Abstract
Measuring the adenoma detection rate (ADR) is critical to providing quality care, however it is also challenging. We aimed to develop a tool using pre-existing electronic health record (EHR) functions to accurately and easily measure total ADR and to provide real-time feedback for endoscopists. We utilized the Epic EHR. With the help of an Epic analyst, using existing tools, we developed a method by which endoscopy staff could mark whether an adenoma was detected for a given colonoscopy. Using these responses and all colonoscopies performed by the endoscopist recorded in the EHR, ADR was calculated in a report and displayed to endoscopists within the EHR. One endoscopist piloted the tool, and results of the tool were validated against a manual chart review. Over the pilot period the endoscopist performed 145 colonoscopies, of which 78 had adenomas. The tool correctly identified 76/78 colonoscopies with an adenoma and 67/67 of colonoscopies with no adenomas (97.4% sensitivity, 100% specificity, 98% accuracy). There was no difference in ADR as determined by the tool compared to manual review (53.1% vs. 53.8%, p = 0.912). We successfully developed and pilot tested a tool to measure ADR using existing EHR functionality.
Similar content being viewed by others
Introduction
Widespread adoption of screening colonoscopy has coincided with a steady decline in the incidence of and mortality associated with colorectal cancer in the United States1. However, post-colonoscopy cancers do occur and are primarily attributed to missed cancers or pre-cancerous polyps on preceding colonoscopy2. Thus, maximizing the quality of endoscopic inspection is essential in reducing missed lesions and post-colonoscopy cancers. The adenoma detection rate (ADR), or the proportion of screening colonoscopies in which at least one adenomatous polyp is found, has become the established surrogate of colonoscopy quality3. Multiple studies have demonstrated that performance of ADR correlates with decreased incidence of post-colonoscopy cancers3,4 and that providing endoscopists with feedback related to their ADR can improve their ADR performance5,6. This has led to the elevation of ADR as a priority quality indicator (QI) and the recommendation that all endoscopists measure their ADR to assure delivery of high-quality and high-value care7.
The data needed to determine ADR requires coupling of endoscopic and pathology findings which are separated by both time and location within the electronic health record (EHR). Thus, measuring ADR requires time-intensive chart review either by individual endoscopists or designated staff. Other methods to measure ADR, including the use of natural language processing (NLP) to automatically extract data from endoscopy and pathology text, have been described 8,9,10,11,12,13,14; though these require complex data management systems that are likely not available to many endoscopists and are not easily transferable from one practice to another15. Alternatives to labor intensive manual review and complex NLP based strategies are needed. We therefore aimed to develop and validate a method using embedded EHR functions in Epic, the most widely adopted EHR in the US marketplace16, to facilitate both ADR reporting and feedback.
Materials and methods
Many EHRs contain functions that allow for automated capture of structed data from EHR text notes17. We worked with an Epic analyst to develop a tool using this EHR functionality to capture total ADR (tADR), or the proportion of all colonoscopies regardless of indication in which at least one adenoma is found. Total ADR has previously been shown to correlate with true ADR18. The primary Epic functions we utilized were the SmartList and Reporting Workbench. A SmartList is an item of text that can be embedded in a note to produce data that can be pulled into a report. In addition to the SmartList, the build elements necessary for our report included a procedure grouper (an Epic umbrella identifier to capture all the different ways colonoscopies are labeled in the EHR) to identify colonoscopy procedures to the report, a custom Epic property known as results interpreter to interrogate the endoscopist who performed the procedure, and a summary view within the report to display tADR to the endoscopist (Table 1). The SmartList was built to record a simple yes or no adenoma response in a note and store it to a SmartData element (Table 1, Fig. 1B). The number of yes responses are calculated as a percentage of all colonoscopies and reported as a simple pie chart visible to the endoscopist in the EHR (Fig. 1C). The total count of colonoscopies is determined by looking to the colonoscopy grouper and pulling in all completed procedures for a rolling 90-day lookback, then using the custom results interpreter property to filter the list to the endoscopist(s) of interest thus providing the endoscopist with a real-time tally of their tADR over the last 90 days. Ninety days was chosen to assure that at least 100 colonoscopies were included in each report tally as previous studies analyzing effects of feedback on ADR improvement have utilized at least 100 colonoscopies/provider to assess change19,20 The report could be personalized to each endoscopist within our system by changing the name assigned in the results interpreter property as well as the lookback time interval based on endoscopist volume. The components of the report needed to determine tADR are displayed in Table 1.
