Abstract
To develop a tool for predicting pathologic complete response (pCR) after neoadjuvant chemoradiotherapy (neoCRT) in patients with esophageal cancer by combining inflammatory status and tumor glucose metabolic activity. This study included 127 patients with locally advanced esophageal cancer who had received neoCRT followed by esophagectomy from 2007 to 2016. We collected their neutrophil–lymphocyte ratio (NLR) and standardized uptake value (SUV) obtained from fluorodeoxyglucose positron emission tomography (PET/CT) before and after neoCRT. Univariate and multivariate logistic regression analyses were performed to identify potential predictive factors for pCR. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of predictors were calculated. Between pCR and non-pCR groups, there were no statistically significant differences in patient characteristics, such as sex, age, site, and clinical T/N stage. Multivariate analyses identified four independent predictors for pCR, including pre-OP NLR < 5.4 [OR 11.179; 95% CI 8.385–13.495; p = 0.003], NLR change (ΔNLR) < 3 [OR 4.891; 95% CI 2.274–9.180; p = 0.005], changes in SUV (ΔSUV) > 7.2 [OR 3.033; 95% CI 1.354–6.791; p = 0.007], and SUV changes ratio (ΔSUV ratio) > 58% [OR 3.585; 95% CI 1.576–8.152; p = 0.002]. ΔNLR had the highest accuracy and NPV (84.3% and 90.3%, respectively). Combined factors of ΔNLR < 3 and ΔSUV ratio > 58% had the best PPV for pCR (84.8%). Inflammatory status (ΔNLR) and tumor glucose metabolic activity (ΔSUV ratio), when considered together, constitute a promising low-invasive tool with high efficacy for prediction of treatment response before surgery.
Similar content being viewed by others
Introduction
Esophageal cancer was the 7th most common cancer worldwide in 2018 and was ranked the 6th most common cause of cancer-related mortality1. The 5-year overall survival rate (OS) of patients with esophageal cancer treated with surgery alone is ≤ 25%. More than 20% of patients have microscopic positive resection margin after primary surgery, and 31% of patients have locoregional failure2. In locally advanced esophageal cancer, neoadjuvant chemoradiotherapy (neoCRT) followed by surgery improves survival and local control3,4,5,6. The pathological complete response rate (pCR) of this approach ranges from 13 to 47%7,8. Esophagectomy is a major surgery, which results in morbidity, a mortality rate of up to 5%, and impaired quality of life9. In older patients with multiple comorbidities, achieving pCR, the benefit of esophagectomy could be less than the risk of complications. A more precise preoperative prediction of treatment response may help decision-making in the organ-preserving approach, thereby potentially reducing postoperative morbidity and mortality.
Changes associated with inflammation are crucial in tumor development and progression10. The neutrophil-to-lymphocyte ratio (NLR) is an inflammatory-based score with prognostic values in some cancers, such as esophageal cancer, cervical cancer, and hepatocellular cancer11,12,13. Elevated NLR is associated with poor survival outcomes in patients with esophageal cancer treated with neoCRT followed by surgery11. However, it remains unclear whether NLR can predict tumor response after neoCRT.
Apart from inflammatory factors, functional images, such as Fluorodeoxyglucose Positron Emission Tomography (FDG-PET/CT), are also indicators of responses to neoCRT. FDG-PET/CT uses the radiolabeled glucose analog 18F-fluorodeoxyglucose (FDG) to detect the glucose metabolic activity of cancer cells. Changes in FDG uptake after neoCRT reflect changes in glucose metabolic activity by tumor cells which can be used to predict treatment responses14,15. A systemic review demonstrated the ratio of change in maximum standardized uptake value at one hour (ΔSUV ratio) after neoCRT is associated with treatment response. However, the cut-off point of the ΔSUV ratio varies, and the sensitivity and specificity of the ΔSUV ratio are heterogeneous14. It is risky to predict treatment response after neoCRT based solely on tumor glucose metabolic activity.
Here we aimed to determine the predictive values of combined inflammatory status and tumor glucose metabolism in patients with esophageal cancer treated with neoCRT followed by surgery.
