Abstract
The relationship between renal impairment and diabetic peripheral neuropathy (DPN) remains inconclusive. We aim to investigate the risk factors for the occurrence of DPN in Taiwanese adults with type 2 diabetes mellitus (T2DM) and focus on renal impairment. A hospital-based study was conducted from 2013 to 2019 and 552 Taiwanese people who had T2DM without DPN at baseline were enrolled. DPN was diagnosed using the Michigan Neuropathy Screening Instrument. Potential risk factors were recorded, including patient’s sociodemographic factors, current medication usage and biochemical markers. As of 2019, 73 developed DPN and 479 had no DPN. The cumulative incidence during the 6-year period was 13.22%. Multivariable logistic regression analysis revealed that lower estimated glomerular filtration rate (eGFR) (odds ratio [OR] 0.98, p = 0.005), advanced age (OR 1.06, p = 0.001), increased body weight (OR 1.04, p = 0.018), duration of DM (OR 1.05, p = 0.036) and male gender (OR 3.69, p = 0.011) were significantly associated with future DPN. In addition, patients with T2DM under the age of 65 with higher serum creatinine concentration (OR 8.91, p = 0.005) and higher baseline HbA1C (OR 1.71, p < 0.001) revealed significantly associated with future DPN. In conclusion, this is the first large scaled hospital-based study with long term follow-up to investigate risk factors for DPN in Taiwanese. Lower eGFR and higher serum creatinine concentration, particularly in people under the age of 65, are predictors of future DPN in Taiwanese people with T2DM. Other predictors included advanced age, increased body weight, duration of DM, male gender for all ages and HbA1c in enrolled patients under the age of 65. Our study not only confirms the association between renal impairment and future DPN but also provides a commonly available assessment to predict the future DPN.
Similar content being viewed by others
Introduction
The global burden of diabetes mellitus (DM) has increased enormously in recent decades and will continue to soar in the next few decades. In fact, the global incidence of diabetes has increased by 102.9% from 11.3 million in 1990 to 22.9 million in 2017. Consequently, the prevalence of the complications resulting from type 2 diabetes (T2DM) is likely to rise1.
DPN is the most common complication, and its lifetime prevalence is up to 50% in adults with T2DM2. DPN is associated with a wide range of clinical manifestations, of which distal sensory neuropathy is predominant. This manifestation contributes to numerous disabling morbidities, such as diabetic foot ulceration, impaired balance, and distressing neuropathic pain, which are often difficult to treat. Furthermore, DPN is the most common cause of non-traumatic lower-limb amputations in most high-income countries3. The current study focuses on distal and symmetric polyneuropathy.
Unfortunately, the early manifestations of this insidious disease are often missed until the disease is well established, at which point it seems to be irreversible2. There is a lack of treatments that target the underlying nerve damage other than serum glucose control, which shows limited efficacy in T2DM4. Thus, prevention is the critical component of diabetes care to reduce the burden of care. Previous studies have reported risk factors that include older age, hyperglycemia, longer diabetes duration, metabolic syndrome and dyslipidemia5,6. For dyslipidemia, increased low-density lipoprotein (LDL)7 and triglycerides (TG)8 have been identified as predictors of diabetic sensory neuropathy in type 1 DM. In contrast, it remained inconclusive in T2DM10,11 and there were some studies reported high level of TG and low level of high-density lipoprotein (HDL) as risk factors3,9.
Apart from these, it attracts much more attention that whether renal impairment was a predictor of future diabetic peripheral neuropathy. Conflicting data have been reported between renal impairment and future DPN12,13. As far as we know, recent studies have not described a definite list of risk factors of DPN, especially renal impairment, which may be due to the majority of studies having cross-sectional designs. Longitudinal studies are the key tools to establish predictors of the development of DPN. Therefore, the objective of the current study was to investigate the predictors for future DPN in Taiwanese adults with T2DM and focus on impaired renal function. Look forward to help improve therapeutic strategies in clinical practice.
