Abstract
Nanaomycin K, derived from Streptomyces rosa subsp. notoensis OS-3966T, has been discovered to have inhibitory bioactivity on epithelial–mesenchymal transition (EMT), an important mechanism of cancer cell invasion and migration. In this study, we examined the anti-EMT and anti-tumor effect of nanaomycin K in bladder cancer, where EMT has important roles in progression. We treated two bladder cancer lines, non-muscle-invasive KK47 and muscle-invasive T24, with nanaomycin K to determine the effects on cell proliferation, apoptosis and expression of EMT markers in vitro. Wound-healing assays were performed to assess cell invasion and migration. We conducted an in vivo xenograft study in which mice were inoculated with bladder cancer cells and treated with intratumoral administration of nanaomycin K to investigate its anti-tumor and EMT inhibition effects. As the results, nanaomycin K (50 µg/mL) significantly inhibited cell proliferation in KK47 (p < 0.01) and T24 (p < 0.01) in the presence of TGF-β, which is an EMT-inducer. Nanaomycin K (50 µg/mL) also significantly inhibited cell migration in KK47 (p < 0.01) and T24 (p < 0.01), and induced apoptosis in both cell lines in the presence of TGF-β (p < 0.01). Nanaomycin K increased the expression of E-cadherin and inhibited the expression of N-cadherin and vimentin in both cell lines. Nanaomycin K also decreased expression of Snail, Slug, phospho-p38 and phospho-SAPK/JNK especially in T24. Intratumoral administration of nanaomycin K significantly inhibited tumor growth in both KK47 and T24 cells at high dose (1.0 mg/body) (p = 0.009 and p = 0.003, respectively) with no obvious adverse events. In addition, nanaomycin K reversed EMT and significantly inhibited the expression of Ki-67 especially in T24. In conclusion, we demonstrated that nanaomycin K had significant anti-EMT and anti-tumor effects in bladder cancer cells, suggesting that nanaomycin K may be a therapeutic candidate for bladder cancer treatment.
Similar content being viewed by others
Introduction
Nanaomycins were isolated from a cultured broth of actinomycete strain Streptomyces rosa subsp. notoensis OS-3966T, known to produce 11 nanaomycin compounds (Nanaomycin A–E and F–J)1,2. In our previous study, we found that nanaomycin H, an 8th analog of nanaomycin, selectively killed mechanically and chemically-induced epithelial–mesenchymal transition (EMT) driven Madin–Darby canine kidney (MDCK) cells in vitro3. Nanaomycin K, an 11th analog of nanaomycin, was discovered from the culture broth of strain OS-3966 and showed higher bioactivity and cytotoxicity against MDCK cells and inhibition of EMT induced by transforming growth factor (TGF)-β1 compared to nanaomycin H in vitro4.
EMT is an important mechanism whereby cancers cells acquire the abilities of invasion and migration. EMT involves the loss of E-cadherin and increased expression of several transcriptional repressors of E-cadherin (Zeb-1, Zeb-2, Twist, Snail, and Slug) and overexpression of N-cadherin and vimentin, with resulting changes that lead to decreased cell adhesion and loss of polarity and tight cell junctions5. At the same time, epithelial cells acquire mesenchymal phenotypes, notable motility and capability for invasion and metastasis5. Transforming growth factor (TGF)-β is a secreted cytokine that promotes cell invasion and migration, and also promotes EMT in many cancer types including breast cancer, gastric cancer and bladder cancer, resulting in metastasis and chemotherapy resistance6.
In the progression of bladder cancer, tumor invasiveness is an important factor for patient outcomes and for deciding on therapeutic options. Muscle-invasive cancer has limited therapeutic options and once metastasis occurs only chemotherapeutic treatments and immune checkpoint inhibitors are currently available7 EMT has important roles in the progression of bladder cancer in from non-muscle invasive to muscle-invasive, resulting in metastasis and poor clinical outcome8. Mesenchymal phenotypes are also correlated with poor prognosis and associated with resistance to chemotherapy9. Therefore, EMT-targeted therapy is a rational therapeutic possibility for treating invasive bladder cancers.
In this study, we examined the anti-tumor effect of nanaomycin K on inhibition of EMT in bladder cancer, as a potential therapeutic candidate for muscle-invasive bladder cancer in vitro and in vivo. We also compared the bioactivity in non-muscle invasive and muscle-invasive cancer to evaluate EMT inhibition.
