Abstract
In vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) is associated with an increased risk of preterm (33rd–37th gestational week) and early preterm birth (20th–32nd gestational week). The underlying general and procedure related risk factors are not well understood so far. 4328 infertile women undergoing IVF/ICSI were entered into this study. The study population was divided into three groups: (a) early preterm birth group (n = 66), (b) preterm birth group (n = 675) and (c) full-term birth group (n = 3653). Odds for preterm birth were calculated by stepwise multivariate logistic regression analysis. We identified seven independent risk factors for preterm birth and four independent risk factors for early preterm birth. Older (> 39) or younger (< 25) maternal age (OR: 1.504, 95% CI 1.108–2.042, P = 0.009; OR: 2.125, 95% CI 1.049–4.304, P = 0.036, respectively), multiple pregnancy (OR: 9.780, 95% CI 8.014–11.935, P < 0.001; OR: 8.588, 95% CI 4.866–15.157, P < 0.001, respectively), placenta previa (OR: 14.954, 95% CI 8.053–27.767, P < 0.001; OR: 16.479, 95% CI 4.381–61.976, P < 0.001, respectively), and embryo reduction (OR: 3.547, 95% CI 1.736–7.249, P = 0.001; OR: 7.145, 95% CI 1.990–25.663, P = 0.003, respectively) were associated with preterm birth and early preterm birth, whereas gestational hypertension (OR: 2.494, 95% CI 1.770–3.514, P < 0.001), elevated triglycerides (OR: 1.120, 95% CI 1.011–1.240, P = 0.030) and shorter activated partial thromboplastin time (OR: 0.967, 95% CI 0.949–0.985, P < 0.001) were associated only with preterm birth. In conclusion, preterm and early preterm birth risk factors in patients undergoing assisted IVF/ICSI are in general similar to those in natural pregnancy. The lack of some associations in the early preterm group was most likely due to the lower number of early preterm birth cases. Only embryo reduction represents an IVF/ICSI specific risk factor.
Similar content being viewed by others
Introduction
There are more than 15 million preterm infants every year among the world, accounting for 10% of the total newborn infants, of which the incidence of preterm delivery in China ranks the second. Preterm delivery accounts for more than 75% of perinatal morbidity and mortality worldwide1. Furthermore, those infants who do survive have higher rates of long-term morbidities, including cardiovascular diseases2 as well as neurologic and developmental disabilities, compared to infants born full term. Known maternal risk factors for preterm birth in the general population include having a previous premature birth, twin pregnancy, an interval of less than six months between pregnancies, history of multiple miscarriages or abortions, smoking cigarettes or using illicit drugs, cardio-metabolic diseases such as hypertension or diabetes, and infections, particularly of the amniotic fluid and lower genital tract3,4,5,6,7. Conceiving through in vitro fertilization represents another risk factor in a subgroup of women undergoing assisted reproduction technologies (ART). The risk factors increasing the likelihood for preterm birth in this particular population are, however, as of today, not well understood. Numerous studies8 analyzed maternal and offspring outcomes after ART, whereas larger sized studies focusing specifically on the risk factors for preterm birth and especially early preterm birth especially associated to poor offspring outcome are lacking. However, this is a clinically important topic, since the understanding of ART related risk factors for preterm birth might identify changeable factors offering potential treatment options to improve offspring short term and long-term outcome in women undergoing ART. The aim of the current study was to identify causes or medical reasons for preterm and early preterm birth in a large cohort of women who underwent ART.
Methods
Ethics, inclusion and exclusion criteria, data collection
The study was approved by the ethics committee of Reproductive & Genetic Hospital of Citic-Xiangya, Changsha, China (approval document number: LL-SC-2019-003). A total of 4349 infertile women who had undergone IVF/ICSI treatment and obtained live birth in the Reproductive & Genetic Hospital of Citic-Xiangya from January 1st, 2016 to December 30th, 2017 were enrolled.
