Abstract
From May 2015 to June 2016, data on 296 patients undergoing 1.5-Tesla MRI for symptoms of acute ischemic stroke were retrospectively collected. Conventional, echo-planar imaging (EPI) and echo train length (ETL)-T2-FLAIR were simultaneously obtained in 118 patients (first group), and conventional, ETL-, and repetition time (TR)-T2-FLAIR were simultaneously obtained in 178 patients (second group). A total of 595 radiomics features were extracted from one region-of-interest (ROI) reflecting the acute and chronic ischemic hyperintensity, and concordance correlation coefficients (CCC) of the radiomics features were calculated between the fast scanned and conventional T2-FLAIR for paired patients (1st group and 2nd group). Stabilities of the radiomics features were compared with the proportions of features with a CCC higher than 0.85, which were considered to be stable in the fast scanned T2-FLAIR. EPI-T2-FLAIR showed higher proportions of stable features than ETL-T2-FLAIR, and TR-T2-FLAIR also showed higher proportions of stable features than ETL-T2-FLAIR, both in acute and chronic ischemic hyperintensities of whole- and intersection masks (p < .002). Radiomics features in fast scanned T2-FLAIR showed variable stabilities according to the sequences compared with conventional T2-FLAIR. Therefore, radiomics features may be used cautiously in applications for feature analysis as their stability and robustness can be variable.
Similar content being viewed by others
Introduction
The paradigm is shifting from qualitative visual assessment of medical imaging to quantitative data analysis with the development of high-throughput mining of low- to high dimensional data. Radiomic features are considered to be an important alternative for interpretation and analysis of medical images and to predict lesion characteristics with numerous features, from first-order to high-order features1,2,3,4.
However, radiomic features can have limitations in their reproducibility or stability. The stability of radiomic features is still challenging with a lack of standardization during image acquisition, reconstruction, segmentation and analyses even though standardized image processing and feature computation have allowed radiomic features to be stable4. Among the various types of medical imaging, magnetic resonance imaging (MRI) has a variety of imaging acquisition methods and combinations of complicated parameters even in the same imaging sequences, which makes it difficult to apply radiomic features to MRI.
Fast scanned techniques are essential in the acquisition of MRI because of the major limitation of MRI, the need for a long scan time, particularly in emergency situations such as after a suspected cerebral acute ischemic stroke5. Fast scanned images have been realized by using echo-planar imaging (EPI), parallel imaging, echo train length (ETL) and recently introduced advanced techniques such as compressed sensing and simultaneous multi-slice acquisition, and so on6,7,8,9,10,11,12,13. The various techniques have resulted in a very complicated combination of imaging parameters, which can hamper the acquisition of stable radiomic features.
T2-Fluid attenuated inversion recovery (FLAIR) is very commonly used and essential sequence for the evaluation of cerebral acute ischemic stroke patients9,10,11,14,15,16,17,18,19,20. Therefore, T2-FLAIR is an important candidate for the application of radiomic features. However, there have been attempts to reduce the scan time of T2-FLAIR for a long time, which resulted in various parameters of T2-FLAIR. The EPI, parallel imaging, and ETL have been widely used6,9,10,11,12. Nevertheless, the stability of the radiomic features have been poorly investigated.
We hypothesized that radiomic features from fast scanned T2-FLAIR show variability relative to conventional T2-FLAIR. Therefore, the aim of our study was to investigate the stability of radiomic features from various fast scanned T2-FLAIR images in patients with acute ischemic stroke, and to compare the agreement of the radiomic features with conventional T2-FLAIR as a reference standard.