Materials and methods
Cells and reagents
Two human urothelial carcinoma cell lines, non-invasive KK47 (Cell Resource Center for Biomedical Research Institute of Development, Aging and Cancer, Tohoku University, Miyagi, Japan) and invasive T24 (American Type Culture Collection, Manassas, VA), were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MI), 1% penicillin and streptomycin at 37 °C and 5% CO210. Nanaomycin K was isolated from a cultured broth of S. rosa subsp. notoensis OS-3966T and purified as previously described4. Nanaomycin K was dissolved and diluted with dimethyl sulfoxide (DMSO).
Cell proliferation assay
We performed cell proliferation assays using KK47 and T24 in the presence of nanaomycin K to determine anti-tumor bioactivity in vitro. Two thousand KK47 and T24 cells were seeded for 24 h and then divided into 3 groups and switched to media10 with or without 5 ng/mL TGF-β (FUJIFILM Wako Pure Chemicals, Osaka, Japan), or 5 ng/mL TGF-β11 and 36 ng/mL SB-431542 (FUJIFILM Wako Chemicals), a TGF-β receptor inhibitor. After switching media, two different concentrations of nanaomycin K (5 µg/mL, 50 µg/mL) or DMSO (0.05%, 0.5%) were added to the cultures. After incubation for 0, 24, 48, 72 and 96 h, cell proliferation was measured by using 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) (Promega Corporation, Madison, WI) according to the manufacturer’s instructions. All experiments were carried out in triplicate.
Wound healing assay
We performed cell proliferation assays using KK47 and T24 in the presence of nanaomycin K to determine anti-tumor bioactivity in vitro. 1 × 105 cells were seeded and incubated overnight and then divided into 2 groups and switched to media with or without 5 ng/mL TGF-β for 24 h. After incubation, nanaomycin K (5 µg/mL or 50 µg/mL) or 0.05% DMSO were added to the culture. After incubation for 48 h, cells were incubated with 10 µg/mL mitomycin C for 2 h to suppress cell proliferation. After washing the cells with medium, cell monolayers were scratched by 200 µL pipette tips and then washed and incubated with fresh medium for an additional 40 h. Microscopic images were taken at time points 0, 18, 24 and 40 h after scratching. Wound closure was calculated by following formula: (wound area at 0 h − wound area at time)/(wound area at 0 h) × 100. All experiments were carried out in quadruplicate.
Real time RT-PCR for EMT markers
We performed real time RT-PCR to determine the gene expression of EMT markers E-cadherin, N-cadherin and vimentin in the presence of nanaomycin K in the tested bladder cancer cell lines. 1 × 105 KK47 and T24 cells were seeded and incubated overnight, and then divided into 2 groups and switched to media with or without 5 ng/mL TGF-β. After incubation for 24 h, 5 µg/mL nanaomycin K or 0.05% DMSO was added to the cultures. After incubation for an additional 48 h, cells were collected and total RNA was extracted using NucleoSpin RNA (TaKaRa Bio, Inc, Kusatsu, Japan). Then cDNAs were synthesized by reverse transcription of the extracted total RNAs with a PrimeScript RT reagent kit with gDNA Eraser (TaKaRa Bio, Inc), and real-time RT-PCR assays were performed with primer sets as described in Table 1, TB Green Premix Ex TaqII (TaKaRa Bio, Inc) and Thermal Cycler Dice Real Time System (TaKaRa Bio, Inc). Data analysis was performed by the ΔΔCt method12.
Western blotting
Cells were seeded and incubated overnight, and then divided into 2 groups and switched to media with or without 5 ng/mL TGF-β. After incubation for 24 h, nanaomycin K (5 µg/mL or 50 µg/mL) or 0.05% DMSO was added to the cultures. After incubation for an additional 48 h, cells were washed and lysed in 8 M urea buffer. Each sample was added into sample buffer (Nacalai Tesque, Kyoto, Japan) and heated at 95 °C for 5 min. The samples were separated by SDS-PAGE and transferred to PVDF membranes. After blocking with Blocking One or Blocking One-P (Nacalai Tesque) followed by washing, the membranes were incubated overnight at room temperature with anti-E-cadherin (Biolegend, San Diego, CA), anti-N-cadherin (Biolegend), anti-vimentin (Biolegend), anti-phospho-p38 MAPK (Thr180/Tyr182) (Cell Signaling Technology: CST, Danvers, MA), anti-phospho-SAPK/JNK (Thr183/Tyr185) (CST), anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (CST), anti-Snail (CST), anti-Slug (CST) or anti-β-actin (Santa Cruz Biotechnology, Dallas, TX), respectively. After another washing, membranes were incubated for 1 h with HRP-conjugated secondary antibodies. Antibody binding to proteins was detected by enhanced chemiluminescence.