Materials and methods
Study population
We first reviewed patients with histologically proven squamous cell carcinoma (SCC) of the esophagus at clinical stage T2-4N0-3M0 based on the 8th edition of the American Joint Committee on Cancer staging manual. Patients who received neoCRT followed by surgery were then included for analysis. The exclusion criteria were: (a) histology other than SCC; (b) distant metastasis at initial diagnosis; (c) synchronous malignancy; (d) previous history of malignancies within five years, except non-melanoma skin cancer or adequately treated carcinoma in situ of the cervix; (e) concurrent chemotherapy other than cisplatin and 5-fluorouracil (5-FU) and (f) incomplete treatment.
Our study was approved by the Institutional Review Board of Taichung Veterans General Hospital. Written informed consent was obtained from all participating patients. All patients were treated according to the National Comprehensive Cancer Network (NCCN) guideline for esophageal cancer.
Treatment protocol
Pretreatment workup included physical examination, hematologic and biochemistry profile, endoscopic esophageal tumor biopsy, chest computed tomography scan (CT), FDG-PET/CT, transesophageal endoscopic ultrasound, and bronchoscopy.
Radiotherapy was given with a total dose from 50 to 50.4 Gy in 1.8 to 2.0 Gy per fraction, five daily fractions per week.
During the treatment period of radiotherapy, concurrent chemotherapy was given intravenously with cisplatin at 20 mg/m2 drip for 2 h and fluorouracil 800 mg/m2 for 24 h on Days 1–4 (cycle 1), and Days 29–32 (cycle 2).
A second PET-CT scan was performed within 3–4 weeks after the completion of neoCRT to evaluate the treatment response before surgery. Patients received surgery within 4–6 weeks after completion of neoCRT. The surgery procedure included esophagectomy, lymph node dissection, and esophagus reconstruction with the gastric tube. Pathologic reports included differentiation, margin status, tumor regression grade (TRG), and nodal metastasis. Pathological complete response (pCR) was defined as the absence of tumor cells in the primary tumor and all dissected lymph nodes.
Inflammatory status and tumor glucose metabolic activity
Data on inflammatory status were extracted from medical records. The neutrophil-to-lymphocyte ratio (NLR) was defined as the absolute neutrophil count divided by the absolute lymphocyte count. All differential counts were taken within 1 week before neoCRT (Pre-CRT NLR) and 1 week before surgery (Pre-OP NLR). The change of NLR (ΔNLR) was defined as pre-OP NLR substrate pre-CRT NLR. Tumor glucose metabolic activity was defined as the maximum standardized uptake value at one hour of primary esophageal tumor which was obtained from PET/CT before neoCRT (pre-CRT SUV) and after neoCRT (pre-OP SUV). The change of SUV (ΔSUV) after neoCRT was defined as pre-CRT SUV minus pre-OP SUV. The ratio of change in SUV (ΔSUV ratio) was defined as ΔSUV divided by pre-CRT SUV.
Statistical analyses
Patients’ characteristics’ included sex, age, histological differentiation, site, clinical T/N classification, pre-treatment tumor length, time interval between neoCRT to surgery, and numbers of dissected lymph nodes. Between pCR and non-pCR groups, the chi-square test or Fisher's exact test was used to compare categorical variables, and the 2-sample t-test was used to compare continuous variables. The associations between clinical parameters and pCR were evaluated by univariate and multivariate analyses using the logistic regression model. Variates with p < 0.05 and confounders, including clinical T stage and clinical N stage in the univariate analysis, were further explored using multivariate analysis. Receiver operating characteristic (ROC) curves for pCR prediction were used to verify the best cut-off point for pre-CRT NLR, pre-OP NLR, ΔNLR, pre-CRT SUV, pre-OP SUV, ΔSUV, and SUV ratio. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated for both tumor glucose metabolic and inflammatory factors.
Survival outcomes were analyzed using the Kaplan–Meier method and log-rank tests. OS was measured from the date of biopsy until death from any cause or time of the most recent follow-up. Progression-free survival (PFS) started from the date of biopsy until death or disease progression. Data were analyzed statistically using SPSS software (version 25.0, IBM, NY).
