Abstract
To determine the effectiveness and safety of amino acids in preventing the mortality and morbidity among preterm infants. We conducted a systematic review and network meta-analysis. We searched MEDLINE, EMBASE, Web of Science, CINAHL, Scopus, Cochrane, and Google Scholar, and grey literature, from databases inception to January 2021. We included randomized trials that evaluated any amino acids on preterm or low-birth weight infants. We performed frequentist pairwise and network meta-analyses and used the GRADE methodology to assess the certainty of the evidence and provide a summary of the results.We included 18 trials (3702 infants). Low certainty evidence showed that there seems to be no benefit for arginine, glutamine, or N-acetylcysteine in reducing all-cause mortality. Oral arginine likely results in reduction of necrotizin enterocolitis (NEC) stage ≥ II (OR 0.48; 95% CI 0.26–0.90; moderate certainty). Oral glutamine may reduce the likelihood of developing late-onset sepsis (LOS) compared to placebo (OR 0.62; 95% CI 0.47–0.82; low certainty); and likely reduces time to reach full enteral feeding (MD = − 2.63 days; 95% CI − 4.99 to − 0.27; moderate certainty). Amino acids may have no effect on mortality. Oral arginine may reduce severe NEC, and oral glutamine may reduce LOS and the time to reach full feeding.
Systematic review registration: PROSPERO registration number: CRD4201603873.
Similar content being viewed by others
Introduction
Preterm birth is the leading cause of neonatal death globally, accounting for 15.9% of the mortality among neonates1,2. Necrotizing enterocolitis (NEC) is the most serious gastrointestinal complication in preterm infants3, and it is among the leading causes of mortality and morbidity in neonatal intensive care units (NICUs)4,5. NEC-related mortality has been reported to range from 16 to 42%, depending on gestational age and birth weight6. Currently, there is no effective treatment for NEC and the surgical management is associated with very high mortality7,8. Late-onset sepsis (LOS) is also a common complication in preterm infants representing a significant healthcare burden in the NICUs worldwide. Given its high incidence, significant morbidity (including long-term consequences on growth and development), and mortality, implementing efforts to reduce the infection rates is a priority in neonatal care9,10.
Amino acids are essential components of parenteral nutrition. Supplementing amino acids such as glutamine, arginine, and N-acetylcysteine may help modulating the pathophysiology of NEC and LOS by their anti-inflammatory, anti-apoptosis, and antioxidant effects11,12,13. Different amino acids supplementation through different administration routes have been studied in preterm infants for the prevention of NEC and LOS. Two Cochrane reviews by Shah et al.14 and Moe-Byrne et al.15 have summarized the evidence of the effectiveness and safety of the supplementation of arginine and glutamine, respectively, in preterm infants. Shah et al. found that arginine may reduce the incidence of NEC, while Moe-Byrne did not find enough evidence to support the supplementation of glutamine. In 2018, a review narratively summarized the evidence from 15 RCTs that evaluated the effectiveness of amino acids supplementation for preventing NEC16. Authors found that some studies showed benefit for amino acids supplementation in preventing NEC or LOS, while others might have no benefit.
Given the contradictory evidence on the effects of amino acids in conferring benefits to preterm infants and a lack of comparative analysis on the effects of different types of amino acids, we aimed to conduct a systematic review and network meta-analysis (NMA) of randomized trials (RCTs) to determine the comparative effectiveness and safety of amino acids supplementation for preventing the mortality and morbidity among preterm infants.
Methods
This systematic review was registered with PROSPERO (CRD4201603873) and a full protocol was published in an open-access journal17. We followed the recommendations provided by the PRISMA-NMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) extension in reporting our review18.
Data sources
Based on the search strategy from the protocol, we systematically searched MEDLINE, EMBASE, Science Citation Index Expanded and Social Sciences Citation Index, CINAHL, Scopus, Cochrane Central Register of Controlled Trials, and Google scholar from inception to January 10th, 2021, without any language restrictions. We performed grey literature searches through trial registries (Clinicaltrials.gov and WHO Clinical Trials Registry Platform). The lists of references of the eligible trials and relevant reviews were scanned for any additional eligible trial. Supplementary table 1 provides our search strategy.
Study selection
We included RCTs enrolling preterm (gestational age < 37 weeks) and/or low birth weight (birth weight < 2500 g) infants randomized to a preventive administration of any amino acid compared to an alternative intervention, placebo, or no treatment. We included studies of any amino acid (including but not limited to glutamine, arginine, and N-acetyl cysteine) with any dose, regimen, frequency, or route of administration. Our outcomes of interest were all-cause mortality, severe NEC (stage ≥ II—Bell's criteria), culture-proven LOS, NEC-related mortality, length of hospitalization (days), time to reach full enteral feeds (days), feed intolerance, weight at 37 weeks’ postnatal age or at discharge, and adverse events.