Detection of apoptosis
To investigate the mechanism of inhibitory effect of nanaomycin K on cell proliferation, 1 × 105 cells were seeded for 24 h, then switched to media containing 5 ng/mL TGF-β. After incubation for 24 h, nanaomycin K (5 µg/mL or 50 µg/mL) or DMSO (0.05% or 0.5%) was added to the cultures. After incubation for 48 h, cells were stained by Annexin V-FITC and PI (Nacalai Tesque) according to the manufacturer’s instructions. Apoptotic cells were determined by flow cytometry using the Guava easyCyte cytometer (Luminex, Austin, TX) and analyzed by InCyte software (version 3.1) (Luminex).
Animal experiments
Animal experiments using a mouse bladder cancer model were done to investigate the anti-tumor effects of nanaomycin K. Male 6–8 week-old BALB/c nu/nu mice were purchased from CLEA Japan, Inc (Tokyo, Japan). 1 × 106 cells were inoculated at day 0 (n = 4, respectively) with Matrigel (Corning, Corning, NY). Mice were randomly assigned to 2 treatment groups (0.5 mg/body and 1.0 mg/body of nanaomycin K) and control groups (DMSO). Each dose of nanaomycin K was intratumorally administered with Gelform (Pfizer, New York, NY). Tumor volume was expressed by the following formula: (longest diameter) × (shortest diameter)2 × 0.5. Nine days after treatment, mice were sacrificed and tumors were collected10.
Immunohistochemical staining
Tumors were fixed and embedded with paraffin. Paraffin-embedded tissue sections were deparaffinized and rehydrated. Antigen retrieval was performed in citrate buffer (pH 6.0) at 120 °C for 5 min. Immunohistochemical staining (IHC) was performed in an automatic tissue processor (Bond-Max; Leica Microsystems, Wetzlar, Germany) following the standard protocol. Briefly, tissue sections were incubated with the following primary antibodies: anti-E-cadherin, anti-N-cadherin, anti-vimentin, and anti-Ki-6710. After washing, sections were exposed to HRP-conjugated secondary antibodies, according to the instrument’s standard protocols. Tissue sections were incubated with diaminobenzidine and counterstained with hematoxylin. The resulting tissue slides were observed under a BZ-X710 microscope (Keyence, Osaka, Japan).
Immunohistochemical analysis
IHC scoring was based on the percentage of positive cells. The staining intensity was scored as 0 (negative), 1+ (weak), 2+ (medium) or 3+ (strong). The percentage of stained cells was categorized as: 1, 0–10%; 2, 11–50%; and 3, more than 50% stained cells. The total IHC score was determined by multiplying the frequency and intensity scores10.
Ethical approval
All procedures in the animal studies were performed in accordance with the institutional ethical standards in compliance with the ARRIVE guidelines (http://www.nc3rs.org.uk/page.asp?id=1357) and all relevant guidelines and regulations. This article does not contain any studies with human participants performed by any of the authors.
Statistical analysis
Comparisons between two different groups were performed using Student’s t test. Comparisons between multiple groups were performed using one-way analysis of variance (ANOVA) followed by the Tukey–Kramer method. Statistical differences among means were considered significant when p < 0.05.
Research involving human participants and/or animals
All aspects of the experimental design and procedure were reviewed and approved by the institutional ethics and animal welfare committees of Kobe University.
