Abstract
Antimicrobial peptides (AMPs) play crucial roles in the innate immunity of diverse organisms, which exhibit remarkable diversity in size, structural property and antimicrobial spectrum. Here, we describe a new AMP, named Pentatomicin, from the stinkbug Plautia stali (Hemiptera: Pentatomidae). Orthologous nucleotide sequences of Pentatomicin were present in stinkbugs and beetles but not in other insect groups. Notably, orthologous sequences were also detected from a horseshoe crab, cyanobacteria and proteobacteria, suggesting the possibility of inter-domain horizontal gene transfers of Pentatomicin and allied protein genes. The recombinant protein of Pentatomicin was effective against an array of Gram-positive bacteria but not against Gram-negative bacteria. Upon septic shock, the expression of Pentatomicin drastically increased in a manner similar to other AMPs. On the other hand, unlike other AMPs, mock and saline injections increased the expression of Pentatomicin. RNAi-mediated downregulation of Imd pathway genes (Imd and Relish) and Toll pathway genes (MyD88 and Dorsal) revealed that the expression of Pentatomicin is under the control of Toll pathway. Being consistent with in vitro effectiveness of the recombinant protein, adult insects injected with dsRNA of Pentatomicin exhibited higher vulnerability to Gram-positive Staphylococcus aureus than to Gram-negative Escherichia coli. We discovered high levels of Pentatomicin expression in eggs, which is atypical of other AMPs and suggestive of its biological functioning in eggs. Contrary to the expectation, however, RNAi-mediated downregulation of Pentatomicin did not affect normal embryonic development of P. stali. Moreover, the downregulation of Pentatomicin in eggs did not affect vertical symbiont transmission to the offspring even under heavily contaminated conditions, which refuted our expectation that the antimicrobial activity of Pentatomicin may contribute to egg surface-mediated symbiont transmission by suppressing microbial contaminants.
Similar content being viewed by others
Introduction
Antimicrobial peptides (AMPs) are ubiquitous in nature, being found in many life forms spanning from microorganisms to humans1,2,3. As of January 2022, 3324 AMPs of various origins have been reported in the AMP database4 (https://aps.unmc.edu/AP/). Most AMPs share cationic and amphipathic properties, but differ extremely in length, structural property, and antimicrobial activity spectrum5,6.
In insects, AMPs play a pivotal role in humoral immunity by clearing microbial infections, being among the important components of innate immunity in addition to physical and cellular immunities7,8. Humoral immunity comprises three stepwise processes, recognition of nonself, signal transduction, and production of effector molecules9,10,11. The upstream pathways of the humoral immune cascade, constituted by microbial recognition and signaling molecules, tend to be highly conserved, which have greatly contributed to our general understanding of the innate immune mechanisms12. On the other hand, the downstream effector molecules, including AMPs, are so diverse and taxon-specific that there is much room for exploring novel immune effector molecules and their functions13. To fully understand the innate immunity of a certain organism, therefore, it is necessary to investigate the spectrum and effectiveness of each AMP from the effector repertoire of the organism14,15. Considering the continuous emergence and spread of pathogenic bacteria resistant to conventional antibiotics16, detailed information on novel AMPs may lead to the development of new type of antibiotics17,18. Additionally, detailed information on insect immunity may be of use for protecting economically important insects from microbial pathogens.
Horseshoe crabs are relatively large-sized marine arthropods of the family Limulidae19. The hemolymph of horseshoe crabs is rich in soluble defense molecules and numerous hemocytes. The coagulation factors constitute a cascade of serine proteases and their inhibitors, sensitively react to bacterial cell wall lipopolysaccharides (LPS) and promptly trigger body fluid clotting, and are commercialized for medical detection of bacterial endotoxins as Limulus amebocyte lysate (LAL) reagent20. In addition, the hemolymph contains a variety of AMPs, lectins and other presumably defense-related proteins, of which some are well characterized and others are not21. Limulus endotoxin-binding protein-protease inhibitor (LEBP-PI) represents the latter type, 12 kDa in size, accounting for ~ 1% of hemocyte proteins, purified by LPS affinity column, capable of binding to LPS and bacterial cells, and inhibitory to proteolytic activity of trypsin22. However, biological function of LEBP-PI has been elusive: its binding to LPS neither inhibits nor enhances the activation of the clotting system by LPS; its anti-trypsin activity is not affected by the presence of LPS; and it does not exhibit conspicuous antimicrobial activities22. Since the first publication in 199122, no study on LEBP-PI has been reported.
Here we report the discovery of an immune-responsive LEBP-PI-related protein in an insect, the brown-winged green stinkbug Plautia stali (Hemiptera: Pentatomidae). P. stali is known as a notorious agricultural pest infesting various fruits and crop plants23, and has attracted much attention in microbiology and evolutionary biology of the gut bacterial symbiosis24,25,26,27,28,29. In this context, P. stali has been established as an experimental model system for investigating host–microbe interactions: continuous laboratory maintenance and aseptic rearing are practicaly feasible28, efficient gene knockdown by RNAi is applicable30,31, and basic information on the innate immune cascade is available32. Thus far, Defensins, Hemiptericin and Lysozymes have been identified as immune-responsive AMPs drastically upregulated upon septic shock in P. stali32. In this study, we identified the LEBP-PI-related gene in the transcriptomic data of P. stali as a remarkably upregulated transcript upon septic shock, uncovered its antimicrobial activities against Gram-positive bacteria, designated it as a new AMP “Pentatomicin”, and investigated its structural, evolutionary, immunological and functional aspects in detail.