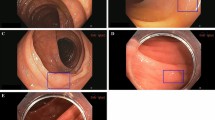
(A) Workflow timeline for utilizing the SmartList tool. When the report is run, all colonoscopies from the previous 90 days are included and linked to instances of SmartList use in the patient’s chart to calculate the tADR in the report. (B) The SmartList as displayed in a simple note. (C) The pie-chart display of the Reporting Workbench results representing the tADR. This figure was constructed from a screenshot from Epic Clinical Software, Version May 2021 (https://www.epic.com/software).
To minimize workflow the clinical staff were instructed to embed the SmartList in a note at time of pathology review, and one provider was selected to prospectively pilot the tool over a 6-month testing timeframe (allowing for 2 90-day Plan Do Study Act (PDSA) cycles). Provider workflow and accompanying EHR functions are displayed in Fig. 1A. Two PDSA cycles were necessary, with iterative expansion of the EHR based colonoscopy grouper until the report captured all colonoscopies performed by our provider over the validation timeframe a greater than 95% sensitivity. A final 90-day audit of colonoscopies performed by the pilot endoscopist was then conducted against a gold standard manual chart review to assess the sensitivity, specificity and accuracy of the report’s ability to capture tADR. Informed consent was waived for this study by the Colorado Multiple Institutions Review Board (COMIRB) because all patient data was de-identified and there was no direct patient contact, nor change in the standard of medical care. This study was reviewed and approved by COMIRB (Protocol Number 19-1378) and was carried out in accordance with all relevant guidelines and regulations.
Results
One hundred and forty-five colonoscopies were included in the final 90-day validation cohort. A manual chart review found that 78 colonoscopies had at least one adenoma (ADR 53.8%). Of the 145 colonoscopies, our EHR method correctly identified 143 (98.6%). The report identified 76/78 of colonoscopies in which at least one adenoma was found and all (67/67) colonoscopies where no adenoma was found (97.4% sensitivity, 100% specificity and 98.6% accuracy for detection of adenomas). The ADR generated by the EHR report was not clinically or statistically different from the ADR determined by gold standard manual chart review (53.1% vs. 53.8%, p = 0.912). The reason the two colonoscopies with adenomas were not captured by our report is because the endoscopist mistakenly did not utilize the SmartList at time of pathology review.
Discussion
The ADR is a high-value quality indicator because it is readily measured and is an established surrogate for the rare outcome post-colonoscopy cancer risk. Thus, it is imperative that all endoscopists have the means to know and track their ADR, and tools to facilitate more widespread measurement of ADR have the potential to support the delivery of high-value care. Here, we describe a novel method, leveraging pre-existing infrastructure within a widely adopted EHR, to do just that. In a pilot testing environment, our tool demonstrated a high sensitivity and accuracy compared to a manual chart review, similar to what studies evaluating NLP methods report8,9,10,11,12,13,14. To our knowledge, this is the first description of a method using EHR functionality to accurately capture endoscopic QIs.
There remain weaknesses to the tool we propose. It still requires effort from clinical staff to open a note and utilize the SmartList at the time of pathology result documentation. This requires several extra clicks beyond the normal workflow and in our pilot study resulted in 2/78 adenoma positive results being incorrectly classified. Additionally, our tool in its present form does not account for colonoscopy indication, completeness or prep quality. However, as previously noted tADR is an accurate surrogate for ADR, and others have even proposed that it may be a preferred colonoscopy QI as it simplifies measurement and may prevent gaming the ADR metric by changing colonoscopy indication18,21. Furthermore, additional macro selections (for instance for indication, bowel preparation quality, polyp histology, such as serrated lesions) can certainly be added to future iterations of our tool. These features can be customized based on a specific practices’ priorities in quality metric tracking and reporting (for instance if this information is not tracked elsewhere). The adaptability and customizability of our tool is a great strength, particularly if professional societies add additional lesion detection rates/benchmarks (such as serrated lesion detection rates) to quality metrics that should be measured.