Results
Nanaomycin K inhibited the cell growth of bladder cancer cells under EMT induced by TGF-β
Both 5 µg/mL and 50 µg/mL of nanaomycin K significantly inhibited KK47 and T24 cancer cell growth in the presence of TGF-β compared to controls after 72 h of culture (p < 0.01, or p < 0.01) (Fig. 1). The higher concentration of nanaomycin K showed early cell cytotoxicity and strongly inhibited cell growth at 72 h in vitro. In contrast, nanaomycin K did not significantly inhibit cell growth in culture without TGF-β or with TGF-β with SB-431542, a TGF-β receptor inhibitor, suggesting that the observed cytotoxicity depended on TGF-β signaling.
Cell cytotoxicity of nanaomycin K against KK47 and T24 in vitro. In vitro cell proliferation assays in KK47 and T24 cell lines were treated with 5 µg/mL nanaomycin K or 50 µg/mL nanaomycin K, with or without TGF-β, and with or without SB-431542 for 72 h. Control cells were treated with DMSO (vehicle control) (n = 3, average ± SE bars, *p < 0.05, **p < 0.01). Graphs show the relative fold changes of cell proliferation normalized by that at 0 h culture.
Migration inhibitory effect of nanaomycin K
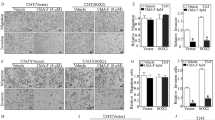
TGF-β promoted wound closure in both KK47 and T24 cells. A low concentration (5 µg/mL) of nanaomycin K did not inhibit wound closure in either cell line with or without TGF-β, but a high concentration (50 µg/mL) of nanaomycin K significantly inhibited wound closure in both KK47 and T24 in the presence of TGF-β, 24 h or 40 h culture after scratching (p < 0.05, p < 0.01) (Fig. 2A,B). These results suggested that nanaomycin K inhibited the cell migration driven by TGF-β.
Wound healing assay. Invasion and migration ability was investigated in the presence of nanaomycin K (5 µg/mL or 50 µg/mL) with or without TGF-β in KK47 and T24 cell lines (100 ×) up to 40 h cultures (A). Control cells were treated with DMSO (vehicle control). Representative figures are shown. Wound closures compared to the wound at 0 h were shown (B) (n = 4, average ± SE bars, *p < 0.05, **p < 0.01).
Changes in EMT properties after culture with nanaomycin K
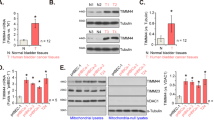
In the presence of TGF-β, nanaomycin K significantly decreased the gene expression of N-cadherin (p < 0.01) and vimentin (p < 0.01), and increased E-cadherin expression (p < 0.01) (Fig. 3). Without TGF-β, nanaomycin K significantly increased the expression of E-cadherin and decreased N-cadherin expression (p < 0.01) in KK47 cells. In T24 cells, nanaomycin K significantly decreased vimentin (p < 0.01) and increased E-cadherin expression (p < 0.01) in the presence of TGF-β. Nanaomycin K also inhibited EMT without TGF-β, but significance was seen only in vimentin (p < 0.01), indicating that T24 already had mesenchymal phenotypes. These results suggested that nanaomycin K inhibited EMT and induced epithelial properties in bladder cancer cell lines.
Gene expression of EMT markers induced by nanaomycin K in vitro. KK47 and T24 cells were treated with 5 µg/mL nanaomycin K with or without TGF-β in vitro for 48 h and the gene expressions of E-cadherin, N-cadherin and vimentin were determined by real time RT-PCR. Control cells were treated with DMSO (vehicle control) and mRNA levels were standardized by the expression levels of control gene β-actin (n = 3, average ± SE bars, *p < 0.05, **p < 0.01).
Expression of EMT-related protein and MAPK signaling after culture with nanaomycin K
A low concentration (5 µg/mL) of nanaomycin K increased the expression of E-cadherin in KK47 without TGF-β while a high concentration (50 µg/mL) of nanaomycin K increased E-cadherin expression in T24 in the presence of TGF-β (Fig. 4). N-cadherin and vimentin were decreased after treatment with a high concentration of nanaomycin K both in cell lines.
Western blotting for EMT and MAPK signaling. The expressions of E-cadherin, N-cadherin, vimentin, Snail, Slug, phopho-p38, phospho-SAPK/JNK, phospho-ERK1/2, and β-actin were determined in the presence of nanaomycin K (5 µg/mL or 50 µg/mL) with or without TGF-β in vitro for 48 h in KK47 and T24 cells. Full-length blots are presented in Supplementary Figure S1.