Inclusion criteria were as follows:
-
(a)
Women treated with super-ovulation protocols exactly as described previously 9
-
(b)
Fresh embryo transfer recipients, who received IVF/ICSI treatment
-
(c)
Giving live birth after ART
Exclusion criteria were as follows:
-
(a)
Using donor sperms or donor eggs for ART
-
(b)
Complete clinical data were not available
-
(c)
Post-term birth (> 42nd week of gestation) cases were excluded
-
(d)
Infertile couples with known female or male genetic causes of infertility
Gestational age was calculated by adding 2 weeks (14 days) to the number of days since fertilization10. Note: Gestational age was determined as the 17th day of gestation when a 6–8 cell embryo was transferred into the uterus and as the 19th day of gestation when a blastocyst was transferred.
Full-term birth was defined as a live birth with a gestational age between 37 but not over 42 weeks (37 weeks ≤ gestational age < 42 weeks). Preterm birth was defined as a live birth with a gestational age of at least 20 but not over 37 weeks (20 weeks ≤ gestational age < 37 weeks)11. Early preterm birth was defined as a live birth with a gestational age between 20 but not over 32 weeks (20 weeks ≤ gestational age < 32 weeks)12.
All patient’s data (clinical data as well as laboratory data) used in our study were extracted from the routine electronic patient records used in our hospital.
Clinic data collection
Informed consent was obtained from all subjects and/or their legal guardian(s). A structured medical history was taken. The following risk factors for preterm birth were examined in this study:
Maternal risk factors: (1) basic parameters before super-ovulation: nationality, education, age, body height, body weight, infertility duration, types of infertility, causes of infertility (maternal causes, paternal causes, maternal and paternal causes, unknown causes), blood pressure readings. (2) Pregnancy history: parity, artificial abortion, drug abortion, spontaneous abortion, ectopic pregnancy, number of deliveries, vaginal delivery, cesarean section (3) blood test result before super-ovulation: liver and kidney function, lipid items, blood coagulation function. (4) Pregnancy related factors: multiple pregnancy, embryo reduction, gestational diabetes, gestational hypertension, placenta previa.
Relevant risk factors during IVF/ICSI procedure: cycle count, fertilization way, embryo transfer type (blastocyst or cleavage stage embryo transfer), ovulation induction scheme, source of sperm, transferred embryo count, dosage of gonadotropin, ovulation inducing days.
Offspring data: gestational age at delivery, gender, birth weight.
Basic parameters about the mother, pregnancy history, gynecological complications, and relevant risk factors during IVF/ICSI procedure came from the case report in the hospital. Furthermore, blood test results from maternal blood taken before the beginning of superovulation was extracted from the case report. Pregnancy related factors and offspring data were followed up strictly by a special nurse.
Patient and public involvement
This study is a retrospective study. Data were obtained through the electronical medical record system of the hospital. Patients were not directly involved in this study. The patients were unaware of the results of the study.
Statistical analysis
Continuous variables are represented as mean ± SD for normally distributed variables and student’s unpaired t-test was used for comparison of variables between two groups. Continuous variables are represented as median and quartiles M (Q1 − Q3) for non-normally distributed variables and Mann–Whitney nonparametric test was used for comparison of variables between groups. Categorical variables are described as frequency and percentages. Pearson’s chi-square test was used for testing qualitative data and Fisher's exact test was used when the expected frequencies were < 5%.
Multivariate logistic regression analyses with step forward selection using the likelihood method were applied to examine the association between the patient’s characteristics and the risk of preterm brith. Analyzed variables with P < 0.05 in the univariate analysis were entered into the multivariate analysis. No other factors were considered as confounders. Results are represented as ORs with corresponding 95% CIs and P values.
Statistical package for social sciences (SPSS version 22.0, Chicago, IL, USA) was used to perform all data analyses and a two-sided P value < 0.05 was considered to be statistically significant.
All methods were carried out in accordance with relevant guidelines and regulations of the People Republic of China.
Ethics approval
This study was approved by the ethics committee of the Reproductive and Genetic Hospital of CITIC-Xiangya, Changsha, China (approval number: ll-sc-2019-003). The data of this study is only used for this study, and the data of patients are strictly confidential. This study will not cause any harm to the patients' body and mind.