Methods
Study design and participants
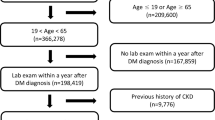
This is a hospital-based, prospective, observational study. Between January 2013 and October 2013, patients over 18 years old with prevalent or newly diagnosed T2DM were eligible for inclusion. The diagnosis of T2DM were based on the criteria of American Diabetes Association (ADA). Data were obtained from patient’s medical records, laboratory examinations, questionnaires and anthropometric measurements at the time of enrollment. Exclusion criteria were as follows: patients having type 1 DM or gestational diabetes, patients had DPN at baseline and whose did not complete the questionnaires or blood sample test at baseline or during the following 6 years. Finally, 552 participants were enrolled in our study.
Participants have been followed observationally via clinical follow-up examination and questionnaires. The blood sample test was performed at least once a year. Our study consequently carried out to 2019—6 years after the trial baseline.
Each of the participants was diagnosed by endocrinologists in the outpatient units at a tertiary medical center in middle Taiwan, which serves approximately 6600 outpatients and 1400 inpatients per day and mainly Han-Chinese population. Before drawn for analysis, the patients’ information was anonymized by computer system, and the researchers were blinded to these data. The study was approved by the Institutional Review Board of Taichung Veterans General Hospital (CG18082B-1). All participants volunteered for the current studies, and provided written informed consent prior to enrolment. Besides, all the methods were performed in accordance with relevant guidelines and regulations.
Anthropometric measurements
While entry the study, all participants received anthropometric measurements, which was performed by a case-management nurse. The sociodemographic factors included height, weight, waist circumference, duration of diabetes, smoking status and body mass index (BMI). For the details of anthropometric measurements, please refers to our published study6. Besides, we recorded the participants’ comorbidities and current medication usage at baseline. Comorbidities obtained from medical record and based on International Classification of Diseases, 9th revision Clinical Modification (ICD-9-CM) and 10th revision (ICD-10) which including hypertension (HTN; ICD-9-CM codes 401–405, ICD-10 codes I10-I15), cerebrovascular disease (CVD; ICD-9-CM codes 430–438, ICD-10 codes I60-I69), ischemic heart disease (IHD; ICD-9-CM codes 410–414, ICD-10 codes I20-I25), liver disease (ICD-9-CM codes 571–573, ICD-10 codes K70-K77). Current medication usage including oral hypoglycemic agent (OHA), insulin, antihypertensive drugs and lipid-lowering drugs such as statins and fibrates.
Biochemical data
Laboratory examination were administrated during endocrinological follow-up. Blood samples were obtained in the morning after an overnight fasting period from the antecubital vein. Fasting plasma glucose (FPG; using standard enzymatic methods), glycated hemoglobin (HbA1c; using high-performance liquid chromatography), serum creatinine concentration and plasma lipid profiles (using standard enzymatic methods), including total cholesterol (TC), HDL, LDL, and TG. For lipid profile, we defined the following cut-off points of pathologic values according to the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III14: TG: > 150 mg/dL; HDL: < 50 mg/dL in female and < 40 mg/dL in male. EGFR was estimated by the six-variable Modification of Diet in Renal Disease (MDRD) equation as the following equation:
For the patients who had received lipid-lowering drugs, their baseline lipid profiles were defined as the mean plasma lipid values (including TC, HDL, LDL and TG) in the 5 years prior to drug prescription and the follow-up lipid profiles were defined as the mean lipid values in the 5 years after medication prescription. For the individuals never received lipid-lowering drugs, the baseline lipid profiles were circumscribed as the 5-year mean plasma lipid values prior to study enrollment, from 2008 to 2012, and the follow-up lipid profiles were defined as the mean lipid values within the 5 years after study entry, from 2013 to 2017.
Assessment of diabetic peripheral neuropathy
All of the included patients received assessment of DPN at the time of enrollment and received second assessment after 6-year follow-up by the same trained and certificated care-management nurse to minimize the inter-rater reliability. DPN was evaluated based on the second component of MNSI. Physical appearance of feet, ulceration, ankle deep tendon reflexes, and the perception of light touch (using Semmes–Weinstein 5.07 10-g monofilament) and distal vibration (using 128-Hz tuning fork) were investigated. As previous validated studies in adults15, individuals whose MNSI examination (MNSIE) score > 2 were diagnosed with DPN.