The expression of Snail, a family of transcription factors that induce EMT, was apparently decreased in T24 by high concentrations of nanaomycin K independently of TGF-β stimulation, but a similar effect was not seen in KK47. High concentrations of nanaomycin K remarkably decreased the expression of Slug in KK47 and T24 independently of TGF-β stimulation.
Regarding the MAPK signaling pathway, a high concentration of nanaomycin K remarkably decreased the expression of phospho-p38 and phospho-SAPK/JNK in both KK47 and T24 cells, but not phospho-ERK1/2. In particular, more intensive EMT and MAPK inhibition by nanaomycin K was seen in T24 cells compared to KK47.
Detection of apoptosis induced by nanaomycin K
High concentration (50 µg/mL) of nanaomycin K induced significantly higher populations of late apoptotic cells compared to controls and low concentrations (5 µg/mL) of nanaomycin K in both KK47 and T24 cells after 48 h treatment (p < 0.01). Apoptosis induction was significantly augmented by TGF-β (p < 0.05) (Fig. 5). These results suggested that nanaomycin K strongly induced apoptosis in cells undergoing EMT.
Apoptosis detection. KK47 and T24 cells were treated with nanaomycin K (5 µg/mL or 50 µg/mL) with or without TGF-β in vitro for 48 h and the proportions of late apoptotic cells were detected by Annexin-V-FITC and propidium iodide (PI) (n = 3, average ± SE bars, *p < 0.05, **p < 0.01). Representative dot plots of each treatment are shown. Data were analyzed by InCyte software (version 3.1, https://www.luminexcorp.com/ja/guava-easycyte-flow-cytometers/#software) (Luminex).
Nanaomycin K inhibited tumor growth in vivo
Intratumoral administration of nanaomycin K at both 0.5 mg and 1.0 mg/body significantly inhibited KK47 tumor growth after 9 days of treatment compared to controls (p = 0.012 and p = 0.009, respectively) (Fig. 6). No obvious adverse events were observed in either group after treatment. In T24 bladder cancer, both low and high doses of nanaomycin K significantly inhibited tumor growth 9 days after treatment compared to controls (p = 0.041 and p = 0.003, respectively), but in both tumor models the higher concentration of nanaomycin K completely suppressed tumor growth, suggesting that anti-tumor effects of nanaomycin K are dose-dependent.
In vivo tumor inhibitory effects of nanaomycin K. Two bladder cancer cell lines, KK47 and T24, were subcutaneously inoculated into BALB/c nu/nu mice. After tumor growth was confirmed, mice were intratumorally treated with 0.5 mg/body nanaomycin K, 1.0 mg/body nanaomycin K, or vehicle control at day 1 (n = 4). After treatment, the tumor volume was measured for 9 days. The tumor volume of each mouse was standardized by that at day 1 and the tumor growth ratio is shown in the graphs (n = 4, average ± SE bars).
Changes of EMT properties and cell proliferation in tumor tissues after treatment with nanaomycin K
The expression of E-cadherin was significantly increased by both 0.5 mg/body and 1 mg/body doses of nanaomycin K compared with control mice in KK47 (p = 0.016 and p = 0.012, respectively). In T24 tumor, 0.5 mg/body nanaomycin K significantly increased the expression of E-cadherin (p = 0.005). The results of IHC scoring were shown Fig. 7A. Representative pictures are shown in Fig. 7B–E. N-cadherin expression were decreased in both tumors, but statistical significance was seen only in T24 treated by 1 mg/body of nanaomycin K (p = 0.001). The expression of vimentin was also decreased by nanaomycin K in both tumors, but no statistical significance was seen. Expression of Ki-67, a cell proliferation marker, was significantly decreased by nanaomycin K in T24 (p = 0.035) (Fig. 7A,E). These results suggested that intratumoral administration of nanaomycin K inhibited EMT and tumor growth, especially in muscle-invasive bladder cancer.
(A–E) Immunohistochemical analysis of KK47 and T24 mouse tumors for EMT markers and Ki-67 after nanaomycin K treatment. Marker expressions of E-cadherin, N-cadherin, vimentin and Ki-67 in tumor tissues were determined by immunohistochemical staining and evaluated by staining score (0–9) (A). Representative images for each marker is shown in (B) E-cadherin, (C) N-cadherin, (D) vimentin, (E) Ki-67, respectively.