Results
Description of the cohort
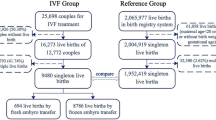
From the primary dataset of 4349 treated women, we excluded 13 cases in which the gestational age was unclear and 8 cases that were post-term pregnancies. Thus finally 4328 cases were included into the study, 3653 of them were full-term deliveries. The prevalence of preterm birth and early preterm birth was 15.5% and 1.8% respectively (Fig. 1). The median age of the participating women was 30 (27, 33) years old. The median BMI of the women was 21.94 (20.08, 23.76) kg/m2. The study cohort consisted mostly of Han Chinese women (87.8%). Caused for infertility in the entire study populations were distributed as follows: primary infertility was present in 42.9% and secondary infertility in 57.1%. The duration of infertility was 4 (2, 6) years. Median birthweight was 3100 (2700, 3500) g, Median gestational age was 38.4 (37.3, 39.4) weeks. For more details see Tables 1 and 2.
Univariate analysis showed that 15 parameters were significantly different between the full-term birth group and the preterm birth group (P < 0.05), including 6 maternal parameters (age, apolipoprotein B, total cholesterol, triglycerides, low density lipoprotein and activated partial thromboplastin time), 5 pregnancy related factors (multiple pregnancy, embryo reduction, placenta previa, gestational diabetes and gestational hypertension), 2 factors related to the IVF/ICSI procedure (embryo transfer type and number of embryos transferred) and 2 offspring related factor (infant sex and intrauterine growth retardation) (Tables 1, 2, 3).
Moreover, univariate analysis comparing early preterm birth and full-term birth showed that 10 factors were significantly different between the full-term birth group and the early preterm birth group (P < 0.05), including 3 maternal parameters (age, apolipoprotein A1, and thrombin time), 3 pregnancy related factors (multiple pregnancy, embryo reduction, and placenta previa), 3 factors related to the IVF/ICSI procedure (embryo transfer type, treatment cycles and number of embryos transferred), 1 offspring related factor (infant sex) (Tables 1, 2, 3).
Multivariate analysis
The above 15 detected factors in the univariate analysis were entered into the multivariate logistic analysis. No other factors were considered as confounders. After stepwise regression analysis, multivariate analysis showed that 7 factors (older or younger maternal age, multiple pregnancy, embryo reduction, placenta previa, gestational hypertension, higher triglycerides and shorter activated partial thromboplastin time) were left in the multivariate analysis model for preterm birth. With regard to early preterm birth, the above described 10 factors, see above, were entered into the multivariate analysis. After stepwise regression analysis, 4 factors (older or younger maternal age, multiple pregnancy, embryo reduction and placenta previa) remained significant in the multivariate analysis model for early preterm birth (Tables 4, 5).
The results of the multivariate analyses showed that compared to the maternal age group of 25–39 years, younger mothers (20–24 years) and also older mothers (> 40 years old) displayed a significantly increased preterm birth ratio by 0.50 times (OR = 1.504, 95% CI 1.108–2.042, P = 0.009) and early preterm birth ratio by 1.13 times (OR = 2.125, 95% CI 1.049–4.304, P = 0.036). Compared to singleton pregnancies, mothers with multiple pregnancies had a significantly increased preterm birth ratio by 8.78 times (OR = 9.780, 95% CI 8.014–11.935, P < 0.001) and early preterm birth ratio by 7.58 times (OR = 8.588, 95% CI 4.866–15.157, P < 0.001). Embryo reduction significantly increased preterm birth ratio by 2.54 times (OR = 3.547, 95% CI 1.736–7.249, P = 0.001) and early preterm birth ratio by 6.14 times (OR = 7.145, 95% CI 1.990–25.663, P = 0.003). Placenta previa increased also significantly both preterm birth ratio by 13.95 times (OR = 14.954, 95% CI 8.053–27.767, P < 0.001) and early preterm birth ratio by 15.48 times (OR = 16.479, 95% CI 4.381–61.976, P < 0.001). The presence of gestational hypertension, higher triglycerides and a shorter activated partial thromboplastin was significantly associated with preterm birth (OR = 2.494, 95% CI 1.770–3.514, P < 0.001; OR = 1.120, 95% CI 1.011–1.240, P = 0.030; OR = 0.967, 95% CI 0.949–0.985, P < 0.001, respectively) but not early preterm birth (P > 0.05) (Tables 4, 5).