Ethical approval
This study was approved by the Institutional Review Board of Taichung Veterans General Hospital. (IRB number: CE19242A).
Informed consent
Written informed consent was obtained from all participating patients. All patients were treated according to the National Comprehensive Cancer Network (NCCN) guideline for esophageal cancer.
Results
We retrospectively analyzed 127 patients who had received neoCRT between October 2007 and July 2016. All of them completed neoCRT and then received surgery. Patients’ characteristics are shown in Table 1. The mean age of the entire cohort was 54.77 ± 8.22 years (mean ± SD). The time interval between the date of completing neoCRT and the date of the surgery was 34.71 ± 7.78 days (mean ± SD). The median time interval between the date of completing neoCRT and the date of the second PET-CT was 22 days (IQR 19–25 days). The median follow-up duration of the surviving patients was 59.7 months. The 5-year OS of the entire cohort was 59.1%. Of the 127 patients, 57 of them showed pathological complete responses after neoCRT with a pCR rate of 44.9%.
Clinical features, which were similar between pCR and non-pCR groups, included sex, age, site, clinical T/N stage, histology, time duration to surgery, and numbers of dissected LN.
The potential predictors of pCR estimated by univariate and multivariate analyses are shown in Table 2. Multivariate analyses identified four independent predictors for pCR, including pre-OP NLR < 5.4 [OR 11.179; 95% CI 8.385–13.495; p = 0.003], NLR change (ΔNLR) < 3 [OR 4.891; 95% CI 2.274–9.180; p = 0.005], changes in SUV (ΔSUV) > 7.2 [OR 3.033; 95% CI 1.354–6.791; p = 0.007], and SUV changes ratio (ΔSUV ratio) > 58% [OR 3.585; 95% CI 1.576–8.152; p = 0.002]. ROC curves for pre-OP NLR, ΔNLR, ΔSUV, and ΔSUV ratio in predicting pCR were plotted in Supplementary Information 1. The optimized cut-off values for pre-OP NLR and ΔNLR were 5.4 and 3, respectively. The best cut-off points for ΔSUV and ΔSUV ratio were 7.2 and 58%, respectively. The prediction efficacy of the inflammatory (pre-OP NLR and ΔNLR) and the tumor glucose metabolic factors (ΔSUV and ΔSUV ratio) are shown in Table 3 and 4. The distribution of ΔNLR and ΔSUV ratio in patients with or without pCR is shown in Fig. 1. ΔNLR showed the highest accuracy and NPV (84.3% and 90.3%, respectively). In tumor glucose metabolic markers, ΔSUV ratio showed great sensitivity and NPV (77.8% and 72.7%, respectively). Combined factors of inflammatory status (ΔNLR < 3) and tumor glucose metabolic marker (ΔSUV ratio > 58%) gave the best PPV for pCR (84.8%). Higher PPV had a lower risk of false positive for the prediction of pCR. The risk of pathological residual tumor was 21.5% if using only ΔNLR < 3. This risk could be reduced to 15.2% with the combination of ΔNLR < 3 and ΔSUV ratio > 58%. The odds ratio of combined condition of ΔNLR < 3 and ΔSUV ratio > 58% was 21.543 (95% CI 8.047–57.670). High ΔNLR and low ΔSUV ratio were associated with residual tumor after neoCRT [OR 11.333, 95% CI 3.196–40.184].
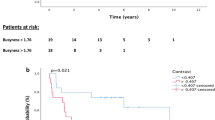
Survival curves of pCR and non-pCR are shown in Figs. 2 and 3. The 5-year OS in the pCR group was 68.4% compared with 51.4% in the non-pCR group (p = 0.010). The 5-year PFS in the pCR group was 63.6% compared with 27.1% in the non-pCR group (p < 0.001).