Results
Stability of radiomic features in fast scanned T2-FLAIR compared with conventional T2-FLAIR
The proportions of stable radiomic features in the first group were 13.8% (82/595) in EPI-T2-FLAIR and 5.5% (33/595) in ETL-T2-FLAIR for acute ischemic hyperintensity and 15.6% (93/595) in EPI-T2-FLAIR and 5.1% (30/595) in ETL-T2-FLAIR for chronic ischemic hyperintensity. Proportions of stable radiomic features in the second group were 16.8% (100/595) in TR-T2-FLAIR and 1.8% (11/595) in ETL-T2-FLAIR for acute ischemic hyperintensity and 13.0% (77/595) in TR-T2-FLAIR and 0.7% (4/595) in ETL-T2-FLAIR for chronic ischemic hyperintensity. EPI-T2-FLAIR and TR-T2-FLAIR showed significantly higher proportions of stable radiomic features than those of ETL-T2-FLAIR (p < 0.001). Proportions of stable radiomic features in the first group were 9.7% (58/595) in the EPI-T2-FLAIR intersection and 3.4% (20/595) in the ETL-T2-FLAIR intersection for acute ischemic hyperintensity and 18.0% (107/595) in the EPI-T2-FLAIR intersection and 11.6% (69/595) in the ETL-T2-FLAIR intersection for chronic ischemic hyperintensity. Proportions of stable radiomic features in the second group were 12.1% (72/595) in the TR-T2-FLAIR intersection and 3.9% (23/595) in the ETL-T2-FLAIR intersection for acute ischemic hyperintensity and 9.6% (57/595) in the TR-T2-FLAIR intersection and 1.2% (7/595) in the ETL-T2-FLAIR intersection for chronic ischemic hyperintensity. EPI-T2-FLAIR and TR-T2-FLAIR showed significantly higher proportions of stable radiomic features than those of ETL-T2-FLAIR (p < 0.002). The detailed results are listed in Table 1.
Stable radiomic features in fast scanned T2-FLAIR compared with conventional T2-FLAIR
There were no stable radiomic features across acute and chronic ischemic hyperintensities in the first and second groups. Stable radiomic features across acute and chronic ischemic hyperintensities in the first group were 1.01% (1/99) in gray-level run-length matrix (GLRLM) and 2.22% (1/45) in neighboring gray tone difference matrix (NGTDM). Stable radiomic features across acute and chronic ischemic hyperintensities in the second group were 1.01% (1/99) in GLRLM. Stable radiomic features across acute ischemic hyperintensities in the first group were 2.17% (1/46) in the first order, 2.53% (5/198) in gray-level co-occurrence matrix (GLCM), 4.04% (4/99) in GLRLM, 2.22% (1/45) in local binary pattern (LBP), 1.71% (2/117) in gray-level size zone matrix (GLSZM), and 6.67% (3/45) in NGTDM. Stable radiomic features across chronic ischemic hyperintensities in the first group were 4.44% (2/45) in the second order, 2.02% (4/198) in GLCM, 6.06% (6/99) in GLRLM, 4.27% (5/117) in GLSZM, and 4.44% (2/45) in NGTDM. Stable radiomic features across acute ischemic hyperintensities in the second group were 2.17% (1/46) in the first order, 2.02% (4/198) in GLCM, 2.02% (2/99) in GLRLM, 2.22% (1/45) in LBP, 0.85% (1/117) in GLSZM, and 4.44% (2/45) in NGTDM. Stable radiomic features across chronic ischemic hyperintensities in the second group were 1.01% (1/99) in GLRLM, 2.22% (1/45) in LBP, and 2.22% (1/45) in NGTDM.
There were no stable radiomic features across acute and chronic ischemic hyperintensities using intersection ROIs in the first group. Stable radiomic features across acute and chronic ischemic hyperintensities in the second group were 1.01% (1/99) in GLRLM and 2.22% (1/45) in LBP. Stable radiomic features across acute ischemic hyperintensities in the first group were 4.35% (2/46) in the first order, 2.22% (1/45) in the second order, 1.01% (2/198) in GLCM, 4.04% (4/99) in GLRLM, 2.22% (1/45) in LBP, and 1.71% (2/117) in GLSZM. Stable radiomic features across chronic ischemic hyperintensities in the first group were 2.22% (1/45) in the second order, 2.53% (5/198) in GLCM, 6.06% (6/99) in GLRLM, 5.13% (6/117) in GLSZM, and 4.44% (2/45) in NGTDM. Stable radiomic features across acute ischemic hyperintensities in the second group were 6.52% (3/46) in the first order, 3.03% (6/198) in GLCM, 5.05% (5/99) in GLRLM, 2.22% (1/45) in LBP, 2.56% (3/117) in GLSZM, and 4.44% (2/45) in NGTDM. Stable radiomic features across chronic ischemic hyperintensities in the second group were 1.01% (1/99) in GLRLM and 4.44% (2/45) in LBP. All of the details mentioned above are shown in Figs. 1, 2 and 3.