Materials and methods
Insects
Adult insects of P. stali were obtained at a forest edge in the National Institute of Advanced Industrial Science and Technology, Tsukuba, Japan, from which an inbred laboratory strain was established. A mass-reared colony of the strain was used as the source of experimental insects. The insects were reared in plastic containers (150 mm in diameter, 60 mm high) with raw peanuts, dry soybeans, and drinking water supplemented with 0.05% ascorbic acid at 25 ± 1 °C under a long-day regime of 16 h light and 8 h dark as described previously28.
Detection and sequencing of Pentatomicin
Total RNAs were extracted from whole adult females approximately 5 days after ecdysis using the RNeasy Mini Kit (Qiagen, Hilden, Germany). Complementary DNAs (cDNAs) were sequenced using Illumina HiSeq 2500 with paired-end 101 bp (Macrogen Japan Corp., Kyoto, Japan), and the generated raw reads were analyzed as previously described32.
Phylogenetic analysis
The deduced amino acid sequences of Pentatomicin of P. stali and other organisms were aligned using the Clustal W program implemented in MEGA 5.233. The molecular phylogenetic analyses were conducted by maximum likelihood method using MEGA 5.2 under the WAG + G model that was inferred as the best model. Bootstrap values based on 1000 replications are indicated as percentages on the nodes.
Construction of the expression vector and recombinant protein expression
To generate the expression vector using In-Fusion HD Cloning Kit (Takara Bio, Shiga, Japan), Pentatomicin-6xHisTag sequence and pGEX-6P-3 (GE Healthcare, Tokyo, Japan) sequence lacking GST except for the first 5 codon were amplified by PCR. The primer sequences are shown in Supplemental Table 1. These PCR products were ligated using In-Fusion HD Cloning Kit. The pGEX-6P-Pentatomicn-Histag plasmid was transformed into Escherichia coli BL21 (DE3) competent cells (Merck, Tokyo, Japan) to express Pentatomicn peptide. After incubation at 37 °C for 4 h in LB medium, the recombinant E. coli was cultured for 20 h at 18 °C with 0.1 mmol/l isopropyl-β-d-thiogalactopyranoside (IPTG).
Purification of recombinant protein
The cultured recombinant E. coli cells were lysed by ca. 40 mg of chicken lysozyme (Sigma-Aldrich Japan, Tokyo, Japan), and ultrasonic vibration. The lysates were centrifuged, and the supernatants were subjected to TALON metal affinity Resin (Takara Bio) for recombinant protein purification, basically following the manufacturer’s instructions except for the elution buffer, wherein 250 mM imidazole instead of 150 mM imidazole was dissolved. The purified recombinant protein was dialyzed using Spectra/Por membrane (Fisher Scientific, Pennsylvania, USA) to remove inadequate salts. The dialyzed recombinant protein was subjected to SDS-PAGE and quantification using ImageJ (https://imagej.nih.gov/ij/). As quantification standards, 2000, 1000, 500, 250, and 150 µg/µl of bovine serum albumin solutions were used.
Assay of antimicrobial activity
The antimicrobial activity assay was performed using eight bacterial strains. E. coli EPI300 was purchased from ARBROWN (Tokyo, Japan). Enterobacter cloacae (MAFF No. 811101)34 and Bacillus subtilis (MAFF No. 301702)35 were obtained from National Agriculture and Food Research Organization (NARO) Genebank, Japan. Micrococcus luteus (NBRC No. 13867), Staphylococcus aureus (NBRC No. 12732), Enterococcus faecalis (NBRC No. 100480) and Enterococcus faecium (NBRC No. 100486) were obtained from Nite Biological Resource Center (NBRC), Japan. The cultivable gut symbiont, Pantoea sp. C, used in this study was isolated from P. stali midgut25. The bacterial media are shown in Supplemental Table 2. The bacterial culture was mixed with the recombinant protein and incubated at 25 °C for 24 h. Bacterial growth was measured using Picoexplorer (Yamato Scientific Co., Tokyo, Japan), by which 200 µl of culture medium was measured at the wavelength of 575–660 nm.
Septic shock experiments
Adult females approximately 5 days after ecdysis were injected using glass capillary tubes (size: 100 µl; Drummond, Alabama, USA). As controls, we performed mock injection, i.e., pricking with a glass capillary without injecting any solution, and saline injection, i.e., injecting 5 µl of 0.9% sodium chloride (physiological saline for insects). The females were injected with ca. 5 µl of 0.9% saline or 108 cells/µl heat-killed bacteria (E. coli or M. luteus) into the ventral septum between the thoracic and abdominal segments32. Total RNA was extracted from the fat body excised 1, 4, 8 or 24 h after injection.
Quantitative RT-PCR
Total RNA samples were prepared from abdominal fat bodies using TRIzol (Life Technologies Japan Ltd, Tokyo, Japan), reverse-transcribed into complementary DNA using High-Capacity cDNA Reverse Transcription Kit (Life Technologies Japan Ltd, Tokyo, Japan). Quantitative reverse transcribed PCR (qRT-PCR) of Pentatomicin was conducted with primers, which were designed based on RNA sequencing data (Supplemental Table 1), using the LightCycler 96 SYBR Green Master (Roche Diagnostics, Tokyo, Japan). In the test of expression levels of Pentatomicin in different body parts of P. stali, mainly female bodies were used, but male-specific organs, genitalia and testis, were obtained by dissecting males.
RNAi experiments on adults
To knockdown the gene expression, adult females approximately 5 days after ecdysis were injected with ca. 3 µl of double-stranded RNA (dsRNA) solution (100 ng/µl) into the ventral septum between the thoracic and abdominal segments. dsRNA was synthesized from a PCR product using primers listed in Supplemental Table 1 and RiboMAX Large Scale RNA production kit (Promega, Madison, USA). The insects were injected with 3 µl of 108 cells/µl heat-killed E. coli or 3.3 ng/µl peptidoglycan of E. coli (Invivogen, San Diego, USA) 3 days after dsRNA injection, and then subjected to RNA extraction on the following day. The extracted RNA samples were subjected to quantitative RT-PCR (as above) to quantify the expression levels of Pentatomicin. pEGFP-L1 (Takara Bio) was used as a template for enhanced green fluorescent protein (EGFP). The concentrations of dsRNA were estimated using Nanodrop Lite (Thermo Fisher Scientific, Wilmington, DE, USA).
