105 ms.
Coronary sinus flow
Images of the coronary sinus were acquired at rest and during adenosine stress for quantification of global perfusion. Rest images were acquired first and stress images were acquired immediately after acquisition of the qFPP images, approximately 5 min later. A breath-hold phase-contrast CMR with retrospective ECG triggering was used. Typical imaging parameters were: TR 5 ms, TE 2.8 ms, flip angle 20°, parallel imaging factor 2, a reconstructed spatial resolution of 1.6 × 1.6 × 8 mm and velocity encoded (VENC) factor 80 cm/s for rest and 120 cm/s for stress.
Fibrosis and extracellular volume
A modified Look-Locker (MOLLI) T1 sequence was used to generate T1 maps before and after gadolinium contrast agent administration. An inline extracellular volume map (ECV-map) was created after manual input of the hematocrit. Three short axis-slices were acquired at a basal, mid-ventricular and apical level. Macroscopic fibrosis was studied using a free breathing, motion corrected, late gadolinium enhancement (LGE) sequence acquired both in short and long axis projections. The inversion time was chosen to null remote myocardium. Specific parameters for the LGE sequence were: TR 2.8 ms, TE 1.2 ms, flip angle 50°, FOV 360 × 270 mm, resolution 1.4 × 1.4 × 8 mm, no slice gap.
CMR image analysis
All images were analyzed using the software Segment (v2.0 R5378), Medviso AB, Lund, Sweden)17, with the observer blinded to subject identity. For all images, endo- and epicardial borders were manually delineated. Left ventricular (LV) volumes, ejection fraction and left ventricular mass (LVM) were quantified from the short-axis cine stack. Interobserver variability for LVM was analyzed in six Iraqi and six Swedish controls. Rate pressure product (RPP) was calculated as heart rate × systolic blood pressure for both rest and stress.
Quantitative first pass perfusion and ECV
For qFPP and ECV images region of interest (ROI) were drawn 10% away from the endo- and epicardial borders to avoid inclusion of blood pool or extra-cardiac structures. Myocardial perfusion (ml/min/g)10,13 and ECV11,12 (%) were assessed manually by drawing a ROI in each short-axis slice. The absolute perfusion could then be extracted directly from the ROI as each pixel contained information on absolute perfusion. Myocardial perfusion and ECV were assessed globally by averaging all acquired short-axis slices. Each short-axis slice in qFPP images at rest and stress was also divided as endocardial (inner 50%) and epicardial (outer 50%) regions. Resting perfusion was corrected for rate pressure product (RPP) as resting perfusion × 10,000/((heart rate at rest) × (systolic blood pressure at rest). Myocardial perfusion reserve (MPR) was calculated as the ratio of stress to rest perfusion and MPR using RPP corrected resting perfusion was also calculated. Transmural endocardial-to-epicardial MPR gradients were also calculated. Recently, a cutoff of global stress qFPP < 2.25 ml/g/min was proposed to be suggestive of microvascular disease19. We also calculated the myocardial perfusion per mass of myocytes as qFPP/(1-ECV).
Sinus coronary flow
Global myocardial perfusion (ml/min/g) was also quantified as CSF (ml/min)/LVM (g)20. LVM for CSF quantification was calculated by including papillary muscles and trabeculae by visual thresholding. Interobserver variability for CSF at rest and stress was analyzed in six Iraqi and six Swedish controls and for the addition of papillary muscles and trabeculae to LVM in all subjects.
Late gadolinium enhancement
Focal fibrosis was assessed on short-axis LGE images by the EWA (expectation maximization weighted intensity a priori information) algorithm [21].
Statistical analysis
Continuous data are expressed as mean ± standard deviation (SD). Mean values between groups were assessed using Fischer’s exact test, paired or unpaired t-test as appropriate in normally distributed data. The relationship between continuous variables were assessed with Pearson’s correlation coefficient. Bias according to Bland–Altman was used to compare qFPP and sinus coronary flow and for interobserver analysis. Univariable association with myocardial perfusion for covariables that differed between groups was analyzed using linear regression. All statistical analyses were performed using IBM SPSS Statistics (IBM SPSS Statistics 23, IBM, New York, USA) and Graph Pad Prism 7.0 software (Graph Pad Software, Inc., La Jolla, CA, USA). Differences with a P value < 0.05 were considered statistically significant.
