Abstract
Genetic diversity of germline variants in breast cancer (BC) predisposition genes is unexplored in miscegenated populations, such those living in Latin America. We evaluated 1663 Brazilian BC patients, who underwent hereditary multigene panel testing (20–38 cancer susceptibility genes), to determine the spectrum and prevalence of pathogenic/likely pathogenic (P/LP) variants and variants of uncertain significance (VUS). Associations between P/LP variants and BC risk were estimated in a case–control analysis of BC patients and 18,919 Brazilian reference controls (RC). In total, 335 (20.1%) participants carried germline P/LP variants: 167 (10.0%) in BRCA1/2, 122 (7.3%) in BC actionable non-BRCA genes and 47 (2.8%) in candidate genes or other cancer predisposition genes. Overall, 354 distinctive P/LP variants were identified in 23 genes. The most commonly mutated genes were: BRCA1 (27.4%), BRCA2 (20.3%), TP53 (10.5%), monoallelic MUTYH (9.9%), ATM (8.8%), CHEK2 (6.2%) and PALB2 (5.1%). The Brazilian variant TP53 R337H (c.1010G>A, p.Arg337His), detected in 1.6% of BC patients and 0.1% of RC, was strongly associated with risk of BC, OR = 17.4 (95% CI: 9.4–32.1; p < 0.0001); monoallelic MUTYH variants c.1187G>A and c.536A>G, detected in 1.2% (0.9% RC) and 0.8% (0.4% RC) of the patients, respectively, were not associated with the odds of BC, the former with OR = 1.4 (95% CI: 0.8–2.4; p = 0.29) and the latter with OR = 1.9 (95% CI: 0.9–3.9; p = 0.09). The overall VUS rate was 46.1% for the entire patient population. Concluding, the use of multigene panel testing almost doubled the identification of germline P/LP variants in clinically actionable predisposition genes in BC patients. In Brazil, special attention should be given to TP53 P/LP variants.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the most common cancer in women worldwide. In Brazil, an average of 66,280 women are diagnosed with carcinoma of the breast every year, accounting for 29.7% of all cancers in the female population1. Inherited pathogenic/likely pathogenic variants (P/LP) in highly penetrant predisposition genes are thought to be involved in about 10% of BC cases. Among the hereditary forms, the most frequent events are germline P/LP variants in BRCA1/2 genes which predispose to hereditary breast and ovarian cancer syndrome (HBOC). The prevalence and spectrum of BRCA1/2 P/LP variants vary among different populations and are responsible for only approximately 25–50% of the familial risk of BC2,3,4. As DNA sequencing technologies evolved, other cancer susceptibility genes have been discovered, including high-penetrant genes such as TP53, CDH1, STK11, PTEN and PALB2 (> 4 fold cancer relative risk), moderate-penetrant genes such as CHEK2 and ATM (1.5–4 fold cancer relative risk), and a number of common low-penetrant BC susceptibility loci identified through genome-wide association studies (1–1.5 fold cancer relative risk)5,6,7. The mutational spectrum of germline mutations in BC predisposition genes have been reported in single populations, with the majority of reports focused on Caucasians from Europe and North America. The population from Southern Hemisphere countries, except for Australia, are underrepresented and understudied in cancer genetic epidemiology research2.
The Brazilian population has unique ethnic characteristics. People miscegenation is a universal phenomenon, due to globalization and large waves of immigration. Brazil is considered an ethnic “melting pot”, reflecting an admixture of European, Native American and Sub-Saharan African people, in addition to immigrants from a large number of European, Asian and Middle Eastern countries. Hence, Brazilian people offer a unique opportunity to advance the understanding of cancer genetic features in a miscegenated population8.
In Brazil, the majority of the inherited BC studies focused on the analyses of BRCA1/2 as well as TP53, given the relatively high population frequency of the TP53 R337H (also known as, c.1010G>A, p.Arg337His) variant in people from the South and Southeast regions of Brazil9. However, the likelihood of carrying P/LP variants in other BC susceptibility genes among BRCA1/2 and TP53-negative patients is largely unexplored.
Recent advances in next generation sequencing (NGS) technology has reduced the cost of massively parallel sequencing, provided to physicians and patients the option of sequencing multiple genes simultaneously and broadened our understanding of the genetic etiology of inherited cancers. Multigene panel testing has proved useful as a diagnostic tool for disorders where similar phenotypes can be influenced by multiple genes such as hereditary predisposition to BC, uncovering potentially actionable findings that may be missed by traditional testing paradigms. Several laboratories have released commercial multigene panel testing ranging from six to > 100 genes10. Panels are cheaper, faster and increase the yield of genetic findings, more than doubling the mutation detection rate in BRCA1/2-negative patients with suspected HBOC11,12,13,14,15,16,17,18. However, finding a mutation in a gene where the cancer risks and/or management strategies are not known, as well as the identification of higher numbers of variants of uncertain significance (VUS), can make the results cumbersome and challenging for a physician to interpret and guide treatment10.
Panels have been widely available in Brazil within the past 7 years, but no study has yet assessed the prevalence and mutational spectrum of germline variants in BC susceptibility genes other than BRCA1/2 and TP53 in a large cohort of individuals with BC, who were referred for genetic evaluation. Given the rapid uptake of multigene panel testing in clinical practice, these data are urgently needed to inform genetic counseling. In this study, we report the results from 1663 consecutive individuals with a history of BC who were referred for multigene panel testing.