Assessment of renal function
We evaluated participants’ baseline renal function with serum creatinine concentration and eGFR in 2013. The eGFR was estimated by MDRD equation which contains elements as serum creatinine, age and gender (a constant in the equation). Because the relationship between serum creatinine, age and eGFR is hyperbolic, we establish a model that do not adjust the serum creatinine, age and gender for eGFR in multivariable logistic regression analysis (Table 3) to statistic the interference between baseline eGFR and the occurrence of DPN.
Besides, it is well-established that serum creatinine had multiple limitations to represent the true renal function and age is an important factor among these16. Furthermore, renal function declines with advancing age. Recent research reported that serum creatinine concentration was not enough to represent a screening test for renal impairment in people aged 65 and above17. Because of there were high percentage (14.4% ~ 17% varied by gender) of people aged 65 and above had a serum creatinine concentration above the laboratory reported upper reference limit of normal18. Serum creatinine might lead to marked under-investigation and under-recognition of renal failure in this population. Thus we stratified the serum creatinine concentration by age group into age≧65 and age < 65. Each groups were carried out the multivariable logistic regression analysis (Table 4).
In addition, some previous studies revealed medications, baseline glycemic control, and comorbidities might bring about renal impairment17,19. Thus we conduct multivariable analysis for renal function which account for these three categories: baseline HbA1c and variables in mediation and comorbidities category that statistical significance level as P value less than 0.1 (P < 0.1) in addition to confounders which had shown a significant correlation. If P value > 0.1, we consider it might play a minor role in incident DPN pathogenesis. Because the relationship between hypertension and antihypertensive drugs is hyperbolic, we choose antihypertensive drugs instead of hypertension. Thus antihypertensive drugs of medication category, cerebrovascular disease of comorbidities category and baseline HbA1c enter the multivariable regression models for baseline eGFR and serum creatinine concentration (Tables 3, 4).
Statistical methods
Descriptive statistics were presented as the mean values ± standard deviation (SD) and as the numbers with percentages. We used Fisher’s exact test or chi-squared test to analyze categorical variables, while the analyses of continuous variables were conducted using independent t-test or paired t-test.
Multivariable logistic regression analyses were carried out to explore the effect of each identified independent variable on DPN. The multivariable regression models included all the confounders and the variables that had shown a significant correlation, and the adjusted odds ratios (OR) with 95% confidence interval (CI) were calculated between the comparison groups. The statistical significance level chosen was P value less than 0.05 (P < 0.05), and all tests were two-sided. All the data were analyzed using statistical package SAS version 9.4 for Windows.
Results
We recruited 681 participants who had T2DM at baseline in 2013. Of these, 116 (17%) who had DPN at baseline and 13 non-T2DM patients were excluded. Thus, 552 were deemed to be eligible to be included in the study. The participants’ median age was 59.7 ± 10.7 years, and 60.1% were males. The mean duration of diabetes was 15.2 ± 6.9 years, and the mean level of HbA1c was 7.4 ± 1.3%. Table 1 summarizes their sociodemographic factors, diabetes-related factors, biochemical factors, comorbidities, and medication usage.
We defined the patients who developed DPN during follow-up as the “incident DPN” group (n = 73). The cumulative incidence of DPN during 6 years of follow-up was 13.22%. The sociodemographic factors revealed that body weight (72.3 ± 11.3 kg vs. 68.3 ± 13.2 kg, p < 0.05), height (165 ± 8.1 cm vs. 163 ± 8.3 cm, p < 0.05) and the measures of SBP (133.5 ± 12.5 mmHg vs. 130.1 ± 13.1 mmHg, p < 0.05) was significantly higher at baseline in patients with incident DPN than in those without incident DPN. Incident DPN were older (65.5 ± 10.7 years vs. 58.8 ± 10.4 years, p < 0.001) and included more males (82.2% vs. 56.8%, p < 0.001). The diabetes-related factors revealed duration of DM was significantly longer in the incident DPN group (17.4 ± 6.9 years vs. 14.9 ± 6.9 years, p < 0.01).