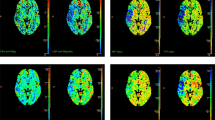
Heat maps of radiomics features extracted from whole- and intersection ROI masks in the first and second groups. Features with CCC > 0.85 were regarded as stable. (a) Features from whole ROI masks in the first group. (b) Features from whole ROI masks in the second group. (c) Features from intersection ROI masks in the first group. (d) Features from intersection ROI masks in the second group. AIH acute ischemic hyperintensity, CIH chronic ischemic hyperintensity, CCC concordance correlation coefficients, ROI region-of-interest, EPI echo-planar imaging, ETL echo train length, TR repetition time.
Proportions of stable radiomic features extracted from whole- and intersection ROI masks on each fast scanned T2-FLAIR sequence in the first and second groups. (a,b) Proportions of stable radiomic features from whole ROI masks (a) and intersection ROI masks (b) according to each fast scanned T2-FLAIR. (c,d) Numbers of stable radiomic features from whole ROI masks (c) and intersection ROI masks (d) according to each fast scanned T2-FLAIR. AIH acute ischemic hyperintensity, CIH chronic ischemic hyperintensity, ROI region-of-interest, EPI echo-planar imaging, ETL echo train length, TR repetition time, GLCM gray-level co-occurrence matrix, GLRLM gray-level run-length matrix, LBP local binary pattern, GLSZM gray-level size zone matric, NGTDM neighboring gray tone difference matrix.
Proportions of stable radiomic features throughout both fast scanned T2-FLAIR sequences extracted from whole- and intersection ROI masks in the first and second groups. (a,b) Proportions of stable radiomic features from whole ROI masks (a) and intersection ROI masks (b). (c,d) Numbers of stable radiomic features from whole ROI masks (c) and intersection ROI masks (d). AIH acute ischemic hyperintensity, CIH chronic ischemic hyperintensity, ROI region-of-interest, EPI echo-planar imaging, ETL echo train length, TR repetition time, GLCM gray-level co-occurrence matrix, GLRLM gray-level run-length matrix, LBP local binary pattern, GLSZM gray-level size zone matric, NGTDM neighboring gray tone difference matrix.
Discussion
This study showed a consistent tendency of higher proportions of reliable features in EPI-T2-FLAIR and TR-T2-FLAIR than ETL-T2-FLAIR in both acute and chronic ischemic hyperintensities and for both whole- and intersection-ROI mask. Therefore, various image acquisitions of T2-FLAIR resulted in unstable radiomic features, which may lead to different radiomic features’ outcomes, such as prediction modeling.
MRI is a useful and sometimes essential imaging modality to identify the infarct core on DWI, and additional useful information can be obtained from various images such as T2-FLAIR or gradient echo (GRE) images, and also allow acquisition of vessel information without the need for contrast media during the evaluation of cerebral acute ischemic stroke patients; however, MRI has a lesser availability and a longer scan time compared to CT5,21,22,23,24,25. Therefore, there have been many attempts to reduce the scan time of MRI in cerebral acute ischemic stroke situations, which has resulted in various MRI sequences and parameters being applied in clinical practice6,9,10,11,12.
Previous studies on fast-scanned T2-FLAIR in acute ischemic stroke showed a consistent superior reliability when compared with that of conventional images6,9,10,11. However, those studies only showed repeatability or reliability in qualitative scoring systems or simple quantitative comparisons, such as signal intensity. In contrast, our study based on radiomic features showed a lower reliability than that of conventional images even though the data was originated from the same registry in the previous study6.