Survival assay on adult insects upon bacterial challenge
Male adults approximately 1 week after ecdysis were subjected to dsRNA injection as described above. Three days after dsRNA injection, the adults were challenged with living E. coli or S. aureus.
To prepare bacterial cells for injection, overnight bacterial cultures were diluted by 40 times and cultured for 6 h at 37 °C for E. coli and at 31 °C for S. aureus. Then, the bacterial cells were centrifuged and washed with PBS. Preliminary experiments were conducted, in which the injected doses were selected such that at least some control individuals (EGFP dsRNA injected) were killed. The bacterial cell titers injected into the insects, which were estimated in terms of colony-forming unit, were, on average, 1.3 × 1010 (high concentration), 8.6 × 109 (medium concentration), and 4.8 × 109 (low concentration) for E. coli, and 5.0 × 108 (high concentration), 3.6 × 108 (medium concentration), and 2.3 × 108 (low concentration) for S. aureus, respectively.
The survival rates upon bacterial challenge were observed every 24 h and the figures for survival rates were drawn using EzR36. P-values were calculated using pairwise comparisons using Log-Rank test.
RNAi experiments on eggs and nymphs
Sexually mature female adults (2 or 3 weeks after ecdysis) were injected with dsRNA of EGFP or Pentatomicin. Then, the eggs laid by injected females were collected daily to analyze the effects of RNAi using qRT-PCR. The effects of RNAi on newly hatched nymphs from those eggs were also tested using qRT-PCR.
Survival assay on eggs and nymphs
To estimate the biological role of Pentatomicin in eggs, the hatching rate of eggs laid by females injected with dsRNA of EGFP or Pentatomicin was inspected. Furthermore, to test the effects on vertical transmission of the obligate symbiont, the rate of attaining the 3rd instar was observed during 10 days after hatching, on the ground that most aposymbiotic nymphs died at 2nd instar or remained at 2nd instar with growth retardation24,25,28. In this experiment, in addition to placing the eggs in a clean dish with sterilized water and peanuts, four different treatments were applied to the eggs for experimental challenge of facilitating microbial contamination: (i) placing the eggs on 20-fold diluted S. aureus culture medium; (ii) soaking the eggs once in S. aureus culture medium (not diluted) and then placing them in a clean dish; (iii) placing the eggs on muddy water; and (iv) soaking the eggs once in muddy water and then placing them in a clean dish. The muddy water was made by suspending 100 g of soil from the forest floor with 400 ml of distilled water.
Diagnostic PCR to detect symbiotic bacteria
DNA samples were extracted from 2nd instar nymphs 3 days after ecdysis using Wizard Genomic DNA Purification Kit (Promega). PCR was conducted using Ex-taq (TaKaRa Bio) and the primers as described previously25 (sequences shown in Supplemental Table 1).
Statistical analysis
Steel–Dwass test was conducted by R3.4.2 (https://www.R-project.org/) and Mann–Whitney U test was done by EzR36.
Results and discussion
Identification of Pentatomicin from P. stali
RNA sequencing was conducted using a control adult insect without treatment, an adult insect injected with heat-killed E. coli, and an adult insect injected with heat-killed M. luteus32. Compared with the non-treated control, 37 of 114,190 contigs were commonly upregulated upon septic shocks by E. coli and M. luteus (over 30 times). The upregulated genes included previously reported AMPs such as Defensin1 and Hemiptericin (Supplemental Table 3). Here, we focused on a transcript of a potential immune effector that was highly upregulated upon septic shocks and exhibited notable sequence and structural similarities to LEBP-PI of the Limulus horseshoe crab22. The 560 bp transcript with polyadenylation signal and poly-A tail (Fig. S1) encoded a polypeptide of 126 amino acid residues with a secretion signal peptide on the C terminus (Fig. 1A). We designated the protein as “Pentatomicin” after the pentatomomorphan stinkbug P. stali.
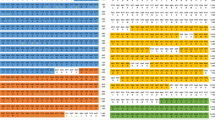
(A) Aligned deduced amino acid sequences of Pentatomicin from nine organisms. Red boxes indicate signal peptide regions. Conserved amino acid residues are shown by asterisks, of which conserved cysteine residues are highlighted by red arrows. (B) Phylogenic tree of Pentatomicin from P. stali and the orthologous genes from other organisms based on the deduced amino acid sequences. The tree was inferred from 71 aligned amino acid sites using the maximum likelihood method (WAG + G model). Bootstrap probabilities based on 1000 replications are indicated on the nodes. A red circle highlights Pentatomicin from P. stali. Accession numbers are indicated in brackets. Group labels are shown on the right side of the phylogeny.
Structural features and diversity of Pentatomicin and allied proteins
Pentatomicin gene identified from the transcriptome of P. stali was subjected to BLASTp searches against non-redundant protein sequences database in NCBI. Among diverse insects, a close Pentatomicin ortholog was identified from a stinkbug Halyomorpha halys (Hemiptera: Pentatomidae). In addition, several Pentatomicin orthologs were detected from a leaf beetle Diabrotica virgifera (Coleoptera: Chrysomelidae) and a click beetle Ignelater luminosus (Coleoptera: Elateridae). Meanwhile, although ample genomic and transcriptomic data are available for many hemipterans, coleopterans and other insect groups, Pentatomicins were not found from them. Notably, LEBP-PI derived from the Limulus horseshoe crab22 was identified as Pentatomicin-allied. While this protein was reported to bind to LPS and inhibit protease activities, its antimicrobial activities has not been documented22. Furthermore, Pentatomicin-allied proteins were detected from diverse prokaryotes encompassing Cyanobacteria, Betaproteobacteria and Deltaproteobacteria (Fig. 1B). These small proteins, 125–159 amino acid residues in size, shared six conserved cysteine residues whereas the other regions exhibited considerable sequence diversity (Fig. 1A). The six cysteine residues were not found in conventional AMPs like defensin, hemiptericin, drosomycin, diptericin and bacteriocin (Fig. S2). More accurate information will be obtained by detailed structural analysis, such as mass spectrometry, which awaits further research.