Results
Study population
In one Iraqi and one Swedish control subject, resting perfusion images were not acquired, however stress images were included in the analysis. In two Iraqi subjects and one Swedish control only two slices qFPP images and T1 maps were acquired. One Iraqi subject with a regional inferior perfusion deficit was excluded. Table 1 shows baseline characteristics for all subjects. High-density lipoprotein and apolipoprotein A1/B were lower in the Iraqi group (P < 0.05) whereas HOMA-β was higher (P < 0.05) compared to the Swedish group, the latter indicating a higher level of insulin secretion. Figure 2 shows representative myocardial perfusion images at rest and stress, corresponding LGE images and extracellular volume maps. Hemodynamics and CMR imaging volumetrics for all subjects are shown in Table 2. Mean RPP were similar for Iraqi males and Swedish healthy controls at rest (7026 ± 1489 vs 7291 ± 2573, P = 0.72) and stress (10403 ± 2563 vs 11016 ± 1437, P = 0.46). The difference between stress and rest RPP did not differ between groups (3377 ± 1609 vs 3724 ± 3077, P = 0.68) indicating similar hemodynamic responses to adenosine stress.
Example of quantitative first pass perfusion at rest and during adenosine stress, late gadolinium enhancement (LGE) and extracellular volume (ECV) CMR images from one Iraqi and one Swedish participant. Note the perfusion defect in the Iraqi subject at stress. No corresponding LGE or increased ECV is seen.
Framingham risk score for coronary heart disease was similar for Iraqi and Swedish males (4.0% vs 4.3%, P = 0.75) reflecting low CVD risk within the next decade in both Iraqi and Swedish born men. The SCORE risk score showed similar results (1.3% vs 1.5%, P = 0.79).
Myocardial perfusion
Quantitative first pass perfusion
Global flow from qFPP analysis demonstrated no statistically significant difference between groups in resting perfusion (0.8 ± 0.2 vs 1.0 ± 0.4 ml/min/g, P = 0.38) or RPP corrected resting perfusion (1.2 ± 0.5 vs 1.1 ± 0.2 ml/min/g × [mmHg × bpm/104]−1, P = 0.59). However, perfusion during adenosine was lower in Iraqi males (2.9 ± 0.7 vs 3.5 ± 0.7 ml/min/g. P = 0.02), see Fig. 3. If a cutoff of 2.25 ml/min/g for a decreased absolute perfusion was used (dashed horizontal line in Fig. 3) three Iraqi subjects demonstrated signs of microvascular disease. MPR did not show any statistically significant difference between groups (3.9 ± 1.0 vs 4.3 ± 1.2 ml/min/g, P = 0.42) or with RPP corrected resting perfusion (2.8 ± 1.0 vs 3.3 ± 0.8 ml/min/g × [mmHg × bpm/104]−1, P = 0.14). There was also a significant difference between Iraqi and Swedish subjects in qFPP per mass of myocytes (4.0 ± 1.0 vs 4.8 ± 1.0 ml/min/g, P = 0.03).
Global quantitative first pass perfusion in Iraqi versus Swedish born males at rest and during adenosine. Perfusion during adenosine was significantly lower in Iraqi males but there was no difference in myocardial perfusion at rest. If a cutoff 2.25 ml/min/g for a decreased absolute perfusion is used (dashed horizontal line) three Iraqi subjects demonstrate signs of microvascular disease. Error bars denote mean ± SD.
Transmural perfusion gradient
In the Iraqi group, using qFPP, the endocardium-to-epicardium ratio at stress was lower compared to rest (0.91 ± 0.09 vs 1.03 ± 0.08 ml/min/g, P = 0.002). In the Swedish control group there was also a lower transmural perfusion gradient during stress (0.97 ± 0.04 vs 1.06 ± 0.10 ml/min/g, P = 0.001). There was no statistical significant difference in endocardial-to-epicardial qFPP stress ratio between the Iraqi and Swedish control group (P = 0.07), nor endocardial-to-epicardial MPR ratio (0.89 ± 0.09 vs 0.96 ± 0.07 P = 0.17).