Discussion
Our study showed that older (> 39) or younger (< 25) maternal age, multiple pregnancy, placenta previa, and embryo reduction surgery were associated with an increased risk for both preterm birth and early preterm birth after IVF/ICSI. Gestational hypertension, higher triglycerides and a shorter activated partial thromboplastin time were only associated with an increased occurrence of preterm birth but not early preterm birth. However, the lack of some associations in the early preterm group was most likely due to the lower sample size.
The variable “preterm birth” used in this study includes spontaneous and iatrogenic preterm birth. In several studies, it was already shown that the risk of preterm birth among singleton IVF/ICSI pregnancies was significantly higher than that occurring in spontaneous conceptions 13. A recent meta-analysis of cohort studies demonstrated that the incidence of preterm birth in IVF/ICSI group and naturally conceived controls are 4.73% and 1.81%, respectively14. The underlying risk factors for preterm birth in this population, however, are not fully established so far. The current study, investigating a cohort of women who underwent ART, identified several factors which were associated with preterm birth. The majority of the identified factors, such as maternal age15, multiple pregnancies, placenta previa, gestational hypertension, high triglycerides and hypercoagulability16,17, have previously been shown to be associated with an increased risk for preterm birth in the general population as well.
Our study showed an increased risk for preterm delivery in association with maternal age. Both younger and older women after ART treatment had an increased risk for preterm birth in our study as it was likewise seen in studies addressing this topic in the general population. Somewhat smaller study done in an ART population mainly reported similar associations 18. However, there is also a study with a similar study design as our study showing that women aged 25–29 were at an increased risk for preterm birth in comparison to women aged 30–34. Women aged ≥ 35 years did not display an increased risk of any type of preterm birth19. These previous findings may suggest that while there is a positive association between maternal age and the risk for preterm birth, younger women who conceived via ART may display a higher risk for preterm birth compared to older women who conceived undergoing ART. As the proportion of women who conceive with ART also shows an age-related increase, these results may also reflect the increased clinical risk of adverse birth outcomes among young women who needed ART to conceive18.
The current study also identified gestational hypertension as risk factor for preterm birth in women who needed ART to conceive. This finding is in line with the current literature, gestational hypertension was shown to be associated with an increased risk for preterm birth in both the general population and women who underwent ART. Moreover, it was shown that ART is associated with a higher frequency of gestational hypertension and preeclampsia as compared to natural pregnancy 20,21. The underlying reasons for a higher frequency of gestational hypertension in ART pregnancies remain incompletely understood. Wang et al. showed in a large cohort comparing ART pregnancies to natural conception that ART is associated with a higher prevalence for gestational hypertension, yet this association disappeared once data was stratified by multiple birth cases22. The authors concluded that multiple pregnancy which is associated with ART is the single most likely explanation for the increased rate of gestational hypertension among ART mothers.
Another risk factor for preterm birth found by the current study is placenta previa. Placenta previa is associated with preterm birth in the general population as well 23. A study that investigated mothers who had conceived both naturally and via ART, showed that the risk of placenta previa was three‐fold higher in the ART pregnancy24. The mechanisms underlying this phenomenon still have to be elucidated. It is hypothesized that ART related procedures, such as an induction of uterine contractions due to transcervical catheter insertion or the unique endocrinological environment with high estradiol concentrations following ART cycles might be responsible25.