Evolutionary dynamics of Pentatomicin and allied proteins
Molecular phylogenetic analysis revealed that Pentatomicin and allied proteins may be classified into the following groups: (i) Insect group, (ii) Cyanobacteria group, (iii) Betaproteobacteria group, and (iv) Deltaproteobacteria + Limulus group (Fig. 1B). It should be noted, however, that the groupings are not necessarily robust with low statistical supports (especially Insect group and Deltaproteobacteria + Limulus group) presumably due to small size and variability of the sequences. In the insect group, the close relationship of the Pentatomicins between P. stali and H. halys must reflect the common ancestry of the gene in the Pentatomidae. On the other hand, the intermingled relationship of the Pentatomicin-like sequences from D. virgifera and I. luminosus suggested dynamic evolutionary trajectories of the genes that may entail both ancestral and recent gene duplications. The clustering of the deltaproteobacterial sequence and the Limulus sequence was suggestive of an inter-domain horizontal gene transfer event. Taken together, the sporadic distribution of Pentatomicin and allied proteins across arthropods and diverse bacteria strongly suggest that Pentatomicin has been moving around within and between the prokaryotes and the eukaryotes.
Antimicrobial activity of Pentatomicin
We expressed Pentatomicin protein in E. coli cells and purified the recombinant protein, 13.6 kDa in size, for antimicrobial activity assay (Fig. S3). Of eight bacterial species tested, all three Gram-negative bacteria (E. coli, Enterobacter cloacae and Pantoea sp. C symbiotic to P. stali25) were not affected, whereas three of five Gram-positive bacteria (Bacillus subtilis, Micrococcus luteus and Staphylococcus aureus, but not Enterococcus faecalis and Enterococcus faecium) were significantly suppressed by the presence of Pentatomicin (Fig. 2; Fig. S4). The minimum inhibitory concentrations (MIC) were 100–1000 µg/ml for B. subtilis, 1–10 µg/ml for M. luteus, and 10–100 µg/ml for S. aureus. These results demonstrated that Pentatomicin is an AMP of P. stali with broad activity against Gram-positive bacteria, except for Enterococcus spp.
Analysis of the activity of Pentatomicin against eight bacterial species. (A) Escherichia coli. (B) Enterobacter cloacae. (C) Pantoea sp. C, the gut symbiont of P. stali. (D) Bacillus subtilis. (E) Micrococcus luteus. (F) Staphylococcus aureus. (G) Enterococcus faecalis. (H) Enterococcus faecium. Each bacterial culture was mixed with the recombinant Pentatomicin and incubated at 25 °C for 24 h. Bacterial growth was measured for 200 µl of culture medium at the wavelength of 575–660 nm. Values represent the means ± SD of three replicates.
Expression patterns and regulatory pathways of Pentatomicin
Among diverse insects in general, microbial challenges promptly induce the expression of an array of AMPs in specific tissues like fat bodies37,38. Here, the expression levels of Pentatomicin upon and after septic shock were monitored using fat bodies of female adults of P. stali (Fig. 3A). The expression levels of Pentatomicin increased nearly 100-fold 4 h after injection and on with heat-killed E. coli or M. luteus. Though less promptly than the bacterial injections, mock and saline injections induced the expression of Pentatomicin at comparable levels (Fig. 3A). Note that the other AMPs of P. stali are not so responsive to mock and saline injections32. These results suggest that Pentatomicin is highly sensitive to low doses of contaminated bacteria and/or mechanical injury.
The expression patterns of Pentatomicin in adult fat bodies. (A) The effects of septic shock treatments on the expression levels of Pentatomicin. Adult females were subjected to the following treatments: N no treatment, M mock injection, S saline injection, Ec heat-killed E. coli injection, Ml heat-killed M. luteus injection. Quantitative RT-PCR was performed for the samples harvested 1, 4, 8, and 24 h after injection (n = 5–8). Different letters show statistically significant differences in each time point (Steel–Dwass test: P < 0.05). (B) Effects of RNAi knockdown of immune pathway genes on septic shock-induced upregulation of Pentatomicin. Adult insects were injected with heat-killed E. coli 3 days after dsRNA injection and subjected to RNA extraction on the following day (n = 9–12). Different letters show statistically significant differences (Steel–Dwass test: P < 0.05). (C) Effects of RNAi knockdown of immune pathway genes on mock injection-induced upregulation of Pentatomicin. Adult insects were injected with a clean needle 3 days after dsRNA injection and subjected to RNA extraction on the following day (n = 4–5). Statistical analysis was done by Mann–Whitney U test (P < 0.05).
The expression of AMPs is regulated by signaling pathways39. In insects, Toll pathway and immune deficiency (IMD) pathway mainly regulate microbe-induced production of AMPs11,40,41. We conducted RNAi-mediated suppression of the immune regulatory genes and examined how the expression levels of Pentatomicin upon septic shock are affected. The upregulation of Pentatomicin was inhibited by the RNAi-mediated suppression of Toll pathway genes MyD88 and Dorsal (Mann–Whitney U test; P < 0.05; ca. 70% and 90% lower than control, respectively), whereas no inhibition was observed with the RNAi-mediated suppression of IMD pathway genes Imd and Relish (Fig. 3B). In P. stali, at least 5 AMPs are known to be regulated by both IMD and Toll pathways, which are more or less entangled32. By contrast, Pentatomicin seems to be regulated by the Toll pathway preferentially (Fig. S5).