Discussion
We reviewed the data of patients with locally advanced esophageal SCC to determine the predictive value of combining the conditions of inflammatory status and tumor glucose metabolism. We found that patients with both low ΔNLR (< 3) and high ΔSUV ratio (> 58%) were associated with a higher probability of pCR [OR 21.543, (95% CI 8.047–57.670)]. The PPV was 84.8% based on the combined condition of a low ΔNLR and a high ΔSUV ratio. Patients with high ΔNLR and low ΔSUV showed a higher probability of having residual tumors. To the best of our knowledge, this is the first study to assess the accuracy of combining both inflammatory status and tumor glucose metabolic activity for predicting pCR in patients with esophageal SCC after neoCRT. NLR was calculated with absolute neutrophil and lymphocyte counts derived from differential counts, which is a low-cost and low-invasive method. SUV obtained from PET-CT is also a minimally invasive procedure. After neoCRT, ΔNLR combined with ΔSUV can help physicians predict the treatment response before surgery.
In previous studies, high NLR was found to be associated with poor survival outcomes in esophageal cancer patients11. However, the time points of NLR vary among different reports. Sharaiha et al. analyzed esophageal cancer patients who received surgery with or without neoCRT. They found that high pre-OP NLR (> 5) was associated with poor OS and PFS16. Feng et al. also confirmed this result with a different cut-off point of NLR (> 3.5)17. In a few studies, high pre-CRT NLR was reported to have a negative impact on survival in patients treated with neoCRT followed by surgery18. Yoo et al. reported that a high pre-CRT NLR was also a negative predictor in the definitive CCRT setting19.
Some recent studies have used NLR to predict pCR after neoadjuvant treatments. McLaren et al. analyzed 60 esophageal cancer patients and found that a high pre-CRT NLR was a negative predictor of pCR20. Hyder et al. reported the smaller the increase in NLR (< 4.6), the higher the probability of achieving pCR21. In these studies, an association was found between NLR and pCR. However, these studies involved only a few cases and their predictive efficacy is unclear. In our analysis, we reviewed 127 patients and found that lower NLR increase (ΔNLR < 3) had a 4- to fivefold probability of achieving pCR [OR 1.891; 95% CI 2.274–9.180; p = 0.005].
In the published reports on esophageal cancer patients, changes in pre-OP PET-CT parameters were found to reflect the tumor response after neoCRT and have an impact on survival outcomes15,22. Patients with ΔSUV > 60% after neoadjuvant CCRT showed better OS and disease-free survival15. However, a systematic review of the predictive value of PET-CT for tumor response, based on 20 reports, showed variable ranges in sensitivity (33–100%), specificity (30–100%), and differences in PPV and NPV14. Hence, prediction of treatment response based on PET-CT parameters alone may be risky as the level of accuracy reported in the literature varies considerably.
To improve the predictive value of treatment response, we herein combined ΔNLR and ΔSUV ratio. Inflammation stimulates angiogenesis, influences immune surveillance on tumor cells, and affects treatment response10. As a marker for systemic inflammation, low NLR reflects a low neutrophil count and a high lymphocyte count. Neutrophils are associated with the production of various inflammatory mediators, which are related to systemic inflammation. Lymphocytes are crucial in cell-mediated immunity to initiate tumor cell death. Low NLR represents a low level of systemic inflammation and better cell-mediated immunity toward tumor cells and consequently better response to treatment.
Generally, the intensity of tumor proliferation is correlated with its glucose metabolic activity. Tumor proliferation affects the clinical behavior, which determines prognosis and treatment response. In clinical practice, PET/CT with a radiolabeled glucose analog can detect glucose metabolic activity, which helps to predict prognosis in several malignancies23. Overexpression of glucose transporter 1 (GLUT-1) in SCC was associated with a higher FDG-uptake and a negative survival impact24. In esophageal SCC, FDG-PET/CT was a useful tool for predicting survival and an indicator of residual tumor burden before surgery15. Greater drops in FDG-uptake, measured as SUV after treatment, reflect a decline in tumor metabolism of glucose, or a better response to treatment. Both NLR and SUV are therefore predictors of anticancer treatment response.