Discussion
In this study, we investigated the anti-tumor activity and EMT inhibitory effect of nanaomycin K against two bladder cancer cell lines, one muscle-invasive and one non-muscle invasive, in vitro and in vivo. Nanaomycin K, isolated from a cultured broth of S. rosa subsp. notoensis OS-3966T, is a new nanaomycin analog with an ergothioneine moiety in the partial structure4. It has stronger bioactivity for killing MDCK cells undergoing EMT than nanaomycin H. Therefore, in this study we explored the bioactivity of nanaomycin K as an EMT inhibitor in bladder cancer cells, where EMT plays a major role in invasion, migration and cancer progression.
In our in vitro study showed that nanaomycin K significantly inhibited tumor proliferation, invasion and migration via EMT in non-muscle invasive KK47 and muscle-invasive T24 cancer cells, especially in the presence of TGF-β. Nanaomycin K showed dose-dependent cell cytotoxicity when cells were stimulated by TGF-β. Many studies have demonstrated that TGF-β family members are potent initiators of EMT in cancer cells13,14. We also demonstrated that SB-431542, a TGF-β signaling inhibitor, suppressed the inhibitory effect of nanaomycin K on cancer cell proliferation in vitro. SB-431542 is a known potent selective inhibitor of TGF-β receptors ALK5 and ALK7, which are responsible for the phosphorylation of Smad215. These findings indicated that nanaomycin K was specifically cytotoxic to cancer cells undergoing EMT in response to TGF-β stimulation.
Cancer cell invasion and migration have important roles in cancer metastasis associated with EMT. Wound healing assay showed that nanaomycin K significantly inhibited the wound healing of KK47 and T24 cells stimulated with TGF-β, suggesting that nanaomycin K had an inhibitory effect on cell migration. TGF-β induced EMT and invasiveness in bladder cancer cells as an EMT model by proteomics analysis16. Aggressive tumors such as T24 often accelerate cell motility, invasion and survival by inducing EMT through TGF-β in an autocrine and paracrine manner17, supporting the finding that T24 had already acquired mesenchymal cell phenotypes.
Regarding EMT-related genes and proteins, we demonstrated that nanaomycin K significantly increased the expression of E-cadherin in both KK47 and T24, especially when the cells were treated with TGF-β. At the protein level, nanaomycin K remarkably increased the expression of E-cadherin and decreased N-cadherin and vimentin expression in both cell lines. We also demonstrated that Snail and Slug, both transcription factors involved in EMT and wound healing18 were apparently decreased by nanaomycin K, especially in T24, supporting the conclusion that nanaomycin K reversed EMT and altered the cancer cells to acquire an epithelial cell phenotype.
At the molecular level, EMT is characterized by loss of E-cadherin. Once wound healing occurs, EMT facilitates repair by promoting epithelial migration to the site of injury. Then, when repair starts, epithelial cells re-express E-cadherin via a process of mesenchymal-to-epithelial transition (MET)8. EMT promoted stemness involving the overexpression of SOX2 and NANOG in muscle-invasive bladder cancer clinical samples. Islam et al. also reported that the EMT induced by TGF-β possibly correlated with the increased stemness phenotype in bladder cancer, and suggested that EMT inhibition may be a possible target to reverse the stemness19. These reports support the conclusion that nanaomycin K reverses TGF-β-induced EMT and possibly stemness in bladder cancer cells.
We experimentally observed later cell apoptosis was induced by nanaomycin K in both KK47 and T24 cells, especially when cells were stimulated with TGF-β. The roles of TGF-β in cell apoptosis, vary during cancer progression and remain very controversial in bladder cancer tumorigenesis. At later apoptosis stages, when cancer cells have undergone oncogenic mutation and/or have lost tumor suppressor gene functions, TGF-β has a role as a promotor by stimulating tumor cells to undergo EMT6. Our data demonstrated that nanaomycin K specifically killed bladder cancer cells which were undergo EMT by induction of late apoptosis.