Meanwhile, we point out the possibility that Toll pathway may be not the main and direct regulator of Pentatomicin. The reduction in Pentatomicin expression by Toll pathway blockage was not so drastic in contrast to the other AMPs: for instance, RNAi knockdown of Toll pathway component, MyD88, results in ca. 95% downregulation of Defensin232. The upregulation of Pentatomicin was induced even by mock or saline injection only, which is extremely sensitive in comparison with the other AMPs. This property of Pentatomicin might be relevant to the fact that it is not a typical insect AMP but is allied to LEBP-PI known as a LPS binding protein of the Limulus horseshoe crab22.
Finally, we investigated whether the effects of mock injection on Pentatomicin could be suppressed by downregulating the Toll pathway. The results showed that RNAi of MyD88 did not cancel the effect of mock, and the expression of Pentatomicin was upregulated by mock injection (Mann–Whitney U test; P < 0.05; Fig. 3C). This strongly suggests that some other effect, not via the Toll pathway, is responsible for the upregulation of Pentatomicin by mock injection.
Biological role of Pentatomicin for adult survival
To examine whether Pentatomicin plays an immunological role in P. stali, we injected dsRNA of Pentatomicin into adult males approximately 1 week after ecdysis. Quantitative RT-PCR confirmed that the dsRNA injection efficiently suppressed the upregulation of Pentatomicin by peptidoglycan injection (Fig. S6). When these insects were challenged by bacterial infections, Gram-positive S. aureus caused significantly higher mortality than control (Fig. 4B), although medium concentration of S. aureus was not affected. On the other hand, Gram-negative E. coli induced no significant mortality in comparison with control (Fig. 4A). These results are consistent with the antimicrobial spectrum of the recombinant Pentatomicin (see Fig. 2) and suggest that Pentatomicin contributes to host insect survival via immune defense against Gram-positive bacterial pathogens.
The effects of Pentatomicin suppression on adult survival against septic challenge by E. coli (A) or S. aureus (B). The average numbers of bacterial cells injected into P. stali adults were 1.3 × 1010 (high concentration), 8.6 × 109 (medium concentration) and 4.8 × 109 (low concentration) E. coli cells per insect, and 5.0 × 108 (high concentration), 3.6 × 108 (medium concentration) and 2.3 × 108 (low concentration) S. aureus cells per insect. Red lines indicate the survival curves of Pentatomicin dsRNA-injected insects, whereas black lines show the survival curves of EGFP dsRNA-injected insects. The number of individuals is given in parentheses. P values are calculated by pairwise comparisons using Log-Rank test.
Discovery of high Pentatomicin expression in developing eggs
In an attempt to explore the roles of Pentatomicin in more depth, we examined the expression levels of Pentatomicin in different body parts of P. stali. Relatively high expression levels in head, wings, legs, pronotum, and genitalia (Fig. 5) seemed likely due to the presence of fat bodies and/or epithelia in these body parts where AMPs are synthesized. Unexpectedly, the highest expression levels of Pentatomicin were detected in eggs 3 days after oviposition, and the relatively high expression levels persisted in eggs 4 and 5 days after oviposition (Fig. 5). This finding was surprising because egg contents are encased in a hard shell and thus considered as aseptic. In Drosophila, it has been established that 16 AMPs (Defensin, Drosocin, DiptericinA, DiptericinB, AttacinA, AttacinB, AttacinC, AttacinD, Metchnikowin, Drosomycin, Drosomycin-like1, CecropinA1, CecropinA2, CecropinB, CecropinC, and Andropin) consistently exhibit no or very low expression in eggs, although AMP-like genes called Bomanins are expressed in late embryonic stages (https://flybase.org/)42,43,44.
The expression levels of Pentatomicin in different body parts of P. stali. Eight individuals were examined for each body part. The highest mean expression was observed in eggs 3 days after oviposition. The top and bottom bars around the boxes indicate the maximum and minimum values of the sample (except for outliers which are shown by circles). The top and bottom lines of the boxes indicate the 75% quartile and 25% quartile of the samples, respectively. The thick lines inside the boxes indicate medians.
Biological role of Pentatomicin for embryonic development?
The high expression of Pentatomicin in eggs suggested the possibility that it may be involved in another biological role in P. stali. Note that, in Drosophila, some AMPs were reported to affect tumor inhibition, memory formation, and aging45. To examine the functional role of Pentatomicin in eggs, we performed maternal RNAi experiments as reported previously30. When dsRNA of Pentatomicin was injected into mated females, significantly lower expression was manifested in eggs laid by females that passed 3 days or more after injection, and the suppressed expression continued for 2 weeks thereafter (Fig. S7). Hatching rates of Pentatomicin-suppressed eggs were not significantly different from those of control eggs (Mann–Whitney U test; P = 0.68; Fig. 6A,B). The expression levels of Pentatomicin in the newborn nymphs were significantly lowered by the maternal RNAi treatment (Fig. S8). These results indicate that Pentatomicin is not essential for embryonic development of P. stali, although the possibility that a very low level of Pentatomicin is sufficient for ensuring embryonic development cannot be excluded.