The biochemical factors revealed serum creatinine concentration (1.1 ± 0.4 mg/dl vs. 0.9 ± 0.3 mg/dl, p < 0.01) were significantly higher at baseline in patients with incident DPN than in those without incident DPN. On the other hand, measures of baseline eGFR (77.8 ± 25.1 mL/min/1.73m2 vs. 87.7 ± 26.2 mL/min/1.73m2, p < 0.01) and HDL (48.4 ± 16.9 mg/dl vs. 52.5 ± 14.9 mg/dl, p < 0.05) were significantly lower for participants with incident DPN.
Patients’ comorbidities at baseline revealed no significant differences between groups, but HTN (75.3% vs. 64.9%, p = 0.08) and CVD (26.0%% vs. 16.9%, p = 0.06) were more common at baseline in patients with incident DPN than in those without it. The DPN and non-DPN groups showed no significant differences in BMI, waist circumference, smoking status, fasting glucose levels, HbA1c levels, OHA and insulin usage, prescriptions of antihypertensive drugs and lipid-lowering drugs, DBP, urine albumin-creatinine ratio (UACR), pathologic high level of TG and LDL, cholesterol nor alanine aminotransferase levels.
Multivariable logistic regression model
Table 2 shows the adjusted odds ratio for risk factors of incident DPN from the multivariable logistic regression model. Advanced age was associated with an increased risk of DPN (odds ratio [OR] 1.06 [95% CI 1.02; 1.09], p = 0.001). Increased weight (OR 1.04 [95% CI 1.01; 1.07], p = 0.018) and male gender (OR 3.69 [95% CI 1.35; 10.09], p = 0.011) were significantly associated with a higher risk of DPN. Duration of DM (OR 1.05 [95% CI 1.00; 1.09], p = 0.036) was significantly associated with a higher risk of DPN as well. In contrast, height, lower HDL and baseline SBP revealed no statistically significant associations with the risk of DPN after adjustment for all confounding factors.
Comparison of baseline renal function between patients with or without incident DPN
After adjusted for height, weight, SBP, duration of diabetes, HbA1c, eGFR, HDL-C, cerebrovascular disease and antihypertensive drugs, we found that higher baseline eGFR (OR 0.98 [95% CI 0.97; 0.99], p = 0.005) was significantly associated with a lower risk of DPN (Table 3). In addition, the stratified analysis also revealed that a higher baseline serum creatinine concentration (OR 8.91 [95% CI 1.92; 41.27], p = 0.005) was independently and significantly associated with incident DPN in enrolled patients under the age of 65 (Table 4). In contrast, there were no significant associations between baseline serum creatinine concentration (OR 0.77 [95% CI 0.17; 3.40] p = 0.728) and incident DPN in the elderly (age≧65 years, Table 4). Besides, baseline HbA1c revealed significantly associated with incident DPN (OR 1.71 [95% CI 1.30; 2.24], p < 0.001) in people under the age of 65 in Table 4.
Discussion
To our knowledge, this is the first large scaled observational study to investigate risk factors for DPN in a Taiwanese adult population. Using MNSIE for the diagnosis of DPN, we found that participants without DPN at baseline had a 13% cumulative incidence of DPN over the 6 years of follow-up (corresponding with an annual incidence of 2.204%) in a population where the duration of DM was as long as 15.2 ± 6.9 years. The incidence of DPN in our study is comparable with that of a previous longitudinal, large-scale, nationwide, population-based study in Taiwan (n = 37,375, annual incidence of 3.2%)20 However, it was lower than that reported Western populations21,22. This discrepancy might be due to differences in the sample size, ethnicity of the study population (the prevalence of DPN is about 32.1% in the UK23 and about 23.5% in Taiwan24), diagnostic criteria, and measurement instruments.