Results
Study population and prevalence of P and LP variants
This study involved a nationwide sample of 1663 consecutive BC patients who underwent germline genetic testing with a multigene cancer panel between 2015 and 2017. Over half of the tests (or 51.9%) were from patients who inhabited the Southeast region of Brazil. Patients from all other regions were also well represented, except for patients from the North region. This information appears on Table 1.
Among all patients, mean age at BC diagnosis was 42.9 ± 11.2 years and median age was 41 years (min: 12 years–max: 87 years) (Table 1). Almost all, or 1650 (99.2%) patients were women. There was a significant age difference between sexes (women: 42.7 ± 11.1 years vs men: 61.1 ± 10.5 years; p < 0.001).
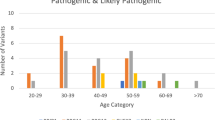
Among all 1,663 patients, 335 or 20.1% carried a P/LP variant in at least one gene (Table 1); among patients aged ≤ 35 years, 25.8% carried a P/LP variant, significantly more than in the whole cohort (25.8% vs. 20.1%; OR = 1.3; 95% CI: 1.0–1.6; p < 0.04).
Overall, 335 (20.1%) participants carried germline P/LP variants, including 223 (13.4%) in high-penetrant BC genes [BRCA1 97 (5.8%), BRCA2 72 (4.3%), TP53 37 (2.2%), PALB2 18 (1.1%), CDH1 1 (0.1%), NF1 1 (0.1%), PTEN 1 (0.1%)] and 69 (4.1%) in moderate-penetrant BC genes [ATM 31 (1.9%), CHEK2 22 (1.3%), RAD51C 7 (0.4%), BRIP1 5 (0.3%), BARD1 1 (0.1%), RAD51D 1 (0.1%)]. Of note, 56 (3.4%) patients had a P/LP variant in candidate genes or genes traditionally associated with other hereditary cancers: MUTYH (n = 35), APC (n = 5), BLM (n = 5), FANCC (n = 3), PMS2 (n = 2), RECQL (n = 2), MEN1 (n = 1), MSH2 (n = 1), MLH1 (n = 1). All these P/LP variants are shown in Fig. 1 and Table 2. Among mutation carriers, there were eight patients carrying exonic deletions, including: BRCA1 (2), MUTYH (2), ATM (1), BRCA (1), MLH1 (1), RAD51C (1); and five presenting Alu insertions in BRCA2. These alterations are listed in Table 2.
Eighteen patients carried P/LP variants in two genes and one patient in three different genes. Of note, two patients presented P/LP variants in both BRCA1 and BRCA2, three patients in TP53 R337H in association with BRCA1 c.5266dupC or monoallelic MUTYH (n = 2). Additionally, mutated monoallelic MUTYH, particularly MUTYH c.1187G>A, was the most frequent partner of other mutated genes (such as, BRCA1, BRCA2, PALB2 and TP53), detected in seven patients (Supplementary Table S1).
MUTYH P/LP variants were detected in 2.1% of the patients, including monoallelic MUTYH c.1187G>A, which was detected in 13 out of 1,068 BC patients, as well as in 170 out of 18,919 reference controls (1.2% vs 0.9%; OR = 1.4; 95% CI: 0.8–2.4; p = 0.29) and MUTYH c.536A>G, detected in 8 patients and 76 reference controls (0.8% vs 0.4%; OR = 1.9; 95% CI: 0.9–3.9; p = 0.09).
Age at BC diagnosis was significantly lower for BRCA1 P/LP variant carriers (38.0 ± 9.2 years) than in patients who were not P/LP germline carriers (43.5 ± 11.3; p < 0.001). Age at diagnosis was not associated with carriers of P/LP variants in any other genes when compared with non-carriers. Among 19 patients older than 75 years, four were P/LP variant carriers (21%), one in ATM, one in BRCA2 and two in CHEK2. Among 13 male patients, two (15.4%) were P/LP variant carriers, both in BRCA2.
Mutation spectrum of P and LP variants
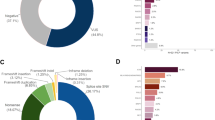
Overall, 354 P/LP variants were identified in 335 patients. Among these P/LP variants (100%), the three most frequently mutated genes were BRCA1 (27.4%), BRCA2 (20.3%) and TP53 (10.5%), followed by MUTYH (9.9%), ATM (8.8%), CHEK2 (6.2%) and PALB2 (5.1%), as shown in Fig. 2.
Allelic heterogeneity among the patients was reflected in the appearance of 188 distinct P/LP variants in 23 genes (Supplementary Table S2). Although the mutational profile was heterogeneous, recurrent variants (detected in three or more individuals) were found in 8 genes: APC c.3920T>A; ATM c.3802delG, c.640delT and c.6975G>A; BARD1 c.176_177delAG; BRCA1 c.5266dupC, 3331_3334delCAAG, c.1687C>T and c.211A>G; BRCA2 c.6405_6409delCTTAA, c.156_157insAlu, c.2808_2811delACAA, c.8488-1G>A, c.6656C>G, c.1813dupA and c.2T>G; CHEK2 c.349A>G, c.470T>C and c.1427C>T; MUTYH c.1187G>A, c.536A>G and c.934-2A>G; and TP53 c.1010G>A (Table 2). The most prevalent BRCA1 recurrent variants, which were the European founder variants c.5266dupC (n = 28) and c.3331_3334delCAAG (n = 13), accounted for 43.2% of all BRCA1 reported variants. The European founder CHEK2 recurrent variant c.349A>G (n = 7) accounted for 41.2% of all CHEK2 reported variants.