The diversity in the image acquisition makes it difficult to apply radiomic features to MRI for cerebral acute ischemic stroke. Imaging acquisition, segmentation, and feature extraction can affect the stability of radiomic features2. Ford et al. demonstrated that changes of imaging parameters could lead to variable radiomic features in a phantom study26. Minjae et al. only showed that the change in acceleration factors on the same images can affect the stability of radiomic features, and two different under-sampling methods on the same images can show different radiomic features even under the same acceleration factors27. Therefore, different imaging parameters even on the same FLAIR sequences may reduce the stability of radiomic features, as in this study. In addition, these obstacles may affect a few published studies on the predictive models developed using MRI radiomic features in cerebral acute ischemic stroke28,29,30. The results from this study showed some stable radiomic features across variable acquisition of T2-FLAIRs and acute and chronic ischemic hyperintensities, but a substantial proportion showed variability. To our knowledge, there is no previous report on the stability of MRI radiomic features according to various imaging parameters in cerebral acute ischemic stroke. The segmentation can affect the stability of radiomic features. Manual segmentation, compared with semi- or automated segmentations, may lead to lower reproducibility in radiomic features2. However, many previous studies on radiomic features or high dimensional quantitative analyses using artificial intelligence relied on manual segmentations. In addition, Haarburger et al. showed poor reproducibility of some radiomic features even under automated segmentation methods31. The segmentation reproducibility can be influenced by the anatomic location and lesion types27,32. In our study, there were some different results between whole and intersection ROI masks, which may be also owing to different sizes of ROI masks and thus different numbers of pixels. Feature extraction can also affect the stability of radiomic features. Li et al. demonstrated the poor stability of radiomic features (no features > 0.85 in concordance correlation coefficient [CCC]) across different extraction combinations33.
Studies evaluating stable radiomic features in cerebral acute or chronic ischemic lesions based on multiparameteric variances appear to be lacking, and the reproducibility of radiomic features in brain tumors, including glioblastoma, has been reported and several features belonging to GLRLM were identified as reproducible features33,34. GLRLM was also the most reproducible feature in cine balanced steady-state free procession and first-order, and GLCM had the most reproducible features on both T1 and T2 maps in the myocardial radiomic features35. However, a phantom study for test–retest reproducibility reported that GLRLM was neither the most robust nor least robust feature class, while GLCM was one of the least robust feature classes across MRI sequences: FLAIR, T1-weighted, and T2-weighted imaging36. Our study also showed that GLCM and GLRLM are common stable features in the numbers but some variability was seen in the proportions of stable radiomic features.
This study has several limitations. First, this study was designed as a retrospective study with a small population in a single center. A study population cannot be free from selection bias, which may have affected the deviations in sex and age, and a specific MR machine was adopted. Therefore, further multi-center studies with a larger sample size are necessary. Second, we did not compare all of the T2-FLAIRs simultaneously because it is hard to obtain all of the T2-FLAIRs at the same time in a cerebral acute ischemic stroke situation. Third, this study presented only the stabilities of radiomic features in T2-FLAIRs of acute ischemic stroke and an evaluation of the stability of identification or prediction models influencing the treatment options for stroke outcomes using radiomic features from variable T2-FLAIRs is necessary.
In conclusion, the fast-scanned T2-FLAIR showed small proportions of stable radiomic features and variable stability of radiomic features among those in terms of the agreements with conventional T2-FLAIR. Therefore, even if the images in the same sequence have different parameters, the radiomic features obtained from the images may be used with caution.
Methods
Study population
From May 2015 to June 2016, data on 296 patients undergoing MRI at a single tertiary hospital for symptoms of acute ischemic stroke were retrospectively collected. Among them, 118 patients underwent echo-planar imaging (EPI)-T2-FLAIR and echo train length (ETL)-T2-FLAIR and 178 patients underwent ETL-T2-FLAIR and repetition time (TR)-T2-FLAIR simultaneously. In total, 79 patients showed acute ischemic hyperintensity and 89 patients showed chronic ischemic hyperintensity on simultaneous acquisition of EPI- and ETL-T2-FLAIR, who were classified to the first group, and 112 patients showed acute ischemic hyperintensity and 127 patients showed chronic ischemic hyperintensity on simultaneous acquisition of ETL- and TR-T2-FLAIR, who were classified to the second group for comparable paired data analysis. The detailed demographics of the patients are presented in Table 2. The data on patients were collected from the fast stroke MRI registry in our institute6. The institutional review board of Asan Medical Center approved the present study, and the requirement for informed consent was waived. The data was analyzed in compliance with the International Council for Harmonization of Technical Requirements for Registration of Pharmaceutical for Human Use: Guideline for Good Clinical Practice (ICH GCP).