Pairs of reviewers (XW, DZ, RM, YC, FF, IF) independently and in duplicate screened the titles and abstracts to assess their eligibility. Potentially eligible studies were reviewed in full text. Reviewers (XW, DZ, RM, YC, FF, IF) independently and in duplicate reviewed the eligible full texts. Reviewers resolved any discrepancies through discussion, or consultation with a third reviewer (BS) when needed. We tried to contact authors of included studies during data extraction and risk of bias assessment for missing information.
Data extraction and risk of bias assessment
Pairs of reviewers (XW, DZ, RM, YC, FF, IF) independently and in duplicate extracted the data and reached consensus through discussion or consultation with a third reviewer (BS) in a piloted format. We collected the basic characteristics of included studies (study design, year, duration of follow-up, sample size), population (gestational age, birth weight, enteral nutrition, and relevant perinatal history), interventions and comparisons (doses, frequency, and regimens), and outcomes (number of events, mean and standard deviation or standard errors). We applied methods proposed in Cochrane Handbook and Hozo et al. to impute mean and standard deviation when median, range, and sample size were reported19. For studies published in duplicate or studies that used data from a similar study population in different publications in part or full, we extracted data from the publication with the most complete dataset.
Pairs of reviewers (XW, DZ, RM, YC, FF, IF) assessed the risk of bias independently and in duplicate, and resolved any discrepancies through discussion, or adjudication by a third reviewer (BS). We used a modified Cochrane risk of bias instrument that addresses the following potential sources of bias: random sequence generation, allocation concealment, blinding study participants (infants’ parents in our study), personnel and outcome assessors, and incomplete outcome data. Responses including “definitely yes” or “probably yes” (considered as low risk of bias), or “definitely no” or “probably no” (considered as high risk of bias) were used rather than the standard response options (high, low, or unclear risk of bias)20,21.
Data synthesis and statistical methods
We performed frequentist pairwise random-effects meta-analyses for all direct comparisons. For dichotomous outcomes, we calculated odds ratio (OR), and for continuous outcomes the weighted mean diffearence (WMD), and their corresponding 95% confidence intervals (95% CIs). We used the Q-statistic and I2 statistic to measure the statistical heterogeneity in each direct comparison22. The I2 measures the percentage of variation across studies due to heterogeneity rather than chance23. We used the I2 value of each direct comparison to evaluate the presence of inconsistency when assessing the certainty of the evidence as described below in the ‘Rating the certainty of the evidence’ subheading. For this purpose, we used a threshold of 50% to define significant inconsistency and rate down the evidence.
A geometry plot was used to present all the available direct comparisons per outcome, in which each node represents one intervention. For those amino acids with different administration forms (e.g., oral, intravenous-i.v.) we separated the evidence in different nodes.
We performed the frequentist random-effects NMA to synthesize the available evidence using the methodology of multivariate meta-analysis assuming a common heterogeneity parameter24,25. We evaluated transitivity by inspection and analysis of the clinical similarities between direct comparisons that informed indirect evidence. For this purpose, we considered the following variables: birth weight, gestational age, mean APGAR scores, and percentage of infants born by C-section. We evaluated the presence of incoherence (also called network inconsistency) by comparing direct evidence with indirect evidence using the node splitting approach22,26. We also assessed the evidence of incoherence in the entire network using the design-by-treatment model27. We calculated ranking probabilities, mean ranks, and rankograms as well as SUCRA values (the Surface Under the Cumulative Ranking curve), for all outcomes.
We performed meta-regression using the Knapp-Hartung modification of the variance regardless of observed heterogeneity to assess the effect modification of gestational age, birth weight and percent infants delivered by C-section28. In sparse networks, heterogeneity estimation across the network using contrast-based random-effects model can have strange results which results in spuriously wide confidence interval (i.e., 95% CI of the network estimates were wider than those of the direct estimates or the indirect estimates)29. In such cases, we performed fixed-effect model NMA and reported the results of random-effects model as sensitivity analysis in the supplementary files. We planned to assess small-study effect using funnel plots when 10 studies or more were available for the direct comparisons.
Rating the certainty of the evidence
We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to assess the certainty of the evidence for effect estimates from direct, indirect, and NMA evidence for each outcome29,30,31,32,33,34,35,36. Initially, we rated the certainty in direct estimates according to the traditional GRADE guidance by considering risk of bias, inconsistency, indirectness, imprecision, and publication bias. Then, we rated the certainty in indirect estimates, starting with the lowest ratings of the direct comparisons contributing to the dominant first-order loop with further rating down, when necessary, for intransitivity. NMA estimate certainty started as the higher of the direct and indirect evidence; however, the relative contribution of direct and indirect evidence to the network estimate was considered when rating the certainty. If incoherence was detected in a specific loop or comparison, we further rated down the certainty of the network estimates.