The TP53 R337H is of particular interest because it is widespread in Brazil due to a founder effect and is present in 0.3% of the southern and southeastern general populations20.
The Brazilian TP53 R337H variant
Overall, TP53 was the third most frequently mutated gene and contributed to 2.2% of BC cases in our cohort. TP53 P/LP variants were detected in 37 out of 1,663 BC patients and in 21 out of 18,919 reference controls (2.2% vs 0.1%; OR = 20.5; 95% CI: 11.6 – 39.9; p < 0.001). It is noteworthy that the TP53 variants were concentrated in the South and Southeast (86.5%; Table 1) compared to the other regions of Brazil (32 vs 5; OR = 2.9; 95% CI: 1.1–7.4; p = 0.03).
The Brazilian TP53 R337H variant accounted for 70.3% of all TP53 reported P/LP variants and was also concentrated in patients from the South and Southeast regions of Brazil (Table 1). This variant was detected in 26 out of 1,663 BC patients, as well as in 17 out of 18,919 reference controls (1.6% vs 0.1%; OR = 17.4; 95% CI: 9.4 – 32.1; p < 0.0001) . Another 10 patients had mutations in the TP53 DNA binding domain. TP53 R337H carriers were diagnosed with BC an average of 10 years older than patients who carried TP53 pathogenic variants within typical DNA-binding domain (42.2 ± 10.9 years vs. 32.3 ± 5.1 years, p < 0.007).
VUS in Brazilian patients with BC
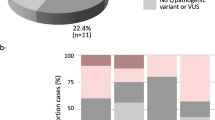
The overall VUS rate was 46.1% for the entire patient population, with 13.4% having two or more VUS (Fig. 3). As expected, the prevalence of VUS increased considerably with the number of genes tested. The chance to detect a VUS was 6.7% if only the BRCA1/2 genes were tested. Comparing to a BRCA1/2 test, this chance was approximately 2 times higher if the 8 high-penetrant BC genes were tested (OR = 2.0; 95% CI: 1.6–2.6; p < 0.0001), 5 times higher if the 14 high/moderate-penetrant BC genes were tested (OR = 5.3; 95% CI: 4.2–6.6; p < 0.0001), and almost 12 times higher if a multigene panel (20–38 genes) was tested (OR = 11.6; 95% CI: 9.4–14.4; p < 0.0001). Among all the genes tested, the highest number of VUS was detected in ATM, followed by BRCA2 (Fig. 4). Approximately 90% of the VUS were missense variants (Supplementary Table S3).
Frequency of variants of unknown significance (VUS). Cumulative fraction of clinical cases with one or more VUS, irrespective of pathogenic variants observed, as the scope of testing increases. High-penetrant genes: BRCA1, BRCA2, CDH1, NF1, PALB2, PTEN, STK11 and TP53; moderate-penetrant genes: ATM, BARD1, BRIP1, CHEK2, RAD51C and RAD51D.
Discussion
This is the largest nationwide cohort of Brazilian BC patients who underwent NGS mutigene panel testing reported to date. In this study, both allelic heterogeneity and founder mutations played a role in inherited BC. The most commonly mutated genes were BRCA1/2, which were identified in 10% of the entire cohort and accounted for almost 50% of all P/LP germline variants identified. In accordance with previous research from different countries, the use of a multigene panel test doubled the yield of P/LP variants detected, as well as increased in 12 times the chance of finding a VUS. Most significantly, this study differs from the others because it highlights the important contribution of Li-Fraumeni syndrome (LFS) to inherited BC burden in Brazil, due to the Brazilian TP53 R337H variant. It is worth emphasizing that the number of patients carrying this mutation is similar to the number of patients with BRCA1 c.5266dupC, which is the most prevalent BRCA1 pathogenic variant in our study.
Patients from all regions of the country were represented, mainly from the Southeast region of Brazil, which is the most densely populated, with more than 89 million people (or 42% of the Brazilian population). Patients from all other regions were also well represented, except for patients from the North region, which is the least densely populated with 8.8 million people in 3.87 million km2, covered mostly by the Amazon Rainforest.
The estimated frequency in the general population of P/LP BRCA1/2 mutations is 1:800–1:1000 per gene21; however, the prevalence of pathogenic variants in BRCA1/2 varies considerably between different ethnic groups and geographic areas. In Brazil, there are no large population studies yet, so we do not have reliable estimates of its prevalence in this scenario. The prevalence of BRCA1/2 pathogenic variants in unselected, under the age of 35 or classified as high-risk BC patients was estimated to be 2.3%22, 16.5–20.4% and 3.4–22.5%, respectively23,24,25,26,27,28,29,30,31,32 (Table 3). Our unselected cohort probably has the bias of comprehending mainly high-risk patients, as they were probably referred for genetic testing due to suspicion of the attending physician, identified a percentage of patients with a BRCA1/2 mutation of approximately 10%. The two most prevalent mutations are in accordance with the largest study of the Brazilian population reported to date: BRCA1 c.5266dupC and BRCA1 c.3331_3334delCAAG33,34. The BRCA1 c.5266dupC founder pathogenic variant is the most frequently reported in Brazil by several independent studies, but has not been observed elsewhere in South America, with the exception of an Ashkenazi community in Argentina. Notwithstanding, the BRCA1 c.3331_3334delCAAG was identified in BC patients in Spain and Portugal, as well as in Brazil, Chile, and Colombia. Despite a significant contribution of African ancestry to the genetic pool of some of the populations of Brazil, no recurrent pathogenic variants were traced back to the African continent in our cohort35.