Regarding maternal laboratory parameters recorded before super-ovulation, the current study demonstrated a positive association between maternal triglycerides, shorter activated partial thromboplastin time and preterm birth. Both high triglycerides and hypercoagulability were already shown to be associated with an increased risk for preterm birth in the general population26,27. Pregnancy is a hypercoagulable state with an increased thrombotic risk throughout gestation and the postpartum period. Women with thrombophilia may have a further increased risk of placental vascular complications, including pregnancy loss, preeclampsia, intrauterine growth restriction, and placental abruption. Accumulating data suggest that maternal antithrombotic prophylaxis may result in improved gestational outcome28. Results from one small prospective study analyzing the relationship between maternal hypercoagulability and preterm labor in 76 women demonstrated a statistically significant procoagulant activity, expressed by a shorter prothrombin time and activated partial thromboplastin time, in pregnant women with premature uterine contractions who gave birth prematurely27. The presence of hypercoagulation before starting an IVF treatment was shown to be associated with negative IVF outcomes such as pregnancy loss. However, the mechanism of hypercoagulability associated with preterm birth is not extensively explored. Our study clearly established hypercoagulability as a risk factor for preterm birth in ART and hence may help to clarify ongoing debates on this subject29.
Regarding triglycerides, pregnancy is typified by an increase in serum levels of total cholesterol and triglycerides pushed by the rise in estrogen, progesterone and placental lactogen. Existing evidence has demonstrated that high maternal triglycerides levels during pregnancy are related to an increased risk for preterm birth in both obese women and in women with normal BMI in the general population conceiving naturally 26. In a retrospective cohort of 2.9 million pregnant women in California, maternal diagnosis of dyslipidemia was significantly associated with increased risk for preterm birth30. Also, some studies investigated lipid levels and ART outcomes and showed that maternal triglyceride was inversely associated with live birth rate. However, data regarding the relationship between maternal triglycerides and preterm birth in the setting of ART are scarce31. Our findings may motivate further study regarding potential benefits of the treatment of hypercoagulability and hyperlipidemia in pregnancy and its effects on maternal–fetal outcomes.
Our study is in good agreement with previous studies indicating that multiple pregnancy is a strong risk factor for preterm birth, both in the general population as well as in women who conceived trough ART32. Multiple pregnancy is considered one of the largest hazards of ART. Until now, ART is associated with a high number of multiple pregnancies due to the current policy transferring multiple embryos simultaneously to achieve a high pregnancy rate33. To reduce the risks associated with multiple pregnancy, embryo reduction is performed frequently. Previous meta-analyses have shown that embryo reduction improves outcomes in triplet pregnancies, but never to that degree of singleton pregnancies. An effective method to reduce the risk of multiple births in ART is an elective single embryo transfer, a policy that is adopted by an increasing number of guidelines. However, studies also demonstrated that elective single embryo transfer is not associated with a reduction in the risk for preterm delivery34.
Our study has limitations. Firstly, the data of maternal pregestational diabetes, chronic hypertension and PE are missing in the electronic database. We mainly focused on causes or medical reasons for preterm birth. Secondly, only fresh embryo transfer recipients who received IVF/ICSI treatment were included in the current study. It is likely that fresh and frozen embryo transfer present different risks of PTB. We will therefore carry out frozen embryo transfers analyses in the future (“Supplementary information”).
In conclusion, our study demonstrated that maternal age, multiple pregnancy, embryo reduction and placenta previa could increase the risk of preterm birth in women undergoing IVF/ICSI. During ART treatment, the numbers of embryo transfers per cycle should be reduced to two or even to one thus reducing the need for embryo reduction procedures or multiple pregnancy. Strengthening antenatal care is necessary during pregnancy, especially for the patients with placenta previa. The finding that coagulation abnormalities are linked to preterm birth needs independent confirmation and if confirmed may stimulate clinical trials testing drugs interfering with the coagulation system as it is for example done in pregnant women suffering from activated protein C resistance35.
References
Slattery, M. M. & Morrison, J. J. Preterm delivery. Lancet (London, England) 360, 1489–1497 (2002).
Tian, M., Reichetzeder, C., Li, J. & Hocher, B. Low birth weight, a risk factor for diseases in later life, is a surrogate of insulin resistance at birth. J. Hypertens. 37, 2123–2134 (2019).
Smid, M. C. et al. Maternal race and intergenerational preterm birth recurrence. Am. J. Obstet. Gynecol. 217(480), e481-480.e489 (2017).