Sinus coronary flow
Global myocardial perfusion assessed by sinus coronary flow demonstrated similar results as qFPP with no difference at rest between groups (0.7 ± 0.2 vs 0.8 ± 0.2 ml/min/g. P = 0.21) or using RPP corrected resting perfusion (1.0 ± 0.3 vs 1.0 ± 0.2 ml/min/g. P = 0.82) but a lower perfusion during adenosine in the Iraqi group (3.0 ± 0.2 vs 3.7 ± 0.6 ml/min/g. P = 0.01), see Fig. 4. MPR did not show any statistically significant difference between groups (4.6 ± 1.3 vs 4.8 ± 1.2 ml/min/g, P = 0.63) or with RPP corrected resting perfusion (3.2 ± 1.2 vs 3.8 ± 1.0 ml/min/g × [mmHg × bpm/104]−1, P = 0.14). The relationship between global qFPP and coronary sinus flow at rest and stress demonstrated a correlation (r) of 0.95 and bias of 0.02 ± 0.5 ml/min/g, see Fig. 5.
The relationship between global quantitative first pass perfusion (qFPP) and coronary sinus flow at rest and stress in Iraqi males (open circles) and Swedish born males as controls (open squares). (A) Global qFPP plotted against coronary sinus flow with the dashed lined representing the line of identity and solid line representing linear regression (r = 0.95, y = 0.89x + 0.20). (B) Agreement between qFPP and sinus coronary flow, bias 0.02 ± 0.5 ml/min/g. Dashed lines represent ± 2SD and solid line represent bias.
Interobserver variability of coronary sinus flow at both rest and stress and LVM in a subset of study participants (n = 12) was 0.1 ± 0.2 ml/min/g and 1 ± 2 g respectively and for inclusion of papillary muscles and trabeculae to LVM − 4 ± 4 g.
None of the variables insulin secretion (HOMA-β), HDL or Apo B/A1 were associated with stress perfusion using qFPP (βHOMA-β = 0.094, βHDL = 0.36, βAPOB/A1 = 0.29, P = 0.1–0.5) or sinus coronary flow (βHOMA-β = 0.091, βHDL = 0.39, βAPOB/A1 = 0.32, P = 0.1–0.5).
Fibrosis and extracellular volume
LGE was not present in any subject. Extracellular volume did not differ between the Iraqi and Swedish group (27 ± 3 vs 26 ± 2%, P = 0.76).
Discussion
This study demonstrates that in healthy males, myocardial perfusion by quantitative first pass perfusion and by coronary sinus flow was lower in Iraqi compared to Swedish born controls, suggesting impaired microvascular function. Despite the absence of clinical cardiovascular risk factors, early signs of CVD are present in Middle Eastern immigrants.
Two independent methods, qFPP and sinus coronary flow, were used for assessing myocardial perfusion and showed similar results and good agreement. All subjects had normal LV ejection fraction and volumes. None of the subjects demonstrated any focal fibrosis on LGE imaging and ECV values were similar between groups and within in normal range22. Thus, myocardial perfusion provided pathophysiological information not evident on either functional, LGE or ECV imaging. Further, these data suggest that the CVD scoring systems may be insufficient in detecting Middle Eastern immigrants at risk for CVD.
Our results in our control group on rest and stress qFPP are in line with previously published data from Nickander et al. (rest 0.80 ± 0.17 and stress 3.2 ± 0.64 ml/min/g)23 and Kellman et al. (0.95 ± 0.16 and 3.4 ± 0.39 ml/min/g)10. The qFPP used in the current study has shown good agreement compared to PET13. The results for sinus coronary flow estimates of myocardial perfusion in our control group were also similar to earlier studies by Carlsson et al.24. Coronary sinus flow can assess global left ventricular perfusion without the use of contrast medium administration, which can be beneficial, and can be used to test the efficacy of drug treatment25, to predict outcome in patients with heart failure with preserved ejection fraction26, with coronary artery disease27 and diabetes respectively28.