Pathogenic variants in the TP53 gene are very relevant for the Brazilian population. In general, the global prevalence estimates of P/LP TP53 variants are within the range of one carrier in 3,555–5,476 individuals36. In Brazil, the TP53 R337H variant is estimated to occur in about 2.7 per 1,000 individuals born in southern Brazil20. In the 2000s, Brazilian researchers associated the TP53 R337H variant, which affects the oligomerization domain, with an increased risk of developing adrenocortical carcinomas. Subsequent studies have shown that the same variant could also increase the risk of other cancers, such as BC, but the penetrance was different37,38,39,40,41. The TP53 R337H variant confers a lifetime cancer risk by age 60 years of 80% in females and 47% in males. In comparison, in classic LFS, those with mutation located in typical DNA-binding domain, the cancer risk is 90% in women and 73% in men42. The reasons concerning the reduced penetrance of this variant is still controversial and usually associated with its location in the gene and biochemistry stability, which is pH dependent. A recent study showed that an extended haplotype cosegregating the TP53 R337H and XAF1 E134* alleles may lead to a more aggressive cancer phenotype than TP53 R337H alone, acting as a functional modifier by attenuating the transactivation of wild-type and hypomorphic TP53 variants, such as R337H. Carriers harboring the extended haplotype were more likely to be diagnosed with sarcomas and multiple tumors, nevertheless this association was not observed in BC patients. Further studies are needed to validate these findings and evaluate their implications on genetic counseling and clinical management of TP53 R337H carriers43. BC is the most common malignancy diagnosed in LFS. In Brazil, in high-risk BC patients, the prevalence of TP53 R337H ranged from 3.4–7.1% in the South/Southeast44,45 and 0.9% in the Northeast region30,46 (Table 4). In a cohort of 815 women affected by BC in southern Brazil who developed the disease before age 45 years, the prevalence of the TP53 R337H variant was 12.1%45. In our cohort, the prevalence of all P/LP TP53 variants was 2.2%, representing the third most commonly mutated gene among BC patients. The TP53 R337H variant was responsible for 70.3% all TP53 mutations identified. Excluding the TP53 R337H variant, it becomes clear that the prevalence of other mutations in TP53 is low in the Brazilian BC patients, approximately 0.7% in the present study, in accordance with other four Brazilian studies that analyzed the entire coding region of TP53 (Table 4), following the same pattern as the worldwide prevalence24,40,46,47,48.
Of note, it should also be emphasized that almost 30% of TP53 P/LP variants occurred on sites other than R337H. Some of these pathogenic variants were already reported in Brazilian BC patients, such as c.733G>A46 and c.818G>A39, while one was detected in patients with Spanish ancestry (c.743G>A)49.
Thus, these results confirm that inheritance of TP53 R337H contribute to a significant number of BC cases in Brazil. These findings reaffirm the need for differentiated guidelines for monitoring and risk reduction strategies in patients with hereditary BC in Brazil. The investigation of the TP53 R337H variant in the Brazilian pre-menopausal patients diagnosed with BC is essential. These patients and their relatives who carry the same variant should receive intensive surveillance which includes at least whole-body magnetic resonance imaging (MRI) and central nervous system MRI, according to Toronto protocol50. In addition, breast MRI should be offered annually from age 20 years and mammography annually after age 30 years. For these patients, risk-reducing bilateral (adeno)mastectomy should be discussed. For BC patients, mastectomy should be the preferred option in an attempt to avoid radiotherapy. Nonetheless, radiotherapy should be considered when the risk of locoregional recurrence is high.
In the present study, germline variants in BC susceptibility genes other than BRCA1/2 and TP53 were also found in approximately 8% of the BC patients. Among BC clinically actionable genes, ATM, CHEK2 and PALB2 were the most frequently mutated. This finding is in accordance with reports from a recent study analyzing BC predisposition genes in a large cohort of patients47. In this work, the cited genes were associated with high or moderate BC risk with similar effect sizes in European and Asian patients which are ancestries well represented in certain regions of Brazil.
ATM was the fifth gene with the highest number of P/LP alterations; no founder mutation was found, but it had the highest number of VUS. The most common variant found in CHEK2 was c.349A>G, representing almost 1/3 of the P/LP variants in this gene. The protein encoded by this allele was found to be defective in functional tests and is likely to be pathogenic51. It was found in men with prostate cancer in Portugal and in women with BC in Europe and Brazil46,52,53.
Pathogenic variants in other genes, such as BARD1 and RAD51C were also detected. The c.176_177delAG in BARD1, was quite common (0.24%) in the present series and, interestingly, it was also detected in other BC Brazilian patients, as well as in Spanish patients, but was not reported in a recent literature review of studies analyzing BARD1 as a cancer predisposing gene, mainly comprehending French or white people46,54,55.