We also found that nanomycin K decreased the protein expression of phospho-p38 and phospho-SAPK/JNK but not phospho-ERK1/2 in T24 cells. Reportedly, ERK1/2, JNKs, and p38, in the MAPK signaling pathway family, regulate EMT in bladder tissues, and the increase of those proteins significantly correlated with EMT in bladder cells20. Our findings suggested that nanaomycin K could inhibit EMT thorough the suppression of JNK and the p38 pathway in muscle-invasive bladder cancer cells. More mechanistic studies are needed to reveal which signaling pathways canonically affect the cancer cell cytotoxicity and cell apoptosis by nanaomycin K.
Our in vivo studies showed that intratumoral administration of nanaomycin K significantly inhibited both KK47 and T24 tumor growth in a dose-dependent fashion in mice xenografts. Consistent with the in vitro studies, nanaomycin K elicited much stronger tumor growth inhibition effects against T24, suggesting that nanaomycin K is more effective in muscle-invasive bladder cancer. In immunohistochemical staining, the expression of E-cadherin was significantly increased while expression of both N-cadherin and vimentin was remarkably decreased by nanaomycin K treatment in both KK47 and T24 tumors. Ki-67, a proliferation marker for cancer cells, was especially significantly decreased in T24 tumors by nanaomycin K treatment. High Ki-67 expression in clinical bladder cancer tissues is associated with cancer progression and poor outcomes in both non-muscle-invasive and muscle-invasive cancers21,22. Our results suggested that nanaomycin K directly reversed EMT and inhibited the cell proliferation of muscle invasive bladder cancer cells and exhibited cytotoxicity to cancer cells undergoing EMT.
Similar to our study, Liang et al. found that ablation of TGF-β signaling by a TGF-β receptor 1 inhibitor, LY364947, inhibited cancer cell proliferation, cancer stem cell population and EMT, and suppressed cancer progression in an orthotopic bladder cancer mouse model23. They also found that TGF-β signaling is important for the invasive and metastatic process in bladder cancer, including increased expression of Ki-67 and anti-apoptosis effects. Their findings support our results that nanaomycin K inhibits TGF-β signaling and suppresses EMT in bladder cancer cells in vivo.
There are no conclusive anti-EMT drugs for advanced bladder cancer. Immune checkpoint inhibitors such as pembrolizumab have been approved for advanced bladder cancer treatment, and therefore combinational therapy using immune checkpoint inhibitors with other drugs may turn out to be effective treatments24. Nanaomycin K may also be a possible anti-EMT drug candidate in combination with immune checkpoint inhibitors.
We would like to emphasize the study limitations. First, we only used two bladder cancer cell lines to investigate the anti-tumor effect of nanaomycin K. Second, the mechanism of apoptosis induced by nanaomycin K should be investigated to reveal which pathways are responsible for the apoptosis. Third, discrepancy between the results of gene expression and protein expression related EMT should be investigated in the future study.
In conclusion, we demonstrated that nanaomycin K had significant anti-EMT and anti-tumor effects in bladder cancer cells. Our findings suggest that nanaomycin K may be a therapeutic candidate for advanced bladder cancer. Further in vivo and in vitro studies are needed to investigate the mechanisms of action of nanaomycin K.
References
Omura, S., Tanaka, H., Koyama, Y., Oiwa, R. & Katagiri, M. Nanaomycins A and B, new antibiotics produced by a strain of Streptomyces. J. Antibiot. (Tokyo) 27, 363–365 (1974).
Nakashima, T. et al. New compounds, nanaomycin F and G, discovered by physicochemical screening from a culture broth of Streptomycesrosa subsp. notoensis OS-3966. J. Biosci. Bioeng. 120, 596–600 (2015).
Nakanishi, J. et al. An application of photoactivatable substrate for the evaluation of epithelial–mesenchymal transition inhibitors. Anal. Sci. 35, 65–69 (2019).
Matsuo, H. et al. Nanaomycin K, a new epithelial–mesenchymal transition inhibitor produced by the actinomycete “Streptomycesrosa subsp. notoensis” OS-3966. J. Biosci. Bioeng. 129, 291–295 (2019).
Pearson, G. W. Control of invasion by epithelial-to-mesenchymal transition programs during metastasis. J. Clin. Med. 8, 646 (2019).
Hao, Y., Baker, D. & Ten Dijke, P. TGF-β-mediated epithelial–mesenchymal transition and cancer metastasis. Int. J. Mol. Sci. 5, 20 (2019).
Knollman, H. et al. Muscle-invasive urothelial bladder cancer: An update on systemic therapy. Ther. Adv. Urol. 7, 312–330 (2015).