Effects of maternal RNAi of Pentatomicin on egg hatching, symbiont transmission and nymphal development under a variety of contaminating conditions. (A–D) Non-contaminated control, in which eggs were placed in a clean dish with sterilized water and peanuts. (E–H) Continuous S. aureus contamination, in which eggs were placed on liquid culture medium of S. aureus in a clean dish with sterilized peanuts. (I–L) Transient S. aureus contamination, in which eggs were soaked in liquid culture medium of S. aureus and then placed in a clean dish with sterilized water and peanuts. (M–P) Continuous muddy water contamination, in which eggs were placed on muddy water in a clean dish with sterilized peanuts. (Q–T) Transient muddy water contamination, in which eggs were soaked in muddy water and then placed in a clean dish with sterilized water and peanuts. (A,E,I,M,Q) Photographs of treated eggs. (B,F,J,N,R) Hatching rate. (C,G,K,O,S) Rate of symbiont acquisition at 2nd instar. (D,H,L,P,T) Rate of attaining 3rd instar. These rates were calculated in each egg mass and the number of egg masses is given in parentheses. In all the treatments, no statistically significant differences were observed between Pentatomicin-suppressed eggs and dsEGFP-injected control eggs for the hatching rates, symbiont acquisition rates, and rates of attaining 3rd instar (Mann–Whitney U test).
Biological role of Pentatomicin for ensuring vertical symbiont transmission?
Finally, we examined the possibility that Pentatomicin may contribute to successful vertical symbiont transmission in P. stali. It should be noted that the obligatory gut bacterial symbiont of P. stali, Pantoea sp. A, is Gram-negative and thus expected to be unaffected by Pentatomicin (Fig. 2). Upon oviposition, adult females of P. stali smear the symbiont-containing secretion onto the egg surface24,25,28,29, where the symbiotic bacteria persist for several days (ca. 5 days at 25 °C) until orally acquired by newborn nymphs. We hypothesized that Pentatomicin may protect the symbiotic bacteria on eggs from Gram-positive bacterial contaminants, which would otherwise outcompete the symbiont and lower the survival of the host insect. Symbiont-free nymphs of P. stali are known to suffer high mortality, especially in the 2nd instar, and severe developmental retardation24,25,28.
When the eggs were placed in a clean dish, suppression of Pentatomicin did not affect the hatching rate, the rate of successful symbiont acquisition at 2nd instar, and the rate of attaining 3rd instar within 10 days after egg hatching (Fig. 6A–D). Then, Pentatomicin-suppressed eggs were subjected to four different contaminating treatments: (i) placing the eggs continuously on liquid culture medium of S. aureus (Fig. 6E–H); (ii) soaking the eggs in liquid culture medium of S. aureus and then transferring them to a clean container (Fig. 6I–L); (iii) placing the eggs continuously on muddy water (Fig. 6M–P); (iv) soaking the eggs in muddy water and then placing them in a clean dish (Fig. 6Q–T). Among dsEGFP-injected controls, the continuous S. aureus treatment significantly reduced the hatching rate (Steel–Dwass test, P < 0.05; Fig. S9) and rates of reaching the 3rd-instar (Steel–Dwass test, P < 0.05; Fig. S9) in comparison with non-contaminated control, but the other treatments did not. In all the treatments, no statistically significant differences were observed between Pentatomicin-suppressed eggs and dsEGFP-injected control eggs for the hatching rates, symbiont acquisition rates, and rates of attaining 3rd instar, although the medians did vary in some cases (Fig. 6). Taken together, the biological role of Pentatomicin in the early life stages was unclear. It is conceivable, though speculative, that Pentatomicin may confer resistance against some natural enemies like parasitic wasps, but further research is required to verify such hypotheses.
Conclusion and perspective
We identified a new antimicrobial peptide, Pentatomicin, from the stinkbug P. stali. Pentatomicin-allied genes were found from hemipteran and coleopteran insects, a horseshoe crab, cyanobacteria, and proteobacteria. Using recombinant protein and conducting RNAi knockdown and quantitative RT-PCR, we uncovered the following properties of Pentatomicin. First, the antimicrobial activity of Pentatomicin was shown against an array of Gram-positive bacteria. Second, the expression of Pentatomicin increased not only upon septic shocks but also upon mock injection and saline injection, which is under the regulation of the Toll pathway, at least partly. Third, Pentatomicin-suppressed adult insects become more vulnerable to infection and pathology of Gram-positive S. aureus. Finally, high Pentatomicin expression was identified in eggs, which is atypical of conventional AMPs known from Drosophila and other insects, although the biological role of Pentatomicin in eggs is currently elusive. These findings extend our understanding of the diversity of AMPs, highlight a dynamic evolutionary aspect of AMPs, and provide a new insight into the innate immune components of insects. Conventionally, studies on insect immunity have been conducted mainly on holometabolous insects such as Drosophila9,11,15,46,47, Manduca48,49 and Bombyx50,51. For better understanding of the diversity of innate immunity, more studies on hemimetabolous insects are needed. In addition to P. stali, the bean bug Riptortus pedestris52,53,54,55,56 and the blood sucking bug Rhodnius prolixus57,58,59,60,61,62 would provide tractable models for understanding the innate immunity of hemimetabolous insects.
Data availability
Data sets generated or analyzed during this study are included in Supplementary Information files.
References
Guaní-Guerra, E., Santos-Mendoza, T., Lugo-Reyes, S. O. & Terán, L. M. Antimicrobial peptides: General overview and clinical implications in human health and disease. Clin. Immunol. 135, 1–11 (2010).
Martinez, F. A. C., Balciunas, E. M., Converti, A., Cotter, P. D. & de Souza Oliveira, R. P. Bacteriocin production by Bifidobacterium spp. A review. Biotechnol. Adv. 31, 482–488 (2013).
Rolff, J. & Schmid-Hempel, P. Perspectives on the evolutionary ecology of arthropod antimicrobial peptides. Philos. Trans. R. Soc. B Biol. Sci. 371, 20150297 (2016).
Wang, G., Li, X. & Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 44, D1087–D1093 (2016).