GRADE approach to summarize results from NMA
To optimize the interpretation of the findings, we applied the GRADE approach for drawing conclusions from NMAs using a minimally contextualized framework37. This approach allows us to categorize the interventions—from the most effective to the least effective—based on the effect estimates obtained from the NMA and their associated GRADE certainty of evidence. For each outcome, we created groups of interventions as follows: Category 0, the reference intervention (placebo) and interventions with no evidence of difference compared to placebo (i.e. 95%CIs include the null value) which we refer to as “among the least effective”; Category 1, interventions superior to placebo, but not superior to any other of the intervention(s) superior to placebo, inferior to the most effective, but superior to the least effective”; category 3, interventions that proved superior to at least one category 2 intervention, termed “among the most effective”. We then divided all categories into two groups: those with moderate- or high- certainty evidence relative to placebo, and those with low- or very low- certainty evidence relative to placebo37.
Results
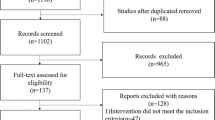
We identified 8634 titles and abstract through our searches, of which 476 full-texts articles were screened for eligibility. We included 18 randomized trials involving 3702 infants38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55. Figure 1 provides the details of the study selection process. Across the included trials, the median of average weight and gestational age were 1089.4 g (interquartile range (IQR) 912.5, 1256.1) and 29.0 weeks (IQR 27.4, 29.6), respectively. Of the 18 included studies, seven studies compared oral glutamine with placebo, six trials compared intravenous (i.v.) glutamine with placebo, three compared oral arginine with placebo, one compared i.v. N-acetylcysteine with placebo, and one three-arm trial, comparing oral arginine, oral glutamine and placebo. Table 1 summarizes study characteristics and Fig. 2 presents network of treatments across outcomes.
Among the included trials, 13 studies39,40,41,44,45,47,48,49,50,51,52,53,55 were judged to be at low risk of bias for allocation concealment. Most studies properly blinded patients and care providers; however, 13 studies had issues in blinding outcome assessors38,39,40,41,42,43,46,47,49,52,53,54,55. Eight trials had issues with incomplete outcome reporting (more than 5% missing participant data)43,46,49,50,51,52,54,55. Table 2 summarizes the risk of bias assessments.
All-cause mortality
The 14 studies reporting mortality enrolled 3407 infants and informed five direct comparisons for 3 of which there were 2 or more studies available for conventional pairwise meta-analysis (Supplementary table 2). We did not observe any evidence of global or loop-specific incoherence in this network (Supplementary table 15). The results of NMA did not show any statistically significant benefit for any of the amino acids compared to placebo (low to moderate certainty—Figs. 3 and 4).
Network meta-analysis results for the primary outcomes. All-cause mortality, severe NEC (stage 2 or more)—top table, and late-onset sepsis—lower table. Results are odds ratio and their corresponding 95% CI. The table should be read from left to right. For all-cause mortality (bottom part of the table), an OR > 1.0 means an increase in mortality. For NEC stage ≥ II (upper part of the table), an OR < 1.0 means a reduction in developing NEC. For culture-proven late-onset sepsis (inferior table), an OR < 1.0 means a reduction in sepsis. Significant results are presented in bold and underlined. The colours represent the GRADE certainty of evidence: green: high; light green: moderate; light orange: low; and orange: very low. *For NEC and sepsis, results are from the fixed-effect model. i.v. intravenous, NAC N-acetyl cysteine, NEC necrotizing enterocolitis.
Network meta-analysis summary results sorted based on GRADE certainty of evidence and treatment effects for the comparisons of active treatments versus placebo for all outcomes. NMA results are sorted based on the GRADE certainty of the evidence for comparing active treatments versus placebo for all outcomes. (see “Summary and Certainty of the Results” subsection in the Methods section for more details about the categories). In the second column, green cells denote high certainty of the evidence, and orange cells denote low certainty of the evidence. In columns three to six, dark green denotes that with high certainty the intervention is among the most effective; light green denotes that with high certainty the intervention is among the least effective; dark orange denotes that with low certainty the intervention is among the most effective; and light orange denotes that with low certainty the intervention is among the least effective. We present the relative and absolute effect estimates for all-cause mortality, NEC ≥ stage II, culture-proven late-onset sepsis, NEC-related mortality, and feed intolerance. For continuous outcomes (Time to reach full enteral feed in days and duration of the hospital stay), results are the mean difference (absolute effect). In bold, interventions that showed statistical significance in comparison to placebo. 95% CI 95% confidence interval, GRADE Grading of Recommendations, Assessment, Development, and Evaluation, i.v. intravenous, NEC necrotizing enterocolitis. *For NEC and sepsis, results are from fixed-effect model.