Apart from these, one of the crucial factors is the baseline duration of DM. One study well established that the prevalence of diabetic neuropathy increased from 8 to 42% in patients with T2DM when patients were monitored for 10 years25. Compared with the previous longitudinal study, patients had newly diagnosed DM with a cumulative incidence of 10% over the 13-year follow-up period and an annual incidence of 0.7%9. The relatively high cumulative incidence over our 6-year follow-up period might be attributable to the longer baseline duration of DM.
The association between renal function and incident DPN
In our study, baseline renal function was found to be an independent risk factor for DPN, including baseline eGFR (Table 3) and baseline serum creatinine concentration (Table 4), particularly in patients under the age of 65. This finding was inconsistent in patients aged 65 and above, which might be due to the decline of renal function in the aging process. This is consistent with the Rochester cohort longitudinal assessment12, in which Dyck et al. reported that the presence of DPN is associated with the severity of nephropathy and might be implicated in its cause. Our previous studies also indicate that the prevalence of DPN increases significantly in patients with impaired renal function6. On the other hand, the baseline UACR did not show the same result, which may be attributed to the large standard deviations (94.9 ± 196.8 mg/g vs. 72.8 ± 259.1 mg/g, p = 0.44).
To date, the mechanisms of neurotoxicity in T2DM patients with renal impairment remains unclear, but they have been demonstrated in some studies26,27. Experimental evidence indicates that renal impairment result in alteration in membrane excitability which is induced by inhibition of the axonal Na+/K+ pump. Consequently, it abolishes the direct contribution of the hyperpolarizing pump current to the membrane potential, leading to an accumulation of extracellular K+ that causes depolarization28. Disruption of these various ionic gradients may affect the Na+/Ca2+ exchanger, leading to increased levels of intracellular Ca2+ and axonal loss29.
In addition, it is clear from previous research that impaired renal function results in microvascular endothelial dysfunction, even in the early stages of chronic kidney disease. Endothelial injury is caused by various factors, including inflammation, hypertension, diabetes-associated factors, and a uremic milieu27,30. Eventually, it leads to neuropathy due to impaired nerve blood flow, epineurial arterio-venous shunting, and reduced nerve oxygen tension31.
Other studies examining nephropathy as a risk factor for DPN have been inconclusive13. However, it is suggested that the selection of disease markers for renal impairment may be important (for example, eGFR or creatinine), and further investigation is needed. Based on the current study, we recommend that increased serum creatinine concentration or lower baseline eGFR be used as an indicator to enhance the awareness of incident DPN.
Other risk factors of future DPN
After adjustment for potential confounding factors, we also found that a higher risk of DPN was linked with increased age, body weight, duration of DM, and male gender. Our findings are consistent with most previous reports from cross-sectional studies and a meta-analysis of patients with T2DM in Western, Korean, and Taiwanese populations5,6,32. Concerning sugar control, previous studies indicated hyperglycemia as a risk factor for the development of DPN5,8, but we found no association between baseline HbA1c levels and incident DPN. This is likely explained by low levels of HbA1c at baseline (7.3 ± 1.2% in the no-DPN group and 7.6 ± 1.7% in the incident-DPN group) compared with the levels usually found in previous studies. These data possibly reflect better medication adherence among Taiwanese DM patients33 compared with worldwide34. Our study also showed equally high numbers of hypoglycemic medication prescriptions in both groups. On the other hand, baseline HbA1c was found to be an independent risk factor for DPN in enrolled patients under the age of 65 (Table 4) but not in all ages. This finding might be attributed to the effect of age on the HbA1c. A possible explanation is that elderly individuals encounter physiologically decreased RBC count thus HbA1c is unsuitable for a marker of glycemic control in elderly35. In summary, the result implies us that baseline glycemic control might play a role in incident DPN pathogenesis in people under the age of 65 but further research is warranted.
In the current study, increased weight was independent risk factor of incident DPN, but no statistically significant associations with incident DPN were found for BMI and waist circumference. This is inconsistent with previous studies5,9,10 but previous studies have not identified a consistent list of risk factors related to markers of obesity10,12. A possible explanation is that previous investigators did not adequately correct the reference cut-off values and the units for tests. This is not to say that markers of obesity may not be risk factors for DPN, but corrections must first be made for these characteristics in the cut-off values and the units12.
