Abstract
Chronic disease of people living with human immunodeficiency virus (HIV) infection are now approaching those of the general population. Previous, in vitro studies shown that HIV causes arterial injuries resulting in inflammation and atherosclerosis but direct relationship between HIV infection clinical stages and lower extremity arterial disease (LEAD) remain controversial. No study assessed, with an accurate method, both the prevalence of LEAD and the influence of HIV severity on LEAD in HIV outpatients in Central Africa. A cross-sectional study was conducted among 300 HIV-infected outpatients, aged ≥ 40 years in Bujumbura, Burundi. All patients underwent ankle-brachial index (ABI) measurement and LEAD was diagnosed by ABI ≤ 0.9. The prevalence of LEAD was 17.3% (CI 95% 13.2–22.1). The mean age was 49.6 ± 7.1 years. On multivariable analysis, factors associated with LEAD were hypertension (OR = 2.42; 95% CI 1.10–5.80), and stage IV HIV clinical infection (OR = 4.92, 95% CI 1.19–20.36). This is the first study performed on a large HIV population in Central Africa, reporting high LEAD prevalence. It underlines the influence of HIV infection on peripheral atherosclerosis at latest clinical stages and the need for LEAD screening in HIV-infected patients.
Similar content being viewed by others
Introduction
During the last decades, the introduction of combined antiretroviral therapy (ART) has led to remarkable reduction in HIV/AIDS-related mortality. Nowadays, the life expectancy and chronic disease of people living with HIV infection (PLWH) are approaching those of the general population1. Among HIV-infected patients, particularly those who receive ART, several atherogenic metabolic disorders including dyslipidaemia, lipodystrophy and insulin resistance are reported2,3,4. Previous studies have also shown that HIV causes direct injury to the arterial wall resulting in inflammation and atherosclerosis5 and that cardiovascular disease (CVD) became one of the main causes of morbidity and mortality among PLWH6,7. Multiple observational cohort studies6,8,9,10,11 have demonstrated elevated rates of coronary artery disease in HIV-infected patients, with an approximate 1.5 to twofold increased relative risk as compared to controls.
A recent meta-analysis estimated that HIV infection confers a 61% increased risk of cardiovascular mortality. Moreover, when limited to HIV-infected patients on ART, the relative-risk of CVD increases twofold as compared to no-HIV and 1.5-fold compared to treatment-naive HIV-infected patients12.
Lower extremity arterial disease (LEAD) is the second most common form of cardiovascular disease after coronary artery disease. Nevertheless, few specific studies assessed the prevalence of LEAD among PLWH. Available data are issued from studies in hospitalized patients13,14, urban population15 or age limited populations, using heterogenous definition of LEAD14,16 or focusing on specific clinical LEAD stages17. Results are controversial18,19 as study populations are not comparable. None of the studies reported any precise, accurate correlation between clinical stages of HIV disease and the prevalence of LEAD in African HIV population.
Within its 11 million inhabitants, Burundi has one of the highest prevalence of PLWH worldwide. In 2016, this country had 2200 new HIV infections and 2900 Acquired Immuno Deficiency Syndrome—related deaths. There were 84,000 people living with HIV, among whom 61% were accessing ART. In Burundi, survey and treatment of HIV infected patients are mainly dispensed in HIV centres in Bujumbura capital.
Due to the paucity of data and controversial results on the prevalence of LEAD in PLWH, our main objective was to determine, using accurate methods in line with the current guidelines, the prevalence of LEAD in HIV outpatients in Burundi and particularly determine the influence of HIV disease clinical stages.
Methods
During 3 months a cross-sectional study, dealing with rural and urban patients, was conducted in four HIV centres in Bujumbura capital (BURUNDI). The aim of our study was to determine the prevalence of LEAD among HIV infected subjects, describe the associated cardiovascular risk factors and analyse the association between HIV clinical stages and LEAD.
Study design
The study population included, successively, all HIV infected outpatients (urban and rural population), aged ≥ 40 years. Refusal to participate was the exclusion criterion. Informed written consent was obtained before inclusion. Data were collected through a face-to-face interview, a clinical examination and laboratory tests.
Ethics
The study obtained the approval of the National Ethics review Committee (NEC) from Burundi (Comité National d’Ethique pour la protection des êtres humains sujets de la recherche biomedicale et comportementale, Burundi).
The research reported in the paper was undertaken in compliance with Helsinki Declaration.
Data collection and definitions
The detailed administered questionnaire, adapted from World Health Organization (WHO) STEPS tools20 included demographic and clinical characteristics: age, gender, lifestyle factors (smoking, alcohol intake), medical history (hypertension, diabetes, cardiovascular disease), current medication (antihypertensive agents, anti-diabetic drugs), past- and current occupation, education (school cycles completion), marital status. Smoking status was classified as never, past (smoking cessation since more than 1 year prior to the survey) or current smoking. Alcohol drinking was assessed based on the frequency and amount of alcohol intake and was categorized as never, occasional (less than 5 days/week) and regular (more than 5 days/week). Body-mass index (BMI) was calculated as weight/height2 and categorized according to the WHO as: underweight (< 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥ 30 kg/m2). Pulse rate, systolic and diastolic blood pressure (average of two measures for each arm at 1-min interval) were recorded. Hypertension was defined in case of self-reported ongoing antihypertensive treatment and/or systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg21. Diabetes was defined according to self-reported diabetes medication or in case of elevated capillary blood glucose level (Accu-Chek Performa, Roche) above 126 mg/dL if the fasting period > 2 h or above 200 mg/dL in non-fasting participants. Furthermore, blood samples were used to determine high-sensitivity C-reactive protein (hs-CRP), cholesterol levels, triglyceride levels, high-density lipoprotein (HDL) and low-density lipoprotein (LDL). Renal dysfunction was defined as end stage renal disease with dialysis, or a glomerular filtration rate (GFR) < 60 mL/minute/1.73 m2 calculated according the Cockcroft-Gault formula.
The ABI was measured using a manual cuff and a hand-held Doppler device (Super Dopplex II, Huntleigh Technology PLC, Luton, UK) to determine, on both arms and legs, the systolic blood pressure in the posterior tibial and dorsalis pedis and brachial arteries. Measurements were done in accordance to the American Heart Association guidelines. The ABI for each leg was calculated as the ratio between the higher of the two systolic blood pressure measurements in each leg (posterior tibial or dorsalis pedis) and the higher of the two systolic blood pressure measurement in the arms. The value of the limb with the lowest ABI was used for each patient. An ABI ≤ 0.90 defined LEAD, and an ABI ≥ 1.40 defined high ABI22.
Presumed duration of HIV infection, duration of antiretroviral therapy (years), HIV detectable load (≥ 200 copies/ml), CD4 + T cell count (cells/ml) were revealed. HIV clinical stages (stage I to IV) were defined following the WHO consolidated guidelines on clinical staging of HIV disease in adults, adolescents and children 201623.
Statistical analysis
The data were analysed with the statistical program Stata 12 (StataCorp, College Station, TX). The Student t-test or the Mann–Whitney test were used for the comparison of the continuous variables