Hancock, R. E. W., Alford, M. A. & Haney, E. F. Antibiofilm activity of host defence peptides: Complexity provides opportunities. Nat. Rev. Microbiol. 19, 786–797 (2021).
Li, W., Separovic, F., O’Brien-Simpson, N. M. & Wade, J. D. Chemically modified and conjugated antimicrobial peptides against superbugs. Chem. Soc. Rev. 50, 4932–4973 (2021).
Gillespie, J. P., Kanost, M. R. & Trenczek, T. Biological mediators of insect immunity. Annu. Rev. Entomol. 42, 611–643 (1997).
Shirasu-Hiza, M. M. & Schneider, D. S. Confronting physiology: How do infected flies die?. Cell. Microbiol. 9, 2775–2783 (2007).
Hultmark, D. Drosophila immunity: Paths and patterns. Curr. Opin. Immunol. 15, 12–19 (2003).
Tanji, T. & Ip, Y. T. Regulators of the Toll and Imd pathways in the Drosophila innate immune response. Trends Immunol. 26, 193–198 (2005).
Lemaitre, B. & Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25, 697–743 (2007).
Lin, S. J. H., Cohen, L. B. & Wasserman, S. A. Effector specificity and function in Drosophila innate immunity: Getting AMPed and dropping Boms. PLoS Pathog. 16, e1008480 (2020).
Hanson, M. A., Lemaitre, B. & Unckless, R. L. Dynamic evolution of antimicrobial peptides underscores trade-offs between immunity and ecological fitness. Front. Immunol. 10, 2620 (2019).
Hanson, M. A. et al. Synergy and remarkable specificity of antimicrobial peptides in vivo using a systematic knockout approach. Elife 8, e44341 (2019).
Lemaitre, B., Reichhart, J.-M. & Hoffmann, J. A. Drosophila host defense: Differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. PNAS 94, 14614–14619 (1997).
Murray, C. J. et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 399, 629–655 (2022).
Mahlapuu, M., Håkansson, J., Ringstad, L. & Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 6, 194 (2016).
Mylonakis, E., Podsiadlowski, L., Muhammed, M. & Vilcinskas, A. Diversity, evolution and medical applications of insect antimicrobial peptides. Philos. Trans. R. Soc. B Biol. Sci. 371, 20150290 (2016).
Walls, E. A., Berkson, J. & Smith, S. A. The horseshoe crab, Limulus polyphemus: 200 million years of existence, 100 years of study. Rev. Fish. Sci. 10, 39–73 (2002).
Muta, T. & Iwanaga, S. The role of hemolymph coagulation in innate immunity. Curr. Opin. Immunol. 8, 41–47 (1996).
Iwanaga, S. The molecular basis of innate immunity in the horseshoe crab. Curr. Opin. Immunol. 14, 87–95 (2002).
Minetti, C. A., Lin, Y. A., Cislo, T. & Liu, T. Y. Purification and characterization of an endotoxin-binding protein with protease inhibitory activity from Limulus amebocytes. J. Biol. Chem. 266, 20773–20780 (1991).
Schaefer, C. W. & Panizzi, A. R. Heteroptera of Economic Importance (CRC Press, 2000).
Abe, Y., Mishiro, K. & Takanashi, M. Symbiont of brown-winged green bug, Plautia stali Scott. Jpn. J. Appl. Entomol. Zool. (1995).
Hosokawa, T. et al. Obligate bacterial mutualists evolving from environmental bacteria in natural insect populations. Nat. Microbiol. 1, 15011 (2016).
Hosokawa, T., Matsuura, Y., Kikuchi, Y. & Fukatsu, T. Recurrent evolution of gut symbiotic bacteria in pentatomid stinkbugs. Zool. Lett. 2, 24 (2016).
Hayashi, T., Hosokawa, T., Meng, X.-Y., Koga, R. & Fukatsu, T. Female-specific specialization of a posterior end region of the midgut symbiotic organ in Plautia splendens and allied stinkbugs. Appl. Environ. Microbiol. 81, 2603–2611 (2015).
Nishide, Y. et al. Aseptic rearing procedure for the stinkbug Plautia stali (Hemiptera: Pentatomidae) by sterilizing food-derived bacterial contaminants. Appl. Entomol. Zool. 52, 407–415 (2017).
Oishi, S., Moriyama, M., Koga, R. & Fukatsu, T. Morphogenesis and development of midgut symbiotic organ of the stinkbug Plautia stali (Hemiptera: Pentatomidae). Zool. Lett. 5, 16 (2019).
Nishide, Y. et al. Diversity and function of multicopper oxidase genes in the stinkbug Plautia stali. Sci. Rep. 10, 3464 (2020).
Nishide, Y. et al. Effectiveness of orally-delivered double-stranded RNA on gene silencing in the stinkbug Plautia stali. PLoS One 16, e0245081 (2021).
Nishide, Y. et al. Functional crosstalk across IMD and Toll pathways: Insight into the evolution of incomplete immune cascades. Proc. R. Soc. B Biol. Sci. 286, 20182207 (2019).
Tamura, K. et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 (2011).
Watanabe, K. & Sato, M. Plasmid-mediated gene transfer between insect-resident bacteria, Enterobacter cloacae, and plant-epiphytic bacteria, Erwinia herbicola, in guts of silkworm larvae. Curr. Microbiol. 37, 352–355 (1998).
Watanabe, K. & Hayano, K. Distribution and identification of proteolytic Bacillus spp. in paddy field soil under rice cultivation. Can. J. Microbiol. 39, 674–680 (1993).
Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48, 452–458 (2013).
Bulet, P., Hetru, C., Dimarcq, J.-L. & Hoffmann, D. Antimicrobial peptides in insects; Structure and function. Dev. Comp. Immunol. 23, 329–344 (1999).
Tzou, P. et al. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity 13, 737–748 (2000).