In terms of dyslipidemia, we found that serum lipid components had no statistically significant associations with the risk of DPN in T2DM. As stated above, these findings were consisted with some previous studies36,37. In fact, accumulated evidence has shown a correlation between DPN and serum lipid profiles but has shown inconsistent results38. The possible underlying mechanisms of dyslipidemia leading to DPN are complex which may include insulin resistance, chronic inflammatory status, oxidative stress induced by elevated LDL, and demyelination38. Nevertheless, these mechanisms are mainly reported in preclinical studies39,40,41. It is well established that DPN is a multifactorial disease and our findings indicate that lipid metabolism may play a minor role in its pathogenesis.
The major strengths of the current study are its large sample size with long term follow-up, the unselected nature of participants, standardized data collection procedures, and inclusion of several potential risk factors at baseline. But despite these strengths, there are still plenty of limitations. First, our results might not apply to treatment-naïve cohorts of early-stage T2DM. A high proportion of medication prescription might have affected the cardiovascular risk factors. Furthermore, we did not use confirmatory tests such as nerve conduction studies or skin biopsy for DPN diagnosis. However, the diagnosis of DPN is principally a clinical one according to ADA recommendations, and the MNSIE is a sensitive, specific, validated clinical screening tool. Lastly, we included participants from a single hospital, which might limit the generalizability of the results.
Conclusion
Lower eGFR and higher serum creatinine concentration, particularly in people under the age of 65, are predictors of future DPN in Taiwanese people with T2DM. Other risk factors included advanced age, increased body weight, duration of DM, male gender for all ages and HbA1c in enrolled patients under the age of 65 which were compatible with most previous studies. These findings not only confirm the association between renal impairment and future DPN but also provides a commonly available assessment to predict the future DPN. Early detection of risk factors and control of the modifiable factors could enrich therapeutic strategies in clinical practice. Thus, we suggest that the therapeutic strategy for diabetes should provide early management of impaired renal function and prevent overweight. Also, these findings could provide useful information for researchers exploring the underlying mechanisms of DPN and inspire disease-modifying therapies in the future.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Lin, X. et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci. Rep. 10, 14790. https://doi.org/10.1038/s41598-020-71908-9 (2020).
Selvarajah, D. et al. Diabetic peripheral neuropathy: Advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 7, 938–948. https://doi.org/10.1016/s2213-8587(19)30081-6 (2019).
Callaghan, B. C., Cheng, H. T., Stables, C. L., Smith, A. L. & Feldman, E. L. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. 11, 521–534. https://doi.org/10.1016/s1474-4422(12)70065-0 (2012).
Nathan, D. M. et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986. https://doi.org/10.1056/nejm199309303291401 (1993).
Papanas, N. & Ziegler, D. Risk Factors And Comorbidities In Diabetic neuropathy: An update 2015. Rev. Diabetes Stud. 12, 48–62. https://doi.org/10.1900/rds.2015.12.48 (2015).
Pai, Y. W., Lin, C. H., Lee, I. T. & Chang, M. H. Prevalence and biochemical risk factors of diabetic peripheral neuropathy with or without neuropathic pain in Taiwanese adults with type 2 diabetes mellitus. Diabetes Metab. Syndr. 12, 111–116. https://doi.org/10.1016/j.dsx.2017.09.013 (2018).
Forrest, K. Y., Maser, R. E., Pambianco, G., Becker, D. J. & Orchard, T. J. Hypertension as a risk factor for diabetic neuropathy: A prospective study. Diabetes 46, 665–670. https://doi.org/10.2337/diab.46.4.665 (1997).
Tesfaye, S. et al. Vascular risk factors and diabetic neuropathy. N. Engl. J. Med. 352, 341–350. https://doi.org/10.1056/NEJMoa032782 (2005).