Image acquisition
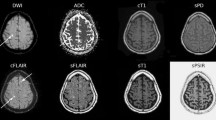
All T2-FLAIR was scanned on a 1.5-T scanner (Magnetom Avanto; Siemens Healthineers). The scan times were 128 s for conventional T2-FLAIR, 45 s for EPI-T2-FLAIR, 74 s for ETL-T2-FLAIR, and 79 s for TR-T2-FLAIR. The detailed scan parameters for the conventional and fast T2-FLAIR were as previously reported6 and are listed in Table 3 and representative images are presented in Fig. 4.
Acute and chronic ischemic hyperintensities on conventional T2-FLAIR and fast scanned T2-FLAIR with respect to DWI. (a) First group with conventional T2-FLAIR, EPI-T2-FLAIR, and ETL-T2-FLAIR. (b) Second group with conventional T2-FLAIR, ETL-T2-FLAIR and TR-T2-FLAIR. DWI diffusion-weighted image, FLAIR fluid attenuated inversion recovery, EPI echo-planar imaging, ETL echo train length, TR repetition time.
Image analysis
The segmentations of acute- and chronic ischemic hyperintensities were conducted as described in a previous report6. We defined acute ischemic hyperintensity as a T2-FLAIR high signal intensity within acute infarcts on diffusion weighted images (DWI)15,17, and chronic ischemic hyperintensity as a hyperintensity outside of acute infarcts on DWI. The segmentation of region-of-interest (ROI) mask was done by one research assistant (K.M.C. with 5 years of experience in stroke imaging) using an in-house program for semiautomatic segmentation (based on ImageJ software; National Institutes of Health, Bethesda, MD). The intersection ROI mask between the respective ROI mask of the conventional and fast T2-FLAIR was obtained after the coregistration and then each intersection ROI mask was transferred into the respective conventional and fast T2-FLAIR images. The intersection ROI mask was used to compare the radiomic features from different T2-FLAIRs without ROI mask differences. A total of 14 kinds of ROI masks were obtained as follows: 3 ROIs from conventional-, EPI-, and ETL-T2-FLAIR in the first group; 2 intersection ROIs from conventional and EPI-T2-FLAIR, and 2 intersection ROIs from conventional and ETL-T2-FLAIR in the first group; 3 ROIs from conventional-, ETL-, and TR-T2-FLAIR in the second group; 2 intersection ROIs from conventional and ETL-T2-FLAIR, and 2 intersection ROIs from conventional and TR-T2-FLAIR in the second group (Fig. 5). From the ROIs, 595 radiomics features were extracted and concordance correlation coefficients (CCC) for radiomic features were calculated between fast scanned and conventional T2-FLAIR in each group.
The outline of the image analysis focused on the types of ROI masks used in this study. Feature extraction was performed from each conventional and fast scanned T2-FLAIR using both whole-(× 3) and intersection ROI masks (× 4) for both the first and second groups (× 2). FLAIR fluid attenuated inversion recovery, ROI region-of-interest, EPI echo-planar imaging, ETL echo train length, TR repetition time.
Radiomic features
Radiomic features were extracted with Matlab R2016a (The Mathworks, Natick, MA): first-order features, texture features, and wavelet-transformed features37,38. The first-order features were acquired based on the histogram analyses of pixel values within the region-of-interest. The second-order features or texture features were as follows: gray-level co-occurrence matrix (GLCM), gray-level run-length matrix (GLRLM), local binary pattern (LBP), gray-level size zone matric (GLSZM), and neighboring gray tone difference matrix (NGTDM). The wavelet transformations extracted additional features. The radiomic features extraction was done under the Imaging Biomarker Standardization Initiative39. Finally, 595 radiomic features were extracted with 46 first-order features, 61 texture features (5 s order, 22 GLCM, 11 GLRLM, 5 LBP, 13 GLSZM, 5 NGTDM) and 488 wavelet features (× 8; 40 s order, 176 GLCM, 88 GLRLM, 40 LBP, 104 GLSZM, 40 NGTDM).