Meister, M., Hetru, C. & Hoffmann, J. A. The antimicrobial host defense of Drosophila. In Origin and Evolution of the Vertebrate Immune System (eds Du Pasquier, L. & Litman, G. W.) 17–36 (Springer, 2000). https://doi.org/10.1007/978-3-642-59674-2_2.
Ferrandon, D., Imler, J.-L., Hetru, C. & Hoffmann, J. A. The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 7, 862–874 (2007).
De Gregorio, E., Spellman, P. T., Tzou, P., Rubin, G. M. & Lemaitre, B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 21, 2568–2579 (2002).
Clemmons, A. W., Lindsay, S. A. & Wasserman, S. A. An effector peptide family required for Drosophila Toll-mediated immunity. PLoS Pathog. 11, e1004876 (2015).
Lindsay, S. A., Lin, S. J. H. & Wasserman, S. A. Short-form bomanins mediate humoral immunity in Drosophila. J. Innate Immun. 10, 306–314 (2018).
Larkin, A. et al. FlyBase: Updates to the Drosophila melanogaster knowledge base. Nucleic Acids Res. 49, D899–D907 (2021).
Hanson, M. A. & Lemaitre, B. New insights on Drosophila antimicrobial peptide function in host defense and beyond. Curr. Opin. Immunol. 62, 22–30 (2020).
Hoffmann, J. A. The immune response of Drosophila. Nature 426, 33–38 (2003).
Hoffmann, J. A. & Reichhart, J.-M. Drosophila innate immunity: An evolutionary perspective. Nat. Immunol. 3, 121–126 (2002).
Kanost, M. R., Jiang, H. & Yu, X.-Q. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol. Rev. 198, 97–105 (2004).
Cao, X. et al. The immune signaling pathways of Manduca sexta. Insect Biochem. Mol. Biol. 62, 64–74 (2015).
Yamakawa, M. & Tanaka, H. Immune proteins and their gene expression in the silkworm, Bombyx mori. Dev. Comp. Immunol. 23, 281–289 (1999).
Tanaka, H. et al. A genome-wide analysis of genes and gene families involved in innate immunity of Bombyx mori. Insect Biochem. Mol. Biol. 38, 1087–1110 (2008).
Futahashi, R. et al. Gene expression in gut symbiotic organ of stinkbug affected by extracellular bacterial symbiont. PLoS One 8, e64557 (2013).
Kim, J. K. et al. Molting-associated suppression of symbiont population and up-regulation of antimicrobial activity in the midgut symbiotic organ of the Riptortus–Burkholderia symbiosis. Dev. Comp. Immunol. 43, 10–14 (2014).
Kim, J. K. et al. Burkholderia gut symbionts enhance the innate immunity of host Riptortus pedestris. Dev. Comp. Immunol. 53, 265–269 (2015).
Kim, J. K. et al. Understanding regulation of the host-mediated gut symbiont population and the symbiont-mediated host immunity in the Riptortus-Burkholderia symbiosis system. Dev. Comp. Immunol. 64, 75–81 (2016).
Park, K.-E. et al. The roles of antimicrobial peptide, rip-thanatin, in the midgut of Riptortus pedestris. Dev. Comp. Immunol. 78, 83–90 (2018).
Feder, D., Mello, C. B., Garcia, E. S. & Azambuja, P. Immune responses in Rhodnius prolixus: Influence of nutrition and ecdysone. J. Insect Physiol. 43, 513–519 (1997).
Garcia, E. S., Castro, D. P., Figueiredo, M. B., Genta, F. A. & Azambuja, P. Trypanosoma rangeli: A new perspective for studying the modulation of immune reactions of Rhodnius prolixus. Parasites Vectors 2, 33 (2009).
Ursic-Bedoya, R., Buchhop, J., Joy, J. B., Durvasula, R. & Lowenberger, C. Prolixicin: A novel antimicrobial peptide isolated from Rhodnius prolixus with differential activity against bacteria and Trypanosoma cruzi. Insect Mol. Biol. 20, 775–786 (2011).
Azambuja, P. et al. Rhodnius prolixus: From physiology by Wigglesworth to recent studies of immune system modulation by Trypanosoma cruzi and Trypanosoma rangeli. J. Insect Physiol. 97, 45–65 (2017).
Salcedo-Porras, N., Guarneri, A., Oliveira, P. L. & Lowenberger, C. Rhodnius prolixus: Identification of missing components of the IMD immune signaling pathway and functional characterization of its role in eliminating bacteria. PLoS One 14, e0214794 (2019).
Salcedo-Porras, N., Noor, S., Cai, C., Oliveira, P. L. & Lowenberger, C. Rhodnius prolixus uses the peptidoglycan recognition receptor rpPGRP-LC/LA to detect Gram-negative bacteria and activate the IMD pathway. Curr. Res. Insect Sci. 1, 100006 (2021).
Acknowledgements
We thank Masae Takashima and Maria Murakami for technical assistance.
Funding
This study was supported by the JSPS KAKENHI Grant JP19K06080 to Y.N. and JP17H06388 to T.F., and the JST ERATO grant JPMJER1902 to T.F. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.N. and K.N. performed recombinant protein experiments. Y.N. performed all the other experiments. Y.N. analyzed experimental data. M.M. and R.F. analyzed RNA sequencing data. Y.N. wrote the draft manuscript. Y.N., D.K., M.M., R.F. and T.F. conceived and designed the experiments. D.K., R.F. and T.F. revised the manuscript. All authors discussed the results and contributed to the article writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nishide, Y., Nagamine, K., Kageyama, D. et al. A new antimicrobial peptide, Pentatomicin, from the stinkbug Plautia stali. Sci Rep 12, 16503 (2022). https://doi.org/10.1038/s41598-022-20427-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20427-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.