Severe NEC
The 18 studies reporting severe NEC (Bell’s stage II or more) enrolled 3702 infants and informed five direct comparisons for 3 of which there were two or more studies available for conventional pairwise meta-analysis (Supplementary table 3). We did not observe any evidence of global or loop-specific incoherence in this network (Supplementary table 16).
The results of random-effect model NMA was associated with spuriously wide confidence interval (i.e., 95% CI of the NMA estimates were wider than those from the direct or the indirect estimates), and therefore we decided to report results of the fixed-effect model for this outcome. Moderate certainty evidence suggested that oral arginine may reduce the likelihood of developing severe NEC in preterm infants compared to placebo (OR 0.48, 95% CI 0.26–0.90; moderate certainty—absolute risk reduction [ARR]: − 5.21% [95% CI − 7.93% to − 0.71%]). The NMA results showed no other amino acids has statistically significant benefit compared to placebo (Figs. 3 and 4). The network estimates from random-effects model are provided in Supplementary table 8.
Culture proven late-onset sepsis
The 15 studies reporting culture proven LOS enrolled 3217 infants and informed four direct comparisons from the pairwise meta-analyses (Supplementary table 4). We did not observe any evidence of incoherence in this network.
The results of random-effect model NMA was associated with spuriously wide confidence interval, and therefore we decided to report results of the fixed-effect model for this outcome. Oral glutamine demonstrated statistically significant reduction in likelihood of LOS compared to placebo (OR 0.62; 95% CI 0.47, 0.82; low certainty—ARR = − 8.32%, 95% CI − 11.84% to − 3.84%) (Figs. 3 and 4). The network estimates from random-effects model are provided in Supplementary table 9.
Other patient-important outcomes
Six studies (1219 infants) reported the NEC-related mortality. Of the four available direct comparisons, two had two or more studies. None of the amino acids showed statistically significant benefit compared to placebo (Fig. 4 and Supplementary table 10).
Time to reach full enteral feeding was reported in seven studies (1,858 infants) informing two direct comparisons, both comparisons had two or more studies. Oral glutamine reduced mean number of days to reach full enteral feeding by 2.63 days compared with placebo (95% CI − 4.99 to − 0.27; moderate certainty) (Supplementary table 11).
The 11 studies reporting duration of hospital stay enrolled 2479 infants and informed 3 direct comparisons of which 2 had 2 or more studies. None of the amino acids showed statistically significant benefit compared to placebo (Fig. 4 and Supplementary table 12).
Networks of length of hospitalization (days) and time to reach full enteral feeding were sparse and there was no global or loop-specific incoherence. We did not observe any evidence of global or loop-specific incoherence in the network of NEC-related mortality (Supplementary tables 17).
Only three studies (2184 infants) reported feeding intolerance. We did not perform NMA for this outcome. The results of conventional meta-analysis showed a reduction in the likelihood of feed intolerance for oral glutamine compared with placebo (OR 0.34; 95% CI 0.19, 0.61; I2 = 0%; moderate certainty—ARR = − 10.71%; 95% CI − 13.54% to − 6.01%) (Supplementary table 13).
The weight at 37 weeks’ postnatal age or at discharge was reported in three studies (770 infants) with two trials comparing oral glutamine to placebo and one trial comparing oral arginine to placebo. Conventional meta-analysis showed no statistically significant benefit for oral glutamine compared to placebo (Supplementary table 14).
Few trials reported on adverse events. Polycarpou et al.48 declared that no adverse effects were observed in neonates receiving oral l-arginine, and Amin et al.39 and El-Shimi et al.41 reported no increase in incidence of hypotension or hyperglycemia among infants supplemented with oral arginine. Ahola et al.38 reported no adverse effects related to i.v. N-acetylcysteine.
Additional analyses
Results of the meta-regression analyses adjusted by mean birth weight, mean gestational age and the proportion of infants delivered by C-section are presented in Supplementary tables 5, 6 and 7. We found a significant effect modification for the comparison of oral glutamine versus placebo for severe NEC and LOS when adjusting for birth weight and gestational age showing more benefit for infants with higher birth weight and older gestational age (Supplementary tables 6 and 7). Supplementary table 18 provides SUCRA values and mean ranks for different amino acids, and Supplemental figures 1 to 6 provide ranking probabilities. For all-cause mortality and severe NEC, SUCRA suggested that oral arginine may be the best intervention, but the mean rank was not remarkably different from oral glutamine and i.v. glutamine. For LOS, oral glutamine showed the highest SUCRA, and the mean rank concurs with this finding (mean rank = 1.1).