Statistical analysis
The stability of the radiomic features was evaluated using CCC between the features extracted from the conventional- and fast scanned T2-FLAIR based on Lin’s definition40. The proportions of stable radiomic features were compared between EPI and ETL in the first group and between TR and ETL in the second group using McNemar’s test. Radiomic features with a CCC of higher than 0.85 were considered to be stable. All statistical analyses were performed using the MedCalc 15.6.1 software package (MedCalc Software) and R version R 3.3.3 (R Foundation for Statistical Computing; http://www.R-project.org, 2016).
Data availability
The datasets collected during and/or analyzed during the current study may be available from the corresponding author on reasonable request and in compliance with ethical standards under an approval of the local institutional review board.
References
Gillies, R. J., Kinahan, P. E. & Hricak, H. Radiomics: Images are more than pictures, they are data. Radiology 278, 563–577. https://doi.org/10.1148/radiol.2015151169 (2016).
Park, J. E., Park, S. Y., Kim, H. J. & Kim, H. S. Reproducibility and generalizability in radiomics modeling: Possible strategies in radiologic and statistical perspectives. Korean J. Radiol. 20, 1124–1137. https://doi.org/10.3348/kjr.2018.0070 (2019).
van Timmeren, J. E., Cester, D., Tanadini-Lang, S., Alkadhi, H. & Baessler, B. Radiomics in medical imaging-"how-to" guide and critical reflection. Insights Imaging 11, 91. https://doi.org/10.1186/s13244-020-00887-2 (2020).
Zwanenburg, A. et al. The image biomarker standardization initiative: Standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 295, 328–338. https://doi.org/10.1148/radiol.2020191145 (2020).
Kohrmann, M. & Schellinger, P. D. Acute stroke triage to intravenous thrombolysis and other therapies with advanced CT or MR imaging: Pro MR imaging. Radiology 251, 627–633. https://doi.org/10.1148/radiol.2513081074 (2009).
Chung, M. S. et al. Reliability of fast magnetic resonance imaging for acute ischemic stroke patients using a 1.5-T scanner. Eur. Radiol. 29, 2641–2650. https://doi.org/10.1007/s00330-018-5812-5 (2019).
Li, B., Li, H., Dong, L. & Huang, G. Fast carotid artery MR angiography with compressed sensing based three-dimensional time-of-flight sequence. Magn. Reson. Imaging 43, 129–135. https://doi.org/10.1016/j.mri.2017.07.017 (2017).
Li, B. et al. Compressed sensing based simultaneous black- and gray-blood carotid vessel wall MR imaging. Magn. Reson. Imaging 38, 214–223. https://doi.org/10.1016/j.mri.2017.01.013 (2017).
Meshksar, A. et al. Role of EPI-FLAIR in patients with acute stroke: A comparative analysis with FLAIR. AJNR Am. J. Neuroradiol. 35, 878–883. https://doi.org/10.3174/ajnr.A3786 (2014).
Nael, K. et al. Six-minute magnetic resonance imaging protocol for evaluation of acute ischemic stroke: Pushing the boundaries. Stroke 45, 1985–1991. https://doi.org/10.1161/STROKEAHA.114.005305 (2014).
Lin, Z., Guo, Z., Qiu, L., Yang, W. & Lin, M. The applied research of MRI with ASSET-EPI-FLAIR combined with 3D TOF MRA sequences in the assessment of patients with acute cerebral infarction. Acta Radiol. 57, 1515–1523. https://doi.org/10.1177/0284185116628338 (2016).
JM, U.K.-I. et al. Utility of an ultrafast magnetic resonance imaging protocol in recent and semi-recent strokes. J. Neurol. Neurosurg. Psychiatry 76, 1002–1005. https://doi.org/10.1136/jnnp.2004.046201 (2005).