Andersen, S. T. et al. Risk factors for incident diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes followed for 13 years: ADDITION-Denmark. Diabetes Care 41, 1068–1075. https://doi.org/10.2337/dc17-2062 (2018).
Callaghan, B. C. et al. Metabolic syndrome components are associated with symptomatic polyneuropathy independent of glycemic status. Diabetes Care 39, 801–807. https://doi.org/10.2337/dc16-0081 (2016).
Jende, J. M. E. et al. Association of serum cholesterol levels with peripheral nerve damage in patients with type 2 diabetes. JAMA Netw. Open 2, e194798–e194798. https://doi.org/10.1001/jamanetworkopen.2019.4798 (2019).
Dyck, P. J. et al. Risk factors for severity of diabetic polyneuropathy: Intensive longitudinal assessment of the Rochester Diabetic Neuropathy Study cohort. Diabetes Care 22, 1479–1486. https://doi.org/10.2337/diacare.22.9.1479 (1999).
Li, J. et al. Correlations among diabetic microvascular complications: A systematic review and meta-analysis. Sci. Rep. 9, 3137. https://doi.org/10.1038/s41598-019-40049-z (2019).
Grundy, S. M. Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 106, 3143–3421 (2002).
Herman, W. H. et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: Results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabetes Med. 29, 937–944. https://doi.org/10.1111/j.1464-5491.2012.03644.x (2012).
Delanaye, P., Cavalier, E. & Pottel, H. Serum creatinine: Not so simple!. Nephron 136, 302–308. https://doi.org/10.1159/000469669 (2017).
Swedko, P. J., Clark, H. D., Paramsothy, K. & Akbari, A. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch. Intern. Med. 163, 356–360. https://doi.org/10.1001/archinte.163.3.356 (2003).
Garg, A. X. et al. Estimating the prevalence of renal insufficiency in seniors requiring long-term care. Kidney Int. 65, 649–653. https://doi.org/10.1111/j.1523-1755.2004.00412.x (2004).
Clemens, K. K., O’Regan, N. & Rhee, J. J. Diabetes management in older adults with chronic kidney disease. Curr. Diabetes Rep. 19, 11. https://doi.org/10.1007/s11892-019-1128-3 (2019).
Yang, C. P. et al. Cardiovascular risk factors increase the risks of diabetic peripheral neuropathy in patients with type 2 diabetes mellitus: The Taiwan diabetes study. Medicine 94, e1783. https://doi.org/10.1097/md.0000000000001783 (2015).
Gaede, P. et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med. 348, 383–393. https://doi.org/10.1056/NEJMoa021778 (2003).
Feldman, E. L. et al. Diabetic neuropathy. Nat. Rev. Dis. Primers 5, 41. https://doi.org/10.1038/s41572-019-0092-1 (2019).
Young, M. J., Boulton, A. J., MacLeod, A. F., Williams, D. R. & Sonksen, P. H. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 36, 150–154. https://doi.org/10.1007/bf00400697 (1993).
Chang, C. et al. Epidemiologic study of type 2 diabetes in Taiwan. Diabetes Res. Clin. Pract. 50(Suppl 2), S49-59. https://doi.org/10.1016/s0168-8227(00)00179-0 (2000).
Partanen, J. et al. Natural history of peripheral neuropathy in patients with non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 333, 89–94. https://doi.org/10.1056/nejm199507133330203 (1995).
Pop-Busui, R. et al. The management of diabetic neuropathy in CKD. Am. J. Kidney Dis. 55, 365–385. https://doi.org/10.1053/j.ajkd.2009.10.050 (2010).
Theodorakopoulou, M. P., Schoina, M. & Sarafidis, P. Assessment of endothelial and microvascular function in CKD: Older and newer techniques, associated risk factors, and relations with outcomes. Am. J. Nephrol. 51, 931–949. https://doi.org/10.1159/000512263 (2020).
Kaji, R. & Sumner, A. J. Ouabain reverses conduction disturbances in single demyelinated nerve fibers. Neurology 39, 1364–1368. https://doi.org/10.1212/wnl.39.10.1364 (1989).