Biallelic MUTYH P/LP variants are associated with an autosomal recessive disorder, characterized by polyposis and increased risk of colorectal carcinoma. However, the cancer risk associated with germline variants in individuals carrying only one MUTYH defective allele is controversial. Studies have shown that risks of colorectal cancer for carriers of monoallelic variants in MUTYH with a first-degree relative with colorectal cancer are sufficiently high to warrant more intensive screening than for the general population, as a consequence NCCN guidelines propose colonoscopy every five years beginning at age 40 years56. Nevertheless, there is no strong evidence of the association of increased BC risk and carriers of monoallelic variants in MUTYH47. In our cohort, the fourth most commonly mutated gene was MUTYH due to the high prevalence of two monoallelic variants: MUTYH c.1187G>A and MUTYH c.536A>G. Our study, in accordance with the majority of previous studies, confirmed that those variants were not associated with increased BC risk. Thus, although it is a frequent finding in patients undergoing multigene panel testing, a monoallelic MUTYH variant should not prompt increased surveillance or risk-reducing strategies for BC57.
The additional pathogenic variants uncovered by multigene panel testing appears clinically relevant, albeit it is also unveiling a large number of variants that we are still not able to clearly define and classify, the VUS. We have found 767 distinctive VUS in 46.1% of our patients and 88.5% were missense variants. Studies have found that particularly among racial/ethnic minorities there is an increased likelihood of VUS results compared to women of European ancestry due to limited understanding of the normal spectrum of genetic variation in understudied groups58. At present, VUS management in the clinical context is challenging. Although it is typically recommended that patients with VUS are managed based on their personal and family history, rather than on the test result, communicating uncertainty has been shown to have the potential to overwhelm patients and increase their worries. In addition, a higher rate of risk reducing surgery among patients with VUS than among patients with negative results has been reported59. In order to overcome the challenge of VUS reclassification, the development and improvement of well represented clinical variants databases, predictive algorithms and in vitro functional assays are urgently needed.
Conclusion
In summary, the largest nationwide cohort of Brazilian BC patients who underwent multigene panel testing identified that BRCA1/2 accounted for almost 50% of all P/LP germline variants. The use of a multigene panel test almost doubled the identification of P/LP germline variants in BC predisposition genes other than BRCA1/2, as well as increased in 12 times the chance of finding a VUS. In general, the spectrum and frequencies of germline variants in non-BRCA1/2 genes mirrored those described in the literature, except for TP53 variants. In our cohort, the third most frequently gene mutated was the TP53 due to the high number of TP53 R337H carriers in the South and Southeast region of Brazil. As a consequence, the high prevalence of this TP53 variant has a significant impact in screening and risk-reducing strategies in Brazil.
Methods
Study population
Patients were eligible to participate if they were 18 years of age or older at testing, had a personal diagnosis of BC, and were referred for a commercial multigene cancer panel testing at a College of American Pathology (CAP)–accredited laboratory (Mendelics Análise Genômica S.A., São Paulo, SP, Brazil). Informed consent for clinical testing was obtained by the ordering physician. All patient data, which comprehended age at BC diagnosis or age at testing and region of sample collection, were obtained from clinician-completed test requisition forms. This information was anonymized before analysis and there was no missing information. Case selection was limited to one individual per family. In the instance where multiple individuals from the same family underwent multigene panel, the first family member to undergo panel testing was selected for inclusion in this study. The protocol was approved by the Faculdade de Medicina da Universidade São Paulo (FMUSP) Institutional Review Board.
Panel composition
A custom targeted NGS panel was chosen at the discretion of the ordering clinician and ranged from 20 to 38 genes. All patients underwent comprehensive germline analysis of 20 genes included on the Mendelics curated BC panel: AKT1, ATM, BARD1, BLM, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, FANCC, NBN, NF1, PALB2, PTEN, TP53, RAD51C, RAD51D, STK11, PIK3CA, RECQL. Other 18 genes could be added in the analysis at the discretion of the attending physician: APC, CDK4, CDKN2A, EGFR, EPCAM, MEN1, MET, MLH1, MSH2, MSH6, MUTYH, NF2, PMS2, POLD1, POLE, RB1, RET, WT1.
Among those genes, 14 were considered clinically actionable because they are referenced in NCCN management guidelines, are commonly included in diagnostic BC panels and there is evidence to support the discussion of personalized BC risk management strategies for patients who test positive19. They were separated in 2 categories: high-penetrant: BRCA1, BRCA2, CDH1, NF1, PALB2, PTEN, STK11, and TP53; and moderate-penetrant: ATM, BARD1, BRIP1, CHEK2, RAD51C, and RAD51D.
Sequencing and variant interpretation
Genomic DNA was obtained from a buccal swab or peripheral blood sample using standard methods. DNA Sequencing was performed by high-end Illumina platforms (HiSeq 2500 and HiSeq 4000). Base calling was performed using original Illumina tools (bcl2fastq). Bioinformatics pipeline followed Broad Institute best practices (https://gatk.broadinstitute.org/hc/en-us/sections/360007226651-Best-Practices-Workflows). After alignment to the reference genome GRCh37 / UCSC hg19, low quality and duplicate readings were removed, and variants (SNPs/indels) were detected with GATK HaplotypeCaller. Enrichment and analysis concentrated on the coding sequences, flanking intronic regions (± 20 bp) and other specific genomic regions previously identified to harbor causing variants. Promoters, untranslated regions and other non-coding regions were not analyzed. Exonic deletions and duplications (CNV) were identified using ExomeDepth, an R package that estimates the number of copies by comparing the reading depth for each target with the mean reading depth for the same target from samples genotyped from the same sequenced library. If a CNV was identified, multiplex ligation-dependent probe amplification (MLPA) assay was employed to confirm the finding. The variants were classified according to algorithms based on machine learning developed by Mendelics Análise Genômica S.A and described with a nomenclature compatible with the norms and guidelines of the American College of Medical Genetics and Genomics (ACMG)/Human Genome Variation Society (HGVS). Variants interpreted as pathogenic (P) and likely pathogenic (LP) were considered positive. All variants were evaluated by a medical geneticist or pathologist or certified oncologist. Frequencies were calculated according to the total number of patients tested.