Feinberg, D. A. & Setsompop, K. Ultra-fast MRI of the human brain with simultaneous multi-slice imaging. J. Magn. Reson. 229, 90–100. https://doi.org/10.1016/j.jmr.2013.02.002 (2013).
Song, S. S. et al. A pragmatic approach using magnetic resonance imaging to treat ischemic strokes of unknown onset time in a thrombolytic trial. Stroke 43, 2331–2335. https://doi.org/10.1161/STROKEAHA.111.630947 (2012).
Ebinger, M. et al. Fluid-attenuated inversion recovery evolution within 12 hours from stroke onset: a reliable tissue clock?. Stroke 41, 250–255. https://doi.org/10.1161/STROKEAHA.109.568410 (2010).
Lee, K. Y. et al. Distal hyperintense vessels on FLAIR: An MRI marker for collateral circulation in acute stroke?. Neurology 72, 1134–1139. https://doi.org/10.1212/01.wnl.0000345360.80382.69 (2009).
Petkova, M. et al. MR imaging helps predict time from symptom onset in patients with acute stroke: Implications for patients with unknown onset time. Radiology 257, 782–792. https://doi.org/10.1148/radiol.10100461 (2010).
Cheng, B. et al. Quantitative signal intensity in fluid-attenuated inversion recovery and treatment effect in the WAKE-UP trial. Stroke 51, 209–215. https://doi.org/10.1161/STROKEAHA.119.027390 (2020).
Scheldeman, L. et al. Different mismatch concepts for magnetic resonance imaging-guided thrombolysis in unknown onset stroke. Ann. Neurol. 87, 931–938. https://doi.org/10.1002/ana.25730 (2020).
Grosch, A. S. et al. Extent of FLAIR hyperintense vessels may modify treatment effect of thrombolysis: A post hoc analysis of the WAKE-UP trial. Front. Neurol. 11, 623881. https://doi.org/10.3389/fneur.2020.623881 (2020).
Vert, C., Parra-Farinas, C. & Rovira, A. MR imaging in hyperacute ischemic stroke. Eur. J. Radiol. 96, 125–132. https://doi.org/10.1016/j.ejrad.2017.06.013 (2017).
Rudkin, S., Cerejo, R., Tayal, A. & Goldberg, M. F. Imaging of acute ischemic stroke. Emerg. Radiol. 25, 659–672. https://doi.org/10.1007/s10140-018-1623-x (2018).
Muir, K. W., Buchan, A., von Kummer, R., Rother, J. & Baron, J.-C. Imaging of acute stroke. Lancet Neurol. 5, 755–768. https://doi.org/10.1016/s1474-4422(06)70545-2 (2006).
Puig, J. et al. From “time is brain” to “imaging is brain”: A paradigm shift in the management of acute ischemic stroke. J. Neuroimaging 30, 562–571. https://doi.org/10.1111/jon.12693 (2020).
Thomalla, G. et al. MRI-guided thrombolysis for stroke with unknown time of onset. N. Engl. J. Med. 379, 611–622. https://doi.org/10.1056/NEJMoa1804355 (2018).
Ford, J., Dogan, N., Young, L. & Yang, F. Quantitative radiomics: Impact of pulse sequence parameter selection on MRI-based textural features of the brain. Contrast Media Mol. Imaging 2018, 1729071. https://doi.org/10.1155/2018/1729071 (2018).
Kim, M. et al. Reproducibility of radiomic features in SENSE and compressed SENSE: Impact of acceleration factors. Eur. Radiol. https://doi.org/10.1007/s00330-021-07760-w (2021).
Cui, H., Wang, X., Bian, Y., Song, S. & Feng, D. D. Ischemic stroke clinical outcome prediction based on image signature selection from multimodality data. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 722–725, 2018. https://doi.org/10.1109/EMBC.2018.8512291 (2018).
Tang, T. Y. et al. Penumbra-based radiomics signature as prognostic biomarkers for thrombolysis of acute ischemic stroke patients: A multicenter cohort study. J. Neurol. 267, 1454–1463. https://doi.org/10.1007/s00415-020-09713-7 (2020).