Craner, M. J., Lo, A. C., Black, J. A. & Waxman, S. G. Abnormal sodium channel distribution in optic nerve axons in a model of inflammatory demyelination. Brain 126, 1552–1561. https://doi.org/10.1093/brain/awg153 (2003).
Malyszko, J. Mechanism of endothelial dysfunction in chronic kidney disease. Clin. Chim. Acta 411, 1412–1420. https://doi.org/10.1016/j.cca.2010.06.019 (2010).
Tesfaye, S., Malik, R. & Ward, J. D. Vascular factors in diabetic neuropathy. Diabetologia 37, 847–854. https://doi.org/10.1007/bf00400938 (1994).
Won, J. C., Kim, S. S., Ko, K. S. & Cha, B. Y. Current status of diabetic peripheral neuropathy in Korea: Report of a hospital-based study of type 2 diabetic patients in Korea by the diabetic neuropathy study group of the Korean diabetes association. Diabetes Metab. J. 38, 25–31. https://doi.org/10.4093/dmj.2014.38.1.25 (2014).
Lin, C. S. et al. A study on the impact of poor medication adherence on health status and medical expense for diabetes mellitus patients in Taiwan: A longitudinal panel data analysis. Medicine 99, e20800. https://doi.org/10.1097/md.0000000000020800 (2020).
Cramer, J. A. A systematic review of adherence with medications for diabetes. Diabetes Care 27, 1218–1224. https://doi.org/10.2337/diacare.27.5.1218 (2004).
Wu, L. et al. Effect of age on the diagnostic efficiency of HbA1c for diabetes in a Chinese middle-aged and elderly population: The Shanghai Changfeng Study. PLoS ONE 12, e0184607. https://doi.org/10.1371/journal.pone.0184607 (2017).
Terekeci, H. M. et al. Plasma osteoprotegerin concentrations in type 2 diabetic patients and its association with neuropathy. Exp. Clin. Endocrinol. Diabetes 117, 119–123. https://doi.org/10.1055/s-0028-1085425 (2009).
Jende, J. M. E. et al. Structural nerve remodeling at 3-T MR neurography differs between painful and painless diabetic polyneuropathy in type 1 or 2 diabetes. Radiology 294, 405–414. https://doi.org/10.1148/radiol.2019191347 (2020).
Cai, Z., Yang, Y. & Zhang, J. A systematic review and meta-analysis of the serum lipid profile in prediction of diabetic neuropathy. Sci. Rep. 11, 499. https://doi.org/10.1038/s41598-020-79276-0 (2021).
Vincent, A. M. et al. Dyslipidemia-induced neuropathy in mice: The role of oxLDL/LOX-1. Diabetes 58, 2376–2385. https://doi.org/10.2337/db09-0047 (2009).
Xie, F. et al. High energy diets-induced metabolic and prediabetic painful polyneuropathy in rats. PLoS ONE 8, e57427. https://doi.org/10.1371/journal.pone.0057427 (2013).
Vincent, A. M., McLean, L. L., Backus, C. & Feldman, E. L. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. Faseb J. 19, 638–640. https://doi.org/10.1096/fj.04-2513fje (2005).
Acknowledgements
The authors appreciate the volunteer’s participation in this study, and the statistical support by Biostatics Taskforce of Taichung Veterans General Hospital.
Funding
This research received grants from Taichung Veterans General Hospital (TCVGH 1083404C, 1093402C and 1103402C), but nothing from commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
C.-S.W.: advisor on writing, manuscript editing. Y.-W.P.: manuscript editing. C.-S.W., Y.-W.P., I-T.L., M.-H.C.: study design, analysis and interpretation of results. C.-H.L.: data analysis and statistical computation. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, CS., Pai, YW., Lin, CH. et al. Renal impairment is one of appropriate predictors of future diabetic peripheral neuropathy: a hospital-based 6-year follow-up study. Sci Rep 12, 5240 (2022). https://doi.org/10.1038/s41598-022-09333-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09333-3
This article is cited by
-
Diabetic Neuropathy: Pathophysiology Review
Current Pain and Headache Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.