Discussion
Main findings
In this systematic review and NMA, we summarized evidence from 18 studies (3702 infants) and found that three different amino acids have been studied to prevent mortality and morbidity in preterm infants. Low to moderate certainty evidence indicated no meaningful impact on all-cause mortality of amino acids relative to placebo (Fig. 2). Low certainty evidence indicated that oral arginine may prevent NEC stage II and III compared with placebo and low certainty evidence suggested that oral glutamine prevented culture-proven sepsis compared with placebo. No evidence supports the preventive effects of other comparisons on NEC ≥ II and culture-proven sepsis. Moderate certainty evidence shows that the number of days to reach full enteral feed was reduced by 2.63 days using oral glutamine relative to placebo; however, it may not include a clinically meaningful reduction. Low certainty evidence of no impact was found for oral arginine or oral glutamine for weight at 37 weeks postnatal age at discharge versus placebo.
We did not find any differences between arginine, glutamine (oral or i.v.) and NAC in all-cause mortality and severe NEC. Nonetheless, with low certainty, we found that oral glutamine may be superior not only to placebo but also to the other amino acids in reducing the incidence of LOS.
Strengths and limitations
Our review has several strengths. This is the most comprehensive systematic review on this topic to date, using a broad search to identify and include all available literature without language restrictions. We applied state-of-the-art methodologies for developing NMA, according to a prespecified protocol and statistical-analysis plan, and we followed the PRISMA-NMA guidance18. Moreover, we used the GRADE approach36,37 to assess the certainty of the NMA effect estimates and to draw conclusions applying the most recent methodological approach, which considers the effect and the certainty of the evidence.
Our review is not free of limitations. A low number of studies relative to the number of comparisons considered, and the scarce direct evidence comparing the amino acids among them resulted in mostly indirect comparisons and low confidence in estimates for many key analyses. Only one trial directly compared two active treatments (oral arginine versus oral glutamine), while the rest of the studies compared one active treatment to placebo (Table 1). Due to this scarcity of direct evidence among active interventions, we were unable to further explore the effect of variability in supplementation details, including dosages administered and durations of the intervention.
Comparison with other studies
Two Cochrane reviews have addressed the effectiveness of amino acids for preterm infants. Moe-Byrne et al.15 included 12 trials and concluded that glutamine did not have an effect on mortality or the incidence of invasive infection or NEC compared to control. Our main findings differ from theirs for two reasons. First, although they performed subgroup analyses by administration route, they drew their conclusions from the syntheses of both oral and i.v. glutamine. Instead, we considered oral and i.v. glutamine as different interventions, and thus, we created one node for each and could find that oral administration may be useful for preventing LOS (low certainty). Lastly, NMA provides effect estimates of interventions not only compared to placebo or no treatment but also among all the interventions. Therefore, we could also describe how oral glutamine was significantly superior to i.v. glutamine.
The reason for this difference according to the administration route may be related to intestinal permeability. As the most abundant free amino acid in the human body, glutamine helps to maintain gut barrier integrity by promoting enterocyte proliferation, regulating tight junction proteins, suppressing pro-inflammatory signaling pathways, and protecting cells against apoptosis and cellular stresses. Glutamine supplementation could balance its depletion during sepsis and improve clinical outcomes56,57. In fact, Sevastiadou et al.49 found a significant reduction of intestinal permeability with oral glutamine. This reduction might be associated with less bacterial translocation and therefore, less intestinal-related sepsis. Although we cannot be sure about the effect of oral glutamine, our results warrant further trials comparing it with i.v. glutamine and other interventions.
In 2017, the review by Shah et al.14 included three trials and reported a positive effect of arginine supplementation in reducing the risk of development of NEC stage I, but not for NEC stages II or III. In our NMA, we found a potential reduction of NEC stage ≥ II when supplementing with oral arginine. However, these findings should be interpreted with caution because of the small number of included studies and the potential of biases in the included trials, which explain the low certainty of the evidence. Similar to glutamine, arginine may have a potential beneficial effect on the immature intestine, which could explain its effect on reducing sepsis occurrence58.
Although some of our findings agree with previous reviews, we present some novel results. First, our review is the first to compare all the available amino acids simultaneously, and thus, we can provide effect estimates of comparisons among them. For instance, our league tables (Fig. 2) show how oral glutamine is not only more effective than placebo but also more effective than i.v. glutamine. Second, we applied GRADE methodology to assess the certainty of the evidence so readers can identify what comparisons benefit from further research more than others. Third, we provide updated evidence from studies that had not been synthesized before and included three new studies in addition to the total 15 studies from the previous two Cochrane reviews, and as such, this work will be useful to inform decision-making and for updating guidelines.
In summary, oral glutamine and arginine seem to reduce culture-proven late-onset sepsis and NEC ≥ II, respectively. These findings need to be confirmed in future large trials. We encourage trialists to design large high-quality RCTs directly comparing oral arginine, oral glutamine, and placebo to confirm these effects.