Brazilian genomic database
Reference control data were obtained from the Mendelics Análise Genômica S.A. database, which contains panel and exome sequencing data from 18,919 Brazilian individuals, sequenced as part of various disease-specific genetic tests, excluding samples from cancer cases. Case–control analysis was performed by variant or pooling P/LP variants to the gene level and comparing the frequency in BC patients relative to Brazilian reference controls.
Statistical analysis
Patients characteristics and sequencing results were tabulated, with descriptive statistics including medians, means, and standard deviations for continuous data and proportions with 95% confidence interval (CI) for categorical data are presented. A χ2 test or Fisher exact test was used to compare proportions among cohorts and P values less than 0.05 were considered significant. Continuous variables were compared by t tests or Anova, followed by Bonferroni post-test, as necessary. Odds ratios (OR) and 95% CI were calculated by established methods. Statistical analysis was performed using SPSS Version 16.
Statement
The authors declare that all methods were carried out in accordance with relevant guidelines and regulations.
Presentation
Preliminary results of this study have been presented at 2018 ASCO Annual Meeting—J Clin Oncol 36, 2018 (suppl; abstr e13610).
References
Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva. Estimativa 2020: Incidência de câncer no Brasil. https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//estimativa-2020-incidencia-de-cancer-no-brasil.pdf (2019).
Rebbeck, T. R. et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum. Mutat. 39, 593–620 (2018).
Kurian, A. W. et al. Breast and ovarian cancer penetrance estimates derived from germline multiple-gene sequencing results in women. JCO Precis. Oncol. 1, 1–12 (2017).
Nielsen, F. C., van Overeem Hansen, T. & Sørensen, C. S. Hereditary breast and ovarian cancer: New genes in confined pathways. Nat. Rev. Cancer. 16, 599–612 (2016).
Michailidou, K. et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat. Genet. 45, 353–361 (2013).
Rivandi, M., Martens, J. W. M. & Hollestelle, A. Elucidating the underlying functional mechanisms of breast cancer susceptibility through post-GWAS analyses. Front. Genet. 9, 280. https://doi.org/10.3389/fgene.2018.00280 (2018).
Easton, D. F. et al. Gene-panel sequencing and the prediction of breast-cancer risk. N. Engl. J. Med. 372, 2243–2257 (2015).
de Souza, A. M., Resende, S. S., de Sousa, T. N. & de Brito, C. F. A. A systematic scoping review of the genetic ancestry of the Brazilian population. Genet. Mol. Biol. 42, 495–508 (2019).
Palmero, E. I. et al. Detection of R337H, a germline TP53 mutation predisposing to multiple cancers, in asymptomatic women participating in a breast cancer screening program in Southern Brazil. Cancer Lett. 261, 21–25 (2008).
Kurian, A. W. & Ford, J. M. Multigene panel testing in oncology practice: How should we respond?. JAMA Oncol. 1, 277–278 (2015).
Cybulski, C. et al. Mutations predisposing to breast cancer in 12 candidate genes in breast cancer patients from Poland. Clin. Genet. 88, 366–370 (2015).
Desmond, A. et al. Clinical actionability of multigene panel testing for hereditary breast and ovarian cancer risk assessment. JAMA Oncol. 1, 943–951 (2015).
Kurian, A. W. et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J. Clin. Oncol. 32, 2001–2009 (2014).
Laduca, H. et al. Utilization of multigene panels in hereditary cancer predisposition testing: Analysis of more than 2,000 patients. Genet. Med. 16, 830–837 (2014).
Maxwell, K. N. et al. Prevalence of mutations in a panel of breast cancer susceptibility genes in BRCA1/2-negative patients with early-onset breast cancer. Genet. Med. 17, 630–638 (2015).
Thompson, E. R. et al. Panel testing for familial breast cancer: Calibrating the tension between research and clinical care. J. Clin. Oncol. 34, 1455–1459 (2016).
Tung, N. et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer 121, 25–33 (2015).
Tung, N. et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J. Clin. Oncol. 34, 1460–1468 (2016).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 1.2020). https://www.nccn.org/professionals/physician_gls/default.aspx. (2020).
Custódio, G. et al. Impact of neonatal screening and surveillance for the TP53 R337H mutation on early detection of childhood adrenocortical tumors. J. Clin. Oncol. 31, 2619–2626 (2013).
Balmaña, J., Díez, O., Rubio, I. T. & Cardoso, F. BRCA in breast cancer: ESMO clinical practice guidelines. Ann. Oncol. 22, vi31–vi41 (2011).
Gomes, M. C. B. et al. Prevalence of BRCA1 and BRCA2 mutations in breast cancer patients from Brazil. Breast Cancer Res. Treat. 103, 349–353 (2007).
Encinas, G. et al. Somatic mutations in early onset luminal breast cancer. Oncotarget 9, 22460–22479 (2018).
Carraro, D. M. et al. Comprehensive analysis of BRCA1, BRCA2 and TP53 germline mutation and tumor characterization: A portrait of early-onset breast cancer in Brazil. PLoS ONE 8, e57581. https://doi.org/10.1371/journal.pone.0057581 (2013).