We believe the tool we propose has several benefits. Because our tool relies largely on pre-existing abilities imbedded within an EHR, it does not require access to specialized data management systems often needed to adopt NLP based solutions. In addition, while NLP methods are often successful at individual institutions, adapting those tools across more diverse clinical settings has proven challenging15. Our tool can be scaled for use by anyone using the Epic EHR. Our tool provides real-time feedback within the EHR related to QI performance, allowing endoscopists to confront their own performance in the same interface in which they regularly manage patient care. While our tool does require some minimal effort from clinical staff, this is largely within normal clinical workflow and remains far less than what is required for manual extraction. Finally, though our tool was built using the Epic EHR, multiple EHRs have similar discrete data macro functionalities which could allow a similar tool to be developed in different systems both in the United States and Europe17,22.
Further work is needed to validate this tool among a greater proportion of endoscopists and ideally among multiple centers using the Epic EHR. Reassuringly, Smartlists are already widely used among everyday documentation in Epic. Additionally, prior research has demonstrated excellent adoption of Smartlists in post-colonoscopy EHR documentation23. Adjusting the structure of the Reporting Workbench algorithm by utilizing other macro data tools like additional SmartLists or flowsheets within the EHR may also allow for capture of additional data such as colonoscopy indication, prep quality and even allow for use of a similar tool to capture QIs in other endoscopic arenas.
This pilot study demonstrates the potential to leverage existing EHR functionality to achieve accurate measurement and feedback of tADR, a reliable surrogate for ADR. This tool may present an easily adoptable alternative to complex NLP based systems or time-intensive chart review to facilitate QI measurement and assure delivery of high-value care.
Abbreviations
- ADR:
-
Adenoma detection rate
- QI:
-
Quality indicator
- EHR:
-
Electronic health record
- NLP:
-
Natural language processing
- tADR:
-
Total adenoma detection rate
References
Richardson, L., Tai, E., Rim, S. H., Joseph, D. & Plescia, M. Vital signs: Colorectal cancer screening, incidence, and mortality–United States, 2002–2010. MMWR Morb. Mortal Wkly. Rep. 60(26), 884–889 (2011).
Pohl, H. & Robertson, D. J. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin. Gastroenterol. Hepatol. 8(10), 858–864 (2010).
Kaminski, M. F. et al. Quality indicators for colonoscopy and the risk of interval cancer. N. Engl. J. Med. 362(19), 1795–1803 (2010).
Corley, D. A. et al. Adenoma detection rate and risk of colorectal cancer and death. N. Engl. J. Med. 370(14), 1298–1306 (2014).
Keswani, R. N. et al. Physician report cards and implementing standards of practice are both significantly associated with improved screening colonoscopy quality. Am. J. Gastroenterol. 110(8), 1134–1139 (2015).
Kahi, C. J., Vemulapalli, K. C., Johnson, C. S. & Rex, D. K. Improving measurement of the adenoma detection rate and adenoma per colonoscopy quality metric: The Indiana University experience. Gastrointest. Endosc. 79(3), 448–454 (2014).
Rex, D. K. et al. Quality indicators for colonoscopy. Gastrointest. Endosc. 81(1), 31–53 (2015).
Gawron, A. J. et al. Simplifying measurement of adenoma detection rates for colonoscopy. Dig. Dis. Sci. 66, 3149–3155 (2020).
Nayor, J., Borges, L. F., Goryachev, S., Gainer, V. S. & Saltzman, J. R. Natural language processing accurately calculates adenoma and sessile serrated polyp detection rates. Dig. Dis. Sci. 63(7), 1794–1800 (2018).
Harkema, H. et al. Developing a natural language processing application for measuring the quality of colonoscopy procedures. J. Am. Med. Inform. Assoc. 18(S1), i150–i6 (2011).
Mehrotra, A. et al. Applying a natural language processing tool to electronic health records to assess performance on colonoscopy quality measures. Gastrointest. Endosc. 75(6), 1233–9.e14 (2012).
Imler, T. D. et al. Multi-center colonoscopy quality measurement utilizing natural language processing. ACG 110(4), 543–52 (2015).