Conclusion
The evidence from RCTs suggests that amino acid supplementation, including glutamine, arginine and N-acetylcysteine, does not have an important effect on mortality in preterm infants. However, in comparison to the placebo, oral glutamine has shown to be superior for reducing the days to reach full enteral feeding (moderate certainty), it may be superior for preventing the culture-proven late-onset sepsis (low certainty), and oral arginine may be effective for reducing NEC stage ≥ II (moderate certainty). Lastly, i.v. glutamine and NAC seem to be similar to placebo for all the measured outcomes. These findings should be interpreted with caution because of the small number of studies included and the potential risk of biases.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
World Health Organization. Preterm Birth. https://www.who.int/news-room/fact-sheets/detail/preterm-birth. Accessed on April 2, 2020.
Liu, L. et al. Global, regional, and national causes of under-5 mortality in 2000–15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet (London, England) 388(10063), 3027–3035. https://doi.org/10.1016/s0140-6736(16)31593-8 (2016).
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364(3), 255–264. https://doi.org/10.1056/NEJMra1005408 (2011).
Battersby, C. et al. Incidence of neonatal necrotising enterocolitis in high-income countries: A systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 103(2), F182–F189. https://doi.org/10.1136/archdischild-2017-313880 (2018).
Eaton, S., Rees, C. M. & Hall, N. J. Current research on the epidemiology, pathogenesis, and management of necrotizing enterocolitis. Neonatology 111(4), 423–430. https://doi.org/10.1159/000458462 (2017).
Fitzgibbons, S. C. et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J. Pediatr. Surg. 44(6), 1072–1075. https://doi.org/10.1016/j.jpedsurg.2009.02.013 (2009) (discussion 75–6).
Hong, C. R., Han, S. M. & Jaksic, T. Surgical considerations for neonates with necrotizing enterocolitis. Semin. Fetal Neonatal. Med. 23(6), 420–425. https://doi.org/10.1016/j.siny.2018.08.007 (2018).
Robinson, J. R. et al. Surgical necrotizing enterocolitis. Semin. Perinatol. 41(1), 70–79. https://doi.org/10.1053/j.semperi.2016.09.020 (2017).
Shah, B. A. & Padbury, J. F. Neonatal sepsis: An old problem with new insights. Virulence 5(1), 170–178. https://doi.org/10.4161/viru.26906 (2014).
Zea-Vera, A. & Ochoa, T. J. Challenges in the diagnosis and management of neonatal sepsis. J. Trop. Pediatr. 61(1), 1–13. https://doi.org/10.1093/tropej/fmu079 (2015).
Faintuch, J., Aguilar, P. B. & Nadalin, W. Relevance of N-acetylcysteine in clinical practice: Fact, myth or consequence?. Nutrition (Burbank, Los Angeles County, Calif) 15(2), 177–179. https://doi.org/10.1016/s0899-9007(98)00060-4 (1999).
Marc Rhoads, J. & Wu, G. Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 37(1), 111–122. https://doi.org/10.1007/s00726-008-0225-4 (2009).
van der Hulst, R. R. et al. Glutamine and the preservation of gut integrity. Lancet (London, England) 341(8857), 1363–1365. https://doi.org/10.1016/0140-6736(93)90939-e (1993).
Shah, P. S., Shah, V. S. & Kelly, L. E. Arginine supplementation for prevention of necrotising enterocolitis in preterm infants. Cochrane Database Syst. Rev. 4, CD004339. https://doi.org/10.1002/14651858.CD004339.pub4 (2017).
Moe-Byrne, T., Brown, J. V. & McGuire, W. Glutamine supplementation to prevent morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 4, CD001457. https://doi.org/10.1002/14651858.CD001457.pub6 (2016).
Garg, B. D. & Kabra, N. S. Role of amino acid supplementation in the prevention of necrotizing enterocolitis in preterm neonates—A review of current evidences. J. Matern. Fetal Neonatal Med. 31(17), 2349–2366. https://doi.org/10.1080/14767058.2017.1342797 (2018).
Sadeghirad, B. et al. Comparative effectiveness of prophylactic therapies for necrotizing enterocolitis in preterm infants: Protocol for a network meta-analysis of randomized trials. Int. J. Prev. Med. 9, 83. https://doi.org/10.4103/ijpvm.IJPVM_328_17 (2018).
Hutton, B. et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 162(11), 777–784. https://doi.org/10.7326/m14-2385 (2015).
Hozo, S. P., Djulbegovic, B. & Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 5, 13. https://doi.org/10.1186/1471-2288-5-13 (2005).
Akl, E. A. et al. Specific instructions for estimating unclearly reported blinding status in randomized trials were reliable and valid. J. Clin. Epidemiol. 65(3), 262–267. https://doi.org/10.1016/j.jclinepi.2011.04.015 (2012).
Higgins, J. P. & Green, S. Cochrane Handbook for Systematic Reviews of Interventions, Version [5.1.0]. The Cochrane Collaboration; 2011. https://handbook-5-1.cochrane.org/ (accessed on April 1, 2020).