Lourenço, J. J. et al. BRCA1 mutations in Brazilian patients. Genet. Mol. Biol. 27, 500–504 (2004).
Dufloth, R. M. et al. Analysis of BRCA1 and BRCA2 mutations in Brazilian breast cancer patients with positive family history. Sao Paulo Med. J. 123, 192–197 (2005).
Silva, F. C. et al. Hereditary breast and ovarian cancer: Assessment of point mutations and copy number variations in Brazilian patients. BMC Med. Genet. 15, 1–11 (2014).
Esteves, V. F. et al. The Brazilian Network of Breast and Ovarian Familial Cancer Aggregation. Prevalence of BRCA1 and BRCA2 gene mutations in families with medium and high risk of breast and ovarian cancer in Brazil. Braz. J. Med. Biol. Res. 42, 453–457 (2009).
Ewald, I. P. et al. Prevalence of the BRCA1 founder mutation c.5266dupin Brazilian individuals at-risk for the hereditary breast and ovarian cancer syndrome. Hered. Cancer Clin. Pract. 9, 1–8 (2011).
Felix, G. E. S. et al. Germline mutations in BRCA1, BRCA2, CHEK2 and TP53 in patients at high-risk for HBOC: Characterizing a Northeast Brazilian Population. Hum. Genome Var. 1, 14012. https://doi.org/10.1038/hgv.2014.12 (2014).
Palmero, E. I. et al. Screening for germline BRCA1, BRCA2, TP53 and CHEK2 mutations in families at-risk for hereditary breast cancer identified in a population-based study from Southern Brazil. Genet. Mol. Biol. 39, 210–222 (2016).
Alemar, B. et al. BRCA1 and BRCA2 mutational profile and prevalence in hereditary breast and ovarian cancer (HBOC) probands from Southern Brazil: Are international testing criteria appropriate for this specific population?. PLoS ONE 12, 1–18 (2017).
Palmero, E. I. et al. The germline mutational landscape of BRCA1 and BRCA2 in Brazil. Sci. Rep. 8, 1–10 (2018).
Fernandes, G. C. et al. Prevalence of BRCA1/BRCA2 mutations in a Brazilian population sample at-risk for hereditary breast cancer and characterization of its genetic ancestry. Oncotarget 7, 80465–80481 (2016).
Dutil, J. et al. The spectrum of BRCA1 and BRCA2 alleles in Latin America and the Caribbean: A clinical perspective. Breast Cancer Res. Treat. 154, 441–453 (2015).
de Andrade, K. C. et al. Variable population prevalence estimates of germline TP53 variants: A gnomAD-based analysis. Hum. Mutat. 40, 97–105 (2018).
Achatz, M. I. W., Hainaut, P. & Ashton-Prolla, P. Highly prevalent TP53 mutation predisposing to many cancers in the Brazilian population: A case for newborn screening?. Lancet Oncol. 10, 920–925 (2009).
Cipriano, N. M. Jr. et al. Mutation screening of TP53, CHEK2 and BRCA genes in patients at high risk for hereditary breast and ovarian cancer (HBOC) in Brazil. Breast Cancer 26, 397–405 (2019).
da Costa, E. et al. Germline variants in DNA repair genes associated with hereditary breast and ovarian cancer syndrome: Analysis of a 21 gene panel in the Brazilian population. BMC Med. Genom. 13, 21. https://doi.org/10.1186/s12920-019-0652-y (2020).
Assumpção, J. G. et al. Association of the germline TP53 R337H mutation with breast cancer in southern Brazil. BMC Cancer 8, 1–6 (2008).
Gomes, M. C. B. et al. The R337H mutation in TP53 and breast cancer in Brazil. Hered. Cancer Clin. Pract. 10, 3. https://doi.org/10.1186/1897-4287-10-3 (2012).
Achatz, M. I. et al. Recommendations for advancing the diagnosis and management of hereditary breast and ovarian cancer in Brazil. JCO Glob. Oncol. 6, 439–452 (2020).
Pinto, E. M. et al. XAF1 as a modifier of p53 function and cancer susceptibility. Sci. Adv. 6, eaba3231. https://doi.org/10.1126/sciadv.aba3231 (2020).
Cury, N. M., Ferraz, V. E. F. & Silva, W. A. TP53 p.R337H prevalence in a series of Brazilian hereditary breast cancer families. Hered. Cancer Clin. Pract. 12, 1–8 (2014).
Giacomazzi, J. et al. Prevalence of the TP53 p.R337H mutation in breast cancer patients in Brazil. PLoS ONE 9, e99893. https://doi.org/10.1371/journal.pone.0099893 (2014).
Sandoval, R. L. et al. Germline molecular data in hereditary breast cancer in Brazil: Lessons from a large single-center analysis. PLoS ONE 16, e0247363. https://doi.org/10.1371/journal.pone.0247363 (2021).
Breast Cancer Association Consortium. Breast cancer risk genes: Association analysis in more than 113,000 women. N. Engl. J. Med. 384, 428–439 (2021).
Gomes, R. et al. Prevalence of germline variants in consensus moderate-to-high-risk predisposition genes to hereditary breast and ovarian cancer in BRCA1/2-negative Brazilian patients. Breast Cancer Res. Treat. 185, 851–861 (2021).
Fonfria, M. et al. Prevalence and clinicopathological characteristics of moderate and high-penetrance genes in non-BRCA1/2 breast cancer high-risk Spanish families. J. Pers. Med. 11, 548. https://doi.org/10.3390/jpm11060548 (2021).