Esophagectomy is a major surgical operation associated with morbidities such as leakage (10%), mortality (3 to 5%), and impairment of life quality9. Elderly patients with multiple comorbidities may have higher risks of post-esophagectomy complications25. A more accurate preoperative assessment of treatment response, such as the ΔNLR and ΔSUV ratio, would allow surgeons to make an individualized treatment plan for their patients. Those who are at high risk for postoperative complications, but have a high probability of achieving a pCR, could potentially be spared the morbidity and mortality of surgery. The organ-preserving approach with active surveillance is an alternative treatment for these patients26,27,28. Van der Wilk et al. reported that patients with esophageal cancer who achieved a clinical complete response after neoCRT and underwent active surveillance had a survival outcome comparable to immediate surgery after neoCRT. The 3-year OS was 77% vs. 55% (p = 0.104) and the 3-year PFS was 60% v. 54% (p = 0.871) in the active surveillance and immediate surgery groups, respectively27. Taketa et al. described similar findings. The median OS was not statistically different between the patients who declined surgery (DS) and those who received surgery (trimodality therapy, TMT) after neoCRT (57.9 months v. 50.8 months, p = 0.28). There was a favorable trend toward TMT in relapse-free survival (RFS), but the difference in 3-year RFS was not statistically significant (23.8% v. 45.2%, p = 0.45)28.
This study had some limitations. The investigation was retrospective in nature, was conducted in a single institution, and the sample size was relatively small in 9 years. One advantage of this study was the highly homogenous treatment protocol that was applied in all reviewed patients. Another advantage was the high compliance of patients who received neoCRT. All of our patients completed radiotherapy and two cycles of concurrent chemotherapy.
Conclusion
Change of both NLR and SUV ratio after neoCRT were good predictors of pCR. This finding may help decision-making with respect to the organ-preserving approach in elderly patients with comorbidities.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Kelsen, D. P. et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N. Engl. J. Med. 339, 1979–1984 (1998).
Tepper, J. et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J. Clin. Oncol. 26, 1086–1092 (2008).
van Hagen, P. et al. CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 366, 2074–2084 (2012).
Shapiro, J. et al. CROSS study group. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 16, 1090–1098 (2015).
Yang, H. et al. AME Thoracic Surgery Collaborative Group. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A Phase III multicenter, randomized, open-label clinical trial. J. Clin. Oncol. 36, 2796–2803 (2018).
Hamai, Y. et al. Results of neoadjuvant chemoradiotherapy with docetaxel and 5-fluorouracil followed by esophagectomy to treat locally advanced esophageal cancer. Ann Thorac Surg. 99, 1887–1893 (2015).
Lin, J. W., Hsu, C. P., Yeh, H. L., Chuang, C. Y. & Lin, C. H. The impact of pathological complete response after neoadjuvant chemoradiotherapy in locally advanced squamous cell carcinoma of esophagus. J. Chin. Med. Assoc. 81, 18–24 (2018).
Kassis, E. S. et al. Predictors of anastomotic leak after esophagectomy: An analysis of the society of thoracic surgeons general thoracic database. Ann. Thorac. Surg. 96, 1919–1926 (2013).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Yodying, H. et al. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: A systematic review and meta-analysis. Ann. Surg. Oncol. 23, 646–654 (2016).
Zheng, J. et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: A meta-analysis and systematic review. Cell Physiol. Biochem. 44, 967–981 (2017).
Zhang, Y. et al. Preoperative neutrophil-lymphocyte ratio before platelet-lymphocyte ratio predicts clinical outcome in patients with cervical cancer treated with initial radical surgery. Int. J. Gynecol. Cancer 24, 1319–1325 (2014).
Kwee, R. M. Prediction of tumor response to neoadjuvant therapy in patients with esophageal cancer with use of 18F FDG PET: A systematic review. Radiology 254, 707–717 (2010).
Huang, J. W. et al. To evaluate the treatment response of locally advanced esophageal cancer after preoperative chemoradiotherapy by FDG-PET/CT scan. J. Chin. Med. Assoc. 78, 229–234 (2015).
Sharaiha, R. Z. et al. Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann. Surg. Oncol. 18, 3362–3369 (2011).