Orbach, H. et al. Hypertension and antihypertensive drugs in pregnancy and perinatal outcomes. Am. J. Obstet. Gynecol. 208(301), e301-306 (2013).
Mutsaerts, M. A. et al. Effects of paternal and maternal lifestyle factors on pregnancy complications and perinatal outcome. A population-based birth-cohort study: the GECKO Drenthe cohort. Hum. Reprod. (Oxford, England) 29, 824–834 (2014).
Margerison-Zilko, C. E., Talge, N. M. & Holzman, C. Preterm delivery trends by maternal race/ethnicity in the United States, 2006–2012. Ann. Epidemiol. 27, 689-694.e684 (2017).
Goisis, A., Remes, H., Barclay, K., Martikainen, P. & Myrskylä, M. Advanced maternal age and the risk of low birth weight and preterm delivery: A within-family analysis using finnish population registers. Am. J. Epidemiol. 186, 1219–1226 (2017).
Sha, T., Yin, X., Cheng, W. & Massey, I. Y. Pregnancy-related complications and perinatal outcomes resulting from transfer of cryopreserved versus fresh embryos in vitro fertilization: A meta-analysis. Fertil. Steril. 109, 330-342.e339 (2018).
Li, Y. et al. Cumulative live birth rates in low prognosis patients according to the Poseidon criteria: An analysis of 26,697 cycles of in vitro fertilization/intracytoplasmic sperm injection. Front. Endocrinol. 10, 642 (2019).
Zegers-Hochschild, F. et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil. Steril. 92, 1520–1524 (2009).
Di Renzo, G. C. et al. Preterm labor and birth management: Recommendations from the European Association of Perinatal Medicine. J. Maternal-Fetal Neonatal Med. 30, 2011–2030 (2017).
Tul, N. et al. The contribution of twins conceived by assisted reproduction technology to the very preterm birth rate: A population-based study. Eur. J. Obstet. Gynecol. Reprod. Biol. 171, 311–313 (2013).
Pinborg, A. et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum. Reprod. Update 19, 87–104 (2013).
Cavoretto, P.I. et al. IVF/ICSI treatment and the risk of iatrogenic preterm birth in singleton pregnancies: Systematic review and meta-analysis of cohort studies. J. Matern. Fetal Neonatal. Med. 35(10), 1987–1996. https://doi.org/10.1080/14767058.2020.1771690 (2022).
Fuchs, F., Monet, B., Ducruet, T., Chaillet, N. & Audibert, F. Effect of maternal age on the risk of preterm birth: A large cohort study. PLoS ONE 13, e0191002 (2018).
Zlatnik, M. G., Cheng, Y. W., Norton, M. E., Thiet, M. P. & Caughey, A. B. Placenta previa and the risk of preterm delivery. J. Maternal-Fetal Neonatal Med. 20, 719–723 (2007).
Khazaeipour, Z., Shirazi, M., Niromanesh, S., Dastgerdy, E. & Sharbaf, F. R. Association of hypertriglyceridaemia with gestational diabetes and adverse pregnancy outcomes. Endocr. Pract. 23, 7A (2017).
Ogawa, K. et al. Association between very advanced maternal age and adverse pregnancy outcomes: A cross sectional Japanese study. BMC Pregnancy Childbirth 17, 349 (2017).
Xiong, X., Dickey, R. P., Pridjian, G. & Buekens, P. Maternal age and preterm births in singleton and twin pregnancies conceived by in vitro fertilisation in the United States. Paediatr. Perinat. Epidemiol. 29, 22–30 (2015).
Almasi-Hashiani, A. et al. Assisted reproductive technology and the risk of preeclampsia: An updated systematic review and meta-analysis. BMC Pregnancy Childbirth 19, 149 (2019).
Xu, X. K., Wang, Y. A., Li, Z., Lui, K. & Sullivan, E. A. Risk factors associated with preterm birth among singletons following assisted reproductive technology in Australia 2007–2009—A population-based retrospective study. BMC Pregnancy Childbirth 14, 406 (2014).