McConkey, D. J. et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 28, 335–344 (2009).
van Staalduinen, J., Baker, D., Dijke, P. T. & van Dam, H. Epithelial–mesenchymal-transition-inducing transcription factors: New targets for tackling chemoresistance in cancer?. Oncogene 37, 6195–6211 (2018).
Kitagawa, K. et al. Possible correlation of sonic hedgehog signaling with epithelial–mesenchymal transition in muscle-invasive bladder cancer progression. J. Cancer Res. Clin. Oncol. 145, 2261–2271 (2019).
Kawata, M. et al. TGF-β-induced epithelial–mesenchymal transition of A549 lung adenocarcinoma cells is enhanced by pro-inflammatory cytokines derived from RAW 264.7 macrophage cells. J. Biochem. 151, 205–216 (2012).
Liao, S. et al. Long non-coding RNA H19 promotes the proliferation and invasion of lung cancer cells and regulates the expression of E-cadherin, N-cadherin, and vimentin. Onco Targets Ther. 12, 4099–4107 (2019).
Syed, I. S., Pedram, A. & Farhat, W. A. Role of sonic Hedgehog (Shh) signaling in bladder cancer stemness and tumorigenesis. Curr. Urol. Rep. 17, 11 (2016).
Stojnev, S. et al. Prognostic impact of canonical TGF-β signaling in urothelial bladder cancer. Medicina (Kaunas) 24, 55 (2019).
DaCosta, B. S., Major, C., Laping, N. J. & Roberts, A. B. SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 65, 744–752 (2004).
Yang, G. et al. Quantitative analysis of differential proteome expression in epithelial-to-mesenchymal transition of bladder epithelial cells using SILAC method. Molecules 21, 84 (2016).
Khin, S. S. et al. BAMBI gene is epigenetically silenced in subset of high-grade bladder cancer. Int. J. Cancer 25, 328–338 (2009).
Ganesan, R., Mallets, E. & Gomez-Cambronero, J. The transcription factors Slug (SNAI2) and Snail (SNAI1) regulate phospholipase D (PLD) promoter in opposite ways towards cancer cell invasion. Mol. Oncol. 10, 663–676 (2016).
Islam, S. S. et al. Sonic hedgehog (Shh) signaling promotes tumorigenicity and stemness via activation of epithelial-to-mesenchymal transition (EMT) in bladder cancer. Mol. Carcinog. 55, 537–551 (2016).
Yu, D. et al. Cigarette smoke induced urocystic epithelial mesenchymal transition via MAPK pathways. Oncotarget 8, 8791–8800 (2017).
Thakur, B., Kishore, S., Dutta, K., Kaushik, S. & Bhardwaj, A. Role of p53 and Ki-67 immunomarkers in carcinoma of urinary bladder. Indian J. Pathol. Microbiol. 60, 505–509 (2017).
Ko, K., Jeong, C. W., Kwak, C., Kim, H. H. & Ku, J. H. Significance of Ki-67 in non-muscle invasive bladder cancer patients: A systematic review and meta-analysis. Oncotarget 8, 100614–100630 (2017).
Liang, Y. et al. Conditional ablation of TGF-β signaling inhibits tumor progression and invasion in an induced mouse bladder cancer model. Sci. Rep. 6, 29479 (2016).
Lopez-Beltran, A. et al. Immune checkpoint inhibitors for the treatment of bladder cancer. Cancers (Basel) 13, 131 (2021).
Funding
This study was supported by funding from the Institute for Fermentation, Osaka (IFO), Japan.
Author information
Authors and Affiliations
Contributions
Conceptualization and design: K.S. and T.N. Methodology: K.K., A.I. and K.S. Data acquisition and analysis: A.I., K.K. and H.M. Statistical analysis: K.K. and A.I. Writing—original draft: K.K., A.I. and K.S. Writing—review and editing: all authors. Supervision: Y.T., S.O., J.N. and M.F.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kitagawa, K., Shigemura, K., Ishii, A. et al. Nanaomycin K inhibited epithelial mesenchymal transition and tumor growth in bladder cancer cells in vitro and in vivo. Sci Rep 11, 9217 (2021). https://doi.org/10.1038/s41598-021-88741-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88741-3
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.