Feng, J. F., Huang, Y. & Chen, Q. X. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J. Surg. Oncol. 12, 58 (2014).
Miyata, H. et al. Prognostic value of an inflammation-based score in patients undergoing pre-operative chemotherapy followed by surgery for esophageal cancer. Exp. Ther. Med. 2, 879–885 (2011).
Yoo, E. J. et al. Prognostic value of neutrophil-to-lymphocyte ratio in patients treated with concurrent chemoradiotherapy for locally advanced oesophageal cancer. Dig. Liver Dis. 46, 846–853 (2014).
McLaren, P. J. et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios can predict treatment response to neoadjuvant therapy in esophageal cancer. J. Gastrointest. Surg. 21, 607–613 (2017).
Hyder, J., Boggs, D. H., Hanna, A., Suntharalingam, M. & Chuong, M. D. Changes in neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios during chemoradiation predict for survival and pathologic complete response in trimodality esophageal cancer patients. J. Gastrointest. Oncol. 7, 189–195 (2016).
Kim, S. J., Koo, P. J. & Chang, S. Predictive value of repeated F-18 FDG PET/CT parameters changes during preoperative chemoradiotherapy to predict pathologic response and overall survival in locally advanced esophageal adenocarcinoma patients. Cancer Chemother. Pharmacol. 77, 723–731 (2016).
Oyama, N. et al. Prognostic value of 2-deoxy-2-[F-18]fluoro-d-glucose positron emission tomography imaging for patients with prostate cancer. Mol. Imaging Biol. 4, 99–104 (2002).
Kato, H. et al. Correlation of 18-F-fluorodeoxyglucose (FDG) accumulation with glucose transporter (Glut-1) expression in esophageal squamous cell carcinoma. Anticancer Res. 4, 3263–3272 (2003).
Alibakhshi, A. et al. The effect of age on the outcome of esophageal cancer surgery. Ann. Thorac. Med. 4, 71–74 (2009).
van der Wilk, B. J. et al. Towards an organ-sparing approach for locally advanced esophageal cancer. Dig. Surg. 36, 462–469 (2019).
van der Wilk, B. J. et al. Active surveillance versus immediate surgery in clinically complete responders after neoadjuvant chemoradiotherapy for esophageal cancer. Ann. Surg. https://doi.org/10.1097/SLA.0000000000003636 (2019) (Epub ahead of print).
Taketa, et al. Propensity-based matching between esophagogastric cancer patients who had surgery and who declined surgery after preoperative chemoradiation. Oncology 85, 95–99 (2013).
Acknowledgements
The authors would like to thank Dr. Paul W.F. Poon and Mr. Peter Wilds for their advice on medical writing.
Author information
Authors and Affiliations
Contributions
Conceptualization: C.C.C., H.L.Y., C.Y.C. Data collection: C.L., J.W.L. Analysis of data and interpretation: C.L., J.W.L, C.C.C. Drafting the manuscript: C.L. Writing–review & editing: C.C.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, C., Lin, JW., Yeh, HL. et al. Good prediction of treatment responses to neoadjuvant chemoradiotherapy for esophageal cancer based on preoperative inflammatory status and tumor glucose metabolism. Sci Rep 11, 11626 (2021). https://doi.org/10.1038/s41598-021-90753-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90753-y
This article is cited by
-
Neoadjuvant Chemoradiation Followed by Surgery for Locally Advanced Squamous Cell Carcinoma Esophagus: Demographics and Evaluation of Prognostic Factors at a Tertiary Care Center in India
Indian Journal of Surgical Oncology (2023)
-
Comparison of the effectiveness of chemotherapy combined with immunotherapy and chemotherapy alone in advanced biliary tract cancer and construction of the nomogram for survival prediction based on the inflammatory index and controlling nutritional status score
Cancer Immunology, Immunotherapy (2023)
-
Pretreatment 18F-FDG PET/CT-Derived Parameters in Predicting Clinical Outcomes of Locally Advanced Upper Third Esophageal Squamous Cell Carcinoma After Definitive Chemoradiation Therapy
Nuclear Medicine and Molecular Imaging (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.