Wang, Y. A. et al. Increased incidence of gestational hypertension and preeclampsia after assisted reproductive technology treatment. Fertil. Steril. 105, 920-926.e922 (2016).
Vahanian, S. A., Lavery, J. A., Ananth, C. V. & Vintzileos, A. Placental implantation abnormalities and risk of preterm delivery: a systematic review and metaanalysis. Am. J. Obstet. Gynecol. 213, S78-90 (2015).
Jackson, R. A., Gibson, K. A., Wu, Y. W. & Croughan, M. S. Perinatal outcomes in singletons following in vitro fertilization: A meta-analysis. Obstet. Gynecol. 103, 551–563 (2004).
Farhi, J. et al. High serum oestradiol concentrations in IVF cycles increase the risk of pregnancy complications related to abnormal placentation. Reprod. Biomed. Online 21, 331–337 (2010).
Lin, X. H. et al. Maternal high triglyceride levels during early pregnancy and risk of preterm delivery: A retrospective cohort study. J. Clin. Endocrinol. Metab. 104, 1249–1258 (2019).
Keren-Politansky, A., Breizman, T., Brenner, B., Sarig, G. & Drugan, A. The coagulation profile of preterm delivery. Thromb. Res. 133, 585–589 (2014).
Brenner, B. Thrombophilia and pregnancy complications. Pathophysiol. Haemost. Thromb. 35, 28–35 (2006).
Ata, B. & Urman, B. Thrombophilia and assisted reproduction technology-any detrimental impact or unnecessary overuse?. J. Assist. Reprod. Genet. 33, 1305–1310 (2016).
Smith, C. J. et al. Maternal dyslipidemia and risk for preterm birth. PLoS ONE 13, e0209579 (2018).
Jamro, E. L. et al. Preconception serum lipids and lipophilic micronutrient levels are associated with live birth rates after IVF. Reprod. Biomed. Online 39, 665–673 (2019).
Stock, S. & Norman, J. Preterm and term labour in multiple pregnancies. Semin. Fetal Neonatal. Med. 15, 336–341 (2010).
Ledger, W. & Johnson, M. H. One plus one equals two: Why fetal reduction is always a second-best solution. Reprod. Biomed. Online 32, 467–468 (2016).
Fechner, A. J. et al. Effect of single embryo transfer on the risk of preterm birth associated with in vitro fertilization. J. Assist. Reprod. Genet. 32, 221–224 (2015).
de Vries, J. I., van Pampus, M. G., Hague, W. M., Bezemer, P. D. & Joosten, J. H. Low-molecular-weight heparin added to aspirin in the prevention of recurrent early-onset pre-eclampsia in women with inheritable thrombophilia: the FRUIT-RCT. J. Thrombosis Haemostasis JTH 10, 64–72 (2012).
Acknowledgements
We would like to thank the hospital staff helping us to get the data from the electronic records. We are also grateful to the key grant of research and development in Hunan Province (2020DK2002), and Huxiang High-Level Talent Innovation Team (2018RS3072).
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was supported by the Hunan province college students research learning and innovative project Grant (S201910542038) and Grant of research and development in Hunan Province (2020DK2002).
Author information
Authors and Affiliations
Contributions
J.L., F.G., G.L. and B.H. designed the study. J.S., S.Z., J.L. collected the data. J.S., X.Z., Y.P., B.H., and Q.Z. checked quality of the data. J.S. and Y.P. performed the statistical analysis. J.S. drafted the manuscript. L.H., C.R. and M.T. contributed to the data interpretation and revised drafts of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Shen, J., Zhang, X. et al. Risk factors associated with preterm birth after IVF/ICSI. Sci Rep 12, 7944 (2022). https://doi.org/10.1038/s41598-022-12149-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12149-w
This article is cited by
-
The effect of fertility treatment and socioeconomic status on neonatal and post-neonatal mortality in the United States
Journal of Perinatology (2024)
-
Nomogram for predicting the risk of preterm birth in women undergoing in vitro fertilization cycles
BMC Pregnancy and Childbirth (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.