Zhang, R. et al. Apparent diffusion coefficient map based radiomics model in identifying the ischemic penumbra in acute ischemic stroke. Ann. Palliat. Med. 9, 2684–2692. https://doi.org/10.21037/apm-20-1142 (2020).
Haarburger, C. et al. Radiomics feature reproducibility under inter-rater variability in segmentations of CT images. Sci. Rep. 10, 12688. https://doi.org/10.1038/s41598-020-69534-6 (2020).
Pavic, M. et al. Influence of inter-observer delineation variability on radiomics stability in different tumor sites. Acta Oncol. 57, 1070–1074. https://doi.org/10.1080/0284186X.2018.1445283 (2018).
Li, Q. et al. A fully-automatic multiparametric radiomics model: Towards reproducible and prognostic imaging signature for prediction of overall survival in glioblastoma multiforme. Sci. Rep. 7, 14331. https://doi.org/10.1038/s41598-017-14753-7 (2017).
Shiri, I. et al. Repeatability of radiomic features in magnetic resonance imaging of glioblastoma: Test-retest and image registration analyses. Med. Phys. 47, 4265–4280. https://doi.org/10.1002/mp.14368 (2020).
Jang, J. et al. Reproducibility of segmentation-based myocardial radiomic features with cardiac MRI. Radiol. Cardiothorac. Imaging 2, e190216. https://doi.org/10.1148/ryct.2020190216 (2020).
Baessler, B., Weiss, K. & Pinto Dos Santos, D. Robustness and reproducibility of radiomics in magnetic resonance imaging: A phantom study. Invest. Radiol. 54, 221–228. https://doi.org/10.1097/RLI.0000000000000530 (2019).
Kickingereder, P. et al. Radiomic profiling of glioblastoma: Identifying an imaging predictor of patient survival with improved performance over established clinical and radiologic risk models. Radiology 280, 880–889. https://doi.org/10.1148/radiol.2016160845 (2016).
Kang, D. et al. Diffusion radiomics as a diagnostic model for atypical manifestation of primary central nervous system lymphoma: Development and multicenter external validation. Neuro Oncol. https://doi.org/10.1093/neuonc/noy021 (2018).
Zwanenburg, A., Leger, S., Vallières, M. & Löck, S. Image biomarker standardisation initiative. arXiv preprint arXiv:1612.07003 (2016).
Lin, L. I. A concordance correlation-coefficient to evaluate reproducibility. Biometrics 45, 255–268. https://doi.org/10.2307/2532051 (1989).
Funding
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2019R1A2C1089939).
Author information
Authors and Affiliations
Contributions
L.J. made contributions to the analysis/interpretation of data and made the first draft of this work. S.C.J. is responsible for the design and conception of this work, supervised the entire process and completed the revision of the first draft. H.L. made contributions to feature extraction and analysis, and completed the revision of the first draft. S.Y.P. took responsibility for the statistical analysis and advice. M.K. made contributions to the analysis/interpretation of the data. J.E.P. made contribution to this work with advice in terms of radiomics. K.M.C. contributed to this work by data acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joo, L., Jung, S.C., Lee, H. et al. Stability of MRI radiomic features according to various imaging parameters in fast scanned T2-FLAIR for acute ischemic stroke patients. Sci Rep 11, 17143 (2021). https://doi.org/10.1038/s41598-021-96621-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96621-z
This article is cited by
-
Radiomics using non-contrast CT to predict hemorrhagic transformation risk in stroke patients undergoing revascularization
European Radiology (2024)
-
Identification of radiomic biomarkers in a set of four skeletal muscle groups on Dixon MRI of the NAKO MR study
BMC Medical Imaging (2023)
-
Effects of MRI scanner manufacturers in classification tasks with deep learning models
Scientific Reports (2023)
-
Feasibility and intra-and interobserver reproducibility of quantitative susceptibility mapping with radiomic features for intracranial dissecting intramural hematomas and atherosclerotic calcifications
Scientific Reports (2023)
-
Brain tumor detection and classification using machine learning: a comprehensive survey
Complex & Intelligent Systems (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.