Kratz, C. P. et al. Cancer screening recommendations for individuals with Li-Fraumeni syndrome. Clin. Cancer Res. 23, e38–e45. https://doi.org/10.1158/1078-0432.CCR-17-0408 (2017).
Le Calvez-Kelm, F. et al. Rare, evolutionarily unlikely missense substitutions in CHEK2 contribute to breast cancer susceptibility: Results from a breast cancer family registry case-control mutation-screening study. Breast Cancer Res. 13, R6. https://doi.org/10.1186/bcr2810 (2011).
Southey, M. C. et al. PALB2, CHEK2 and ATM rare variants and cancer risk: Data from COGS. J. Med. Genet 53, 800–811 (2016).
Brandão, A. et al. The CHEK2 variant C.349A>G is associated with prostate cancer risk and carriers share a common ancestor. Cancers 12, 3254. https://doi.org/10.3390/cancers12113254 (2020).
Alenezi, W. M., Fierheller, C. T., Recio, N. & Tonin, P. N. Literature review of BARD1 as a cancer predisposing gene with a focus on breast and ovarian cancers. Genes 11, 856. https://doi.org/10.3390/genes11080856 (2020).
Rofes, P. et al. BARD1 pathogenic variants are associated with triple-negative breast cancer in a Spanish hereditary breast and ovarian cancer cohort. Genes 12, 150. https://doi.org/10.3390/genes12020150 (2021).
Win, A. K. et al. Risk of colorectal cancer for carriers of mutations in MUTYH, with and without a family history of cancer. Gastroenterology 146, 1208–1211 (2014).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN guidelines): Breast Cancer 1.2022. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (2021).
Kurian, A. W. et al. Uptake, results, and outcomes of germline multiple-gene sequencing after diagnosis of breast cancer. JAMA Oncol. 4, 1066–1072 (2018).
Mighton, C., Shickh, S., Uleryk, E., Pechlivanoglou, P. & Bombard, Y. Clinical and psychological outcomes of receiving a variant of uncertain significance from multigene panel testing or genomic sequencing: A systematic review and meta-analysis. Genet. Med. 23, 22–33 (2021).
de Souza Timoteo, A. R. et al. A portrait of germline mutation in Brazilian at-risk for hereditary breast cancer. Breast Cancer Res. 172, 637–646 (2018).
Bandeira, G. et al. Germline variants of Brazilian women with breast cancer and detection of a novel pathogenic ATM deletion in early-onset breast cancer. Breast Cancer 28, 346–354 (2021).
Nagy, T. R. et al. Germline and Somatic mutations in postmenopausal breast cancer patients. Clinics 76, e2837. https://doi.org/10.6061/clinics/2021/e2837 (2021).
Acknowledgements
The authors acknowledge the assistance of Maria Cristina Pinero Grandal for figure edition. MAAKF received research support from Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (CNPq—308876/2017-2).
Author information
Authors and Affiliations
Contributions
R.S.C.G. and M.A.A.K.F.: conceived and designed the analysis. R.S.C.G., D.V.V and J.P.F.W.K.: collected the data. E.F., D.V.V., J.P.F.W.K., F.P.M.M, A.V., D.S. and F.K.: contributed data. Y.Z., O.I.O, J.P.F.W.K., R.V.M.L, M.A.A.K.F. and R.S.C.G.: performed the analysis. All authors discussed the results and contributed to the final manuscript. R.S.C.G. wrote the manuscript with support from M.A.A.K.F., D.V.V and V.M.R.
Corresponding author
Ethics declarations
Competing interests
Founders and employees of Mendelics Análise Genômica served as coinvestigators in this study and provided material support, including germline testing and interpretation, as described in the manuscript. The specific coinvestigators listed as authors participated in the review and final approval of the submitted manuscript. R.S.C.G. acted as a consultant for AstraZeneca, GlaxoSmithKline and Igenomix; received speaker honoraria from AstraZeneca, Bristol Myers Squibb, GlaxoSmithKline, Merck Sharpe & Dohme Brasil, Novartis, and Roche outside the submitted work; and has equity in Mendelics Análise Genômica. J.P.F.W.K., A.V., D.S. and F.K. are co-founders at Mendelics Análise Genômica. O.I.O. is co-founder at CancerIQ; serves as scientific advisor at Tempus; and is on the advisory board of 54gene. All the other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guindalini, R.S.C., Viana, D.V., Kitajima, J.P.F.W. et al. Detection of germline variants in Brazilian breast cancer patients using multigene panel testing. Sci Rep 12, 4190 (2022). https://doi.org/10.1038/s41598-022-07383-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07383-1
This article is cited by
-
Systematic review of the molecular basis of hereditary breast and ovarian cancer syndrome in Brazil: the current scenario
European Journal of Medical Research (2024)
-
Germline variants of uncertain significance, their frequency, and clinico-pathological features in a cohort of Sri Lankan patients with hereditary breast cancer
BMC Research Notes (2023)
-
Neoadjuvant carboplatin in triple-negative breast cancer: results from NACATRINE, a randomized phase II clinical trial
Breast Cancer Research and Treatment (2023)
-
Familial history and prevalence of BRCA1, BRCA2 and TP53 pathogenic variants in HBOC Brazilian patients from a public healthcare service
Scientific Reports (2022)
-
Frequency of germline genetic variants in women with a personal or family history of breast cancer from Brazil
Molecular Biology Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.