Higgins, J. P. et al. Measuring inconsistency in meta-analyses. BMJ (Clin. Res. Ed.) 327(7414), 557–560. https://doi.org/10.1136/bmj.327.7414.557 (2003).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21(11), 1539–1558. https://doi.org/10.1002/sim.1186 (2002).
Ir, W. Network meta-analysis. Stand. Genomic Sci. 15(4), 951–985 (2015).
White, I. R. et al. Consistency and inconsistency in network meta-analysis: Model estimation using multivariate meta-regression. Res. Synth. methods 3(2), 111–125. https://doi.org/10.1002/jrsm.1045 (2012).
Lu, G. & Ades, A. E. Assessing evidence inconsistency in mixed treatment comparisons. J. Am. Stat. Assoc. 101(474), 447–459. https://doi.org/10.1198/016214505000001302 (2006).
Higgins, J. P. et al. Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Res. Synth. Methods 3(2), 98–110. https://doi.org/10.1002/jrsm.1044 (2012).
Harbord, R. M. & Higgins, J. P. T. Meta-regression in Stata. Stata J 8(4), 493–519 (2008).
Brignardello-Petersen, R. et al. GRADE approach to rate the certainty from a network meta-analysis: Avoiding spurious judgments of imprecision in sparse networks. J. Clin. Epidemiol. 105, 60–67. https://doi.org/10.1016/j.jclinepi.2018.08.022 (2019).
Guyatt, G. H. et al. GRADE guidelines 6. Rating the quality of evidence—Imprecision. J. Clin. Epidemiol. 64(12), 1283–1293. https://doi.org/10.1016/j.jclinepi.2011.01.012 (2011).
Guyatt, G. H. et al. GRADE guidelines: 8. Rating the quality of evidence—Indirectness. J. Clin. Epidemiol. 64(12), 1303–1310. https://doi.org/10.1016/j.jclinepi.2011.04.014 (2011).
Guyatt, G. H. et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J. Clin. Epidemiol. 64(12), 1294–1302. https://doi.org/10.1016/j.jclinepi.2011.03.017 (2011).
Guyatt, G. H. et al. GRADE guidelines: 5. Rating the quality of evidence–Publication bias. J. Clin. Epidemiol. 64(12), 1277–1282. https://doi.org/10.1016/j.jclinepi.2011.01.011 (2011).
Guyatt, G. H. et al. GRADE guidelines: 4. Rating the quality of evidence—Study limitations (risk of bias). J. Clin. Epidemiol. 64(4), 407–415. https://doi.org/10.1016/j.jclinepi.2010.07.017 (2011).
Guyatt, G. H. et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clin. Res. Ed.) 336(7650), 924–926. https://doi.org/10.1136/bmj.39489.470347.AD (2008).
Puhan, M. A. et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ (Clin. Res. Ed.) 349, g5630. https://doi.org/10.1136/bmj.g5630 (2014).
Brignardello-Petersen, R. et al. GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. BMJ (Clin. Res. Ed.) 371, m3900. https://doi.org/10.1136/bmj.m3900 (2020).
Ahola, T. et al. N-acetylcysteine does not prevent bronchopulmonary dysplasia in immature infants: A randomized controlled trial. J. Pediatr. 143(6), 713–719. https://doi.org/10.1067/s0022-3476(03)00419-0 (2003).
Amin, H. J. et al. Arginine supplementation prevents necrotizing enterocolitis in the premature infant. J. Pediatr. 140(4), 425–431. https://doi.org/10.1067/mpd.2002.123289 (2002).
Bober-Olesinska, K. & Kornacka, M. K. Effects of glutamine supplemented parenteral nutrition on the incidence of necrotizing enterocolitis, nosocomial sepsis and length of hospital stay in very low birth weight infants. Med. Wieku Rozwoj. 9(3 Pt 1), 325–333 (2005).
El-Shimi, M. S. et al. Enteral l-arginine and glutamine supplementation for prevention of NEC in preterm neonates. Int. J. Pediatr. 2015, 856091. https://doi.org/10.1155/2015/856091 (2015).
Korkmaz, A. et al. Long-term enteral glutamine supplementation in very low birth weight infants: Effects on growth parameters. Turk. J. Pediatr. 49(1), 37–44 (2007).
Lacey, J. M. et al. The effects of glutamine-supplemented parenteral nutrition in premature infants. JPEN J. Parenter. Enteral Nutr. 20(1), 74–80. https://doi.org/10.1177/014860719602000174 (1996).
Mohamad Ikram, I. et al. A randomised controlled trial of glutamine-enriched neonatal parenteral nutrition in Malaysia. Singapore Med. J. 52(5), 356–360 (2011).