Using a cutoff for global qFPP of 2.25 ml/min/g three Iraqi subjects showed signs of microvascular dysfunction. It has been proposed that a transmural myocardial MPR ratio < 0.7229 and < 0.830 from endocardium-to-epicardium can be suggestive of microvascular dysfunction in microvascular angina patients using semi-quantitative myocardial perfusion. With these cut-offs three Iraqi subjects showed signs of microvascular dysfunction (all with ratios < 0.72). However, in our study, patients were asymptomatic and a different CMR perfusion technique was used making a comparison somewhat difficult. In addition, it has been debated if a single cut-off value for transmural gradients should be used given that normal physiological mechanisms influence the endocardium-to epicardium MPR gradient31.
Previously, first-generation Iraqi immigrants living in Sweden have shown to be more insulin resistant5, have a higher prevalence of type 2 diabetes14 but paradoxically an overall beneficial lipid profile than native Swedes32. The Iraqi group in our study had higher beta-cell function assessed by HOMA-β, suggesting early compensatory insulin secretion for the higher insulin resistance. Also, the Iraqi group had a more unfavorable lipid profile with lower levels of HDL and higher APO B/A1. However, differences in stress perfusion were not associated with either HOMA-B or lipid profile. We could not find any cardiovascular risk factors explaining the variation in myocardial perfusion across males of Middle Eastern and Caucasian origin. From the same MEDIM cohort as the current study is based on two studies on differences in fat metabolism have recently demonstrated that (1) healthy Iraqi male immigrants displayed a higher triglyceride peak and a delayed postprandial triglyceride clearance compared with Swedes after an oral fat tolerance test33 and (2) Iraqi-born men presented a more favorable abdominal fatty-acid composition compared to Swedish-born men using MRI of the abdomen34.
The results in this study should also be interpreted in the light of both groups being a priori cardiovascular healthy, without any symptoms of CVD and with normal ten-year risk scores for myocardial infarction or cardiovascular death. All subjects had normal 12-lead electrocardiograms. Thus, according to standard assessment for CVD risk none of the subjects in the Iraqi group would be expected to have signs of myocardial microvascular disease. From a clinical perspective, assessment of cardiometabolic risk factors is crucial in estimating CVD risk. Previous CVD risk calculators are primarily based on Caucasian populations, but our data indicate these scores may be insufficient when estimating CVD risk in other ethnic populations.
The results of this study should be interpreted in the light of some limitations. There was a limited number of subjects in this study and the results should be confirmed in larger cohorts. None of the subjects performed an assessment of the coronary epicardial vessels. No females were included in this study and therefore results cannot be generalized to both sexes. Also recently, it was shown by Nickander et al. that there is a difference in myocardial perfusion between sexes23 which warrant further caution in interpretation of results. A high number of invited participants declined to participate even though they got follow up phone calls and contact with the primary investigator. The high dropout is a potential inclusion bias to the study.
This study on healthy Iraqi immigrants living in Sweden with no established risk factors for coronary artery disease or microvascular disease demonstrate significantly lower myocardial perfusion during adenosine stress compared to Swedish controls. Our results demonstrate the value of non-invasive CMR imaging to detect potential early alterations to the myocardial microvascular system. Despite the absence of clinical cardiovascular risk factors, early signs of CVD may be present in Middle Eastern immigrants. Thus, myocardial perfusion provided incremental pathophysiological information to both functional, LGE and ECV imaging. Also, CVD scoring systems may be insufficient in detecting people of Middle Eastern origin at risk for CVD. This calls for future altered preventive strategies in detecting cardiovascular disease in this high-risk population.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Timmis, A. et al. European society of cardiology: Cardiovascular disease statistics 2019. Eur. Heart J. 41, 12–85 (2020).
Wändell, P. E. et al. Estimation of diabetes prevalence among immigrants from the Middle East in Sweden by using three different data sources. Diabetes Metab. 34, 328–333 (2008).
Bennet, L., Agardh, C. D. & Lindblad, U. Cardiovascular disease in relation to diabetes status in immigrants from the Middle East compared to native Swedes: A cross-sectional study. BMC Public Health 13, 1 (2013).
Statistics Sweden. Available at http://www.scb.se. Stat. Sweden.
Bennet, L., Groop, L. & Franks, P. W. Ethnic differences in the contribution of insulin action and secretion to type 2 diabetes in immigrants from the Middle East compared to native Swedes. Diabetes Res. Clin. Pract. 105, 79–87 (2014).
Bennet, L. et al. Adult-onset diabetes in Middle Eastern immigrants to Sweden: Novel subgroups and diabetic complications—The All New Diabetes in Scania cohort diabetic complications and ethnicity. Diabetes Metab. Res. Rev. 29, e3419 (2020).
Di Carli, M. F. et al. Coronary circulatory function in patients with the metabolic syndrome. J. Nucl. Med. 52, 1369–1377 (2011).
Cuocolo, A. et al. Resting technetium-99m methoxyisobutylisonitrile cardiac imaging in chronic coronary artery disease: Comparison with rest-redistribution thallium-201 scintigraphy. Eur. J. Nucl. Med. 20, 1186–1192 (1993).
Petretta, M., Storto, G., Pellegrino, T., Bonaduce, D. & Cuocolo, A. Quantitative assessment of myocardial blood flow with SPECT. Prog. Cardiovasc. Dis. 57, 607–614 (2015).
Kellman, P. et al. Myocardial perfusion cardiovascular magnetic resonance: Optimized dual sequence and reconstruction for quantification. J. Cardiovasc. Magn. Reson. 19, 1–14 (2017).
Arheden, H. et al. Measurement of the distribution volume of gadopentetate dimeglumine at echo-planar MR imaging to quantify myocardial infarction: Comparison with 99mTc-DTPA autoradiography in rats. Radiology 211, 698–708 (1999).
Kellman, P., Wilson, J. R., Xue, H., Ugander, M. & Arai, A. E. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J. Cardiovasc. Magn. Reson. 14, 63 (2012).
Engblom, H. et al. Fully quantitative cardiovascular magnetic resonance myocardial perfusion ready for clinical use: A comparison between cardiovascular magnetic resonance imaging and positron emission tomography. J. Cardiovasc. Magn. Reson. 19, 1–9 (2017).
Bennet, L. et al. High prevalence of type 2 diabetes in Iraqi and Swedish residents in a deprived Swedish neighbourhood - A population based study. BMC Public Health 11, 1 (2011).
Wilson, P. W. F. et al. Prediction of coronary heart disease using risk factor categories. Circulation 97, 1837–1847 (1998).
Piepoli, M. F. et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts), Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 37, 2315–2381 (2016).
Heiberg, E. et al. Design and validation of Segment—freely available software for cardiovascular image analysis. BMC Med. Imaging. 10, 1–13 (2010).
Cerqueira, M. D. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the american heart association. Circulation 105, 539–542 (2002).
Kotecha, T. et al. Automated pixel-wise quantitative myocardial perfusion mapping by CMR to detect obstructive coronary artery disease and coronary microvascular dysfunction: Validation against invasive coronary physiology. JACC Cardiovasc. Imaging. 12, 1958–1969 (2019).
Schwitter, K. et al. Magnetic resonance—based assessment of global coronary flow and flow reserve and its relation to left ventricular parameters: A comparison with positron emission tomography. Circulation 101, 2696–2702 (2000).
Engblom, H. et al. A new automatic algorithm for quantification of myocardial infarction imaged by late gadolinium enhancement cardiovascular magnetic resonance: experimental validation and comparison to expert delineations in multi-center, multi-vendor patient data. J. Cardiovasc. Magn. Reson. 18, 27 (2016).
Nordlund, D. et al. Measuring extracellular volume fraction by MRI : First verification of values given by clinical sequences. Magn. Reson. 1, 662–672 (2020).
Nickander, J. et al. Females have higher myocardial perfusion, blood volume and extracellular volume compared to males—an adenosine stress cardiovascular magnetic resonance study. Sci. Rep. 10, 103809 (2020).
Carlsson, M. et al. Submaximal adenosine-induced coronary hyperaemia with 12 h caffeine abstinence: Implications for clinical adenosine perfusion imaging tests. Clin. Physiol. Funct. Imaging. 35, 49–56 (2015).
Schwitter, J. et al. Oral administration of 17beta-estradiol over 3 months without progestin co-administration does not improve coronary flow reserve in post-menopausal women: A randomized placebo-controlled cross-over CMR study. J. Cardiovasc. Magn. Reson. 9, 665–672 (2007).
Kato, S. et al. Cardiovascular magnetic resonance assessment of coronary flow reserve improves risk stratification in heart failure with preserved ejection fraction. J. Cardiovasc. Magn. Reson. 18(23), 112 (2021).
Kato, S. et al. Prognostic value of resting coronary sinus flow determined by phase-contrast cine cardiovascular magnetic resonance in patients with known or suspected coronary artery disease. J. Cardiovasc. Magn. Reson. 19(23), 97 (2021).
Kato, S. et al. Coronary flow reserve by cardiac magnetic resonance imaging in patients with diabetes mellitus. JACC Cardiovasc. Imaging. 12, 2579–2580 (2019).
Panting, J. R. et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N. Engl. J. Med. 20(346), 1948–1953 (2002).
Jaarsma, C. et al. Non-invasive assessment of microvascular dysfunction in patients with microvascular angina. Int. J. Cardiol. 248, 433–439 (2017).
Larghat, A. et al. Endocardial and epicardial myocardial perfusion determined by semi-quantitative and quantitative myocardial perfusion magnetic resonance. Int. J. Cardiovasc. Imaging. 28, 1499–1511 (2012).
Al-Majdoub, M., Spégel, P. & Bennet, L. Metabolite profiling paradoxically reveals favorable levels of lipids, markers of oxidative stress and unsaturated fatty acids in a diabetes susceptible group of Middle Eastern immigrants. Acta Diabetol. 57, 597–603 (2020).
Stenkula, K. G. et al. Postprandial triglyceride levels rather than fat distribution may reflect early signs of disturbed fat metabolism in Iraqi immigrants. Lipids Health Dis. 4(21), 68 (2022).
Trinh, L. et al. Favorable fatty acid composition in adipose tissue in healthy Iraqi- compared to Swedish-born men—a pilot study using MRI assessment. Adipocyte. 11, 153–163 (2022).
Acknowledgements
The authors would like to thank technicians Ann-Helen Arvidsson, Christel Carlander and Johanna Koul for assistance with CMR-scans, Ahmed Salih and Josefin Gode Khan, for recruiting participants, Margit Bergström, Sophie Örjansdottir and Ulrika Biörklund Brogren for conducting the health investigations at the Unit of Diabetes Studies, Skåne University Hospital in Lund.
Funding
Open access funding provided by Lund University. Grants awarded L.B.: The Swedish Research Council, Strategic Research Area Exodiab, Dnr 2009-1039; the Swedish Foundation for Strategic Research Dnr IRC15-0067; the Swedish Research Council, Linnaeus grant, Dnr 349-2006-237; Grants awarded HA: The Swedish Heart and Lung Foundation, The Medical Faculty of Lund University and Region Skane, Sweden.
Author information
Authors and Affiliations
Contributions
R.J., L.B. and H.A. conceived the study and study design. R.J., H.E., A.H.A., H.X., P.K., M.C. and H.A. designed the CMR. protocol. R.J. and L.B. included patients. R.J., L.B. and H.E. analysed the data. R.J., H.E., A.H.A., H.X., P.K., M.C. and H.A. interpreted the patient data regarding qFPP and sinus coronary flow. All authors gave significant input into writing the manuscript. All authors read and approved the final manuscript. Consent for publication was granted from all study participants.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jablonowski, R., Bennet, L., Engblom, H. et al. Quantitative myocardial perfusion during stress using CMR is impaired in healthy Middle Eastern immigrants without CV risk factors. Sci Rep 12, 18237 (2022). https://doi.org/10.1038/s41598-022-23131-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23131-x
This article is cited by
-
Early left ventricular microvascular dysfunction in diabetic pigs: a longitudinal quantitative myocardial perfusion CMR study
Cardiovascular Diabetology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.