Raju, G. S. et al. Natural language processing as an alternative to manual reporting of colonoscopy quality metrics. Gastrointest. Endosc. 82(3), 512–519 (2015).
Sohn, D. K. et al. Validation of an automated adenoma detection rate calculating system for quality improvement of colonoscopy. Ann. Surg. Treat. Res. 97(6), 319–325 (2019).
Carrell, D. S. et al. Challenges in adapting existing clinical natural language processing systems to multiple, diverse health care settings. J. Am. Med. Inform. Assoc. 24(5), 986–991 (2017).
Roth, M. Epic Dominates EMR Market Share Wars; Cerner Loses Ground. HealthLeaders; 2020 [updated April 30]. https://www.healthleadersmedia.com/innovation/epic-dominates-emr-market-share-wars-cerner-loses-ground.
Flint, A. et al. Automated extraction of structured data from text notes in the electronic medical record. J. Gen. Intern. Med. 36, 2880–2882 (2020).
Kaltenbach, T. et al. Adenoma detection rate (ADR) irrespective of indication is comparable to screening ADR: Implications for quality monitoring. Clin. Gastroenterol. Hepatol. 19, 1883–1889 (2021).
Kahi, C. J. M. D. M., Ballard, D. M. D., Shah, A. S. M. D., Mears, R.M.S.N.A.-B.C.R.N. & Johnson, C. S. M. A. Impact of a quarterly report card on colonoscopy quality measures. Gastrointest. Endosc. 77(6), 925–31 (2013).
Coe, S. G., Crook, J. E., Diehl, N. N. & Wallace, M. B. An endoscopic quality improvement program improves detection of colorectal adenomas. Am. J. Gastroenterol. 108(2), 219–26 (2013) (quiz 27).
Rex, D. K. & Ponugoti, P. L. Calculating the adenoma detection rate in screening colonoscopies only: Is it necessary? Can it be gamed?. Endoscopy 49(11), 1069–1074 (2017).
Bodagh, N. et al. Feasibility of real-time capture of routine clinical data in the electronic health record: A hospital-based, observational service-evaluation study. BMJ Open 8(3), e019790 (2018).
Dorn, S. D. et al. An integrated electronic health record-based workflow to improve management of colonoscopy-generated pathology results. Clin. Exp. Gastroenterol. 11, 391–397 (2018).
Funding
Funding for this work provided by Clinical Effectiveness and Patient Safety Grant from the University of Colorado Department of Medicine and assistance from the Colorado Clinical & Translational Sciences Institute (CCTSI) with the Development and Informatics Service Center (DISC) grant support (NIH/NCRR Colorado CTSI Grant Number UL1 RR025780).
Author information
Authors and Affiliations
Contributions
B.J. and S.G.P. had full access to all of the data and in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and Design: B.J., J.P., B.G.P. Acquisition, analysis, or interpretation of data: B.J., J.E., B.G.P., F.I.S., S.W., G.A., S.E., M.C. Drafting of the manuscript: B.J., B.G.P. Critical revision of the manuscript for important intellectual content: F.I.S., S.W., J.P., S.E., G.A. Statistical analysis: B.J., B.G.P. Obtained funding: B.J., B.G.P. Administrative, technical, or material support: J.E., S.L., M.C., G.A., S.E. Supervision: S.G.P. Final Review and Approval of the Manuscript: B.J., F.I.S., S.E., S.L., M.C., S.W., S.E., G.A., J.P., S.G.P.
Corresponding author
Ethics declarations
Competing interests
Scott: Research funding from Janssen Pharmaceuticals, Takeda Pharmaceuticals USA, and the Crohn’s and Colitis Foundation; and has received personal fees from PRIME Incorporated, Janssen Pharmaceuticals, Takeda Pharmaceuticals, and IBD REMEDY. Wani: Consultant Boston Scientific, Medtronic, Cernostics, Interpace. Edmundowicz: Consultant for and on the advisory board for Provation and Olympus. Patel: Freenome Inc (research support), Olympus America (research support), ERBE USA (honorarium). The other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jones, B., Scott, F.I., Espinoza, J. et al. Leveraging electronic medical record functionality to capture adenoma detection rate. Sci Rep 12, 9679 (2022). https://doi.org/10.1038/s41598-022-13943-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13943-2