Neu, J. et al. Enteral glutamine supplementation for very low birth weight infants decreases morbidity. J. Pediatr. 131(5), 691–699. https://doi.org/10.1016/s0022-3476(97)70095-7 (1997).
Pawlik, D. et al. The effects of enteral administration of glutamine enriched solution in very low birth weight infants on reducing the symptoms of feeding intolerance. A prospective, randomized pilot study. Med. Wieku Rozwoj. 16(3), 205–211 (2012).
Poindexter, B. B. et al. Parenteral glutamine supplementation does not reduce the risk of mortality or late-onset sepsis in extremely low birth weight infants. Pediatrics 113(5), 1209–1215. https://doi.org/10.1542/peds.113.5.1209 (2004).
Polycarpou, E. et al. Enteral L-arginine supplementation for prevention of necrotizing enterocolitis in very low birth weight neonates: A double-blind randomized pilot study of efficacy and safety. JPEN J. Parenter. Enteral Nutr. 37(5), 617–622. https://doi.org/10.1177/0148607112471561 (2013).
Sevastiadou, S. et al. The impact of oral glutamine supplementation on the intestinal permeability and incidence of necrotizing enterocolitis/septicemia in premature neonates. J. Matern. Fetal Neonatal Med. 24(10), 1294–1300. https://doi.org/10.3109/14767058.2011.564240 (2011).
Thompson, S. W., McClure, B. G. & Tubman, T. R. A randomized, controlled trial of parenteral glutamine in ill, very low birth-weight neonates. J. Pediatr. Gastroenterol. Nutr. 37(5), 550–553. https://doi.org/10.1097/00005176-200311000-00008 (2003).
van den Berg, A. et al. Glutamine-enriched enteral nutrition in very-low-birth-weight infants and effects on feeding tolerance and infectious morbidity: A randomized controlled trial. Am. J. Clin. Nutr. 81(6), 1397–1404. https://doi.org/10.1093/ajcn/81.6.1397 (2005).
Vaughn, P. et al. Enteral glutamine supplementation and morbidity in low birth weight infants. J. Pediatr. 142(6), 662–668. https://doi.org/10.1067/mpd.2003.208 (2003).
Wang, Y. et al. Protective effect of parenteral glutamine supplementation on hepatic function in very low birth weight infants. Clin. Nutr. (Edinburgh, Scotland) 29(3), 307–311. https://doi.org/10.1016/j.clnu.2010.03.009 (2010).
Maamouri, G. et al. The impact of oral glutamine supplementation on prevention of nosocomial infections in preterm infants. Iran. J. Neonatol. IJN 7(1), 19–24 (2016).
Memişoğlu, A. Ç. et al. Long-term enteral arginine supplementation in very low birth weight infants: Effects on growth parameters, morbidity and mortality. Çocuk Sağlığı ve Hastalıkları Dergisi 52(1), 9–19 (2009).
Nolan, L. S., Parks, O. B. & Good, M. A review of the immunomodulating components of maternal breast milk and protection against necrotizing enterocolitis. Nutrients https://doi.org/10.3390/nu12010014 (2019).
Kim, M. H. & Kim, H. The roles of glutamine in the intestine and its implication in intestinal diseases. Int. J. Mol. Sci. https://doi.org/10.3390/ijms18051051 (2017).
Di Lorenzo, M., Bass, J. & Krantis, A. Use of l-arginine in the treatment of experimental necrotizing enterocolitis. J. Pediatr. Surg. 30(2), 235–240. https://doi.org/10.1016/0022-3468(95)90567-7 (1995) (discussion 40–1).
Funding
BS reports funding from Mitacs Canada Accelerate internship in partnership with Nestlé Canada to support his graduate student stipend from 2016 to 2018. Mitacs is a national, not-for-profit organization that has designed and delivered research and training programs in Canada, working with universities, companies, and federal and provincial governments. All other authors have no financial relationships relevant to this article to disclose.
Author information
Authors and Affiliations
Contributions
Conceptualized and designed the study: X.W., B.S., I.D.F.; Designed and ran the search strategy: R.C.; Selected the articles, extracted the data and assessed the quality of the evidence: X.W., R.M., Y.C., D.Z., R.C., H.C., F.F., I.D.F.; Analysed and interpreted the data: X.W., B.S., I.D.F.; Manuscript Drafting: X.W., B.S., I.D.F.; Critically reviewed the manuscript for important intellectual content: X.W., B.S., R.M., Y.C., D.Z., R.C., H.C., F.F., I.D.F.; All authors read and met the ICMJE criteria for authorship and agree with the results and conclusions of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Sadeghirad, B., Morgan, R.L. et al. Amino acids for the prevention of mortality and morbidity in preterm infants: a systematic review and network meta-analysis. Sci Rep 12, 18333 (2022). https://doi.org/10.1038/s41598-022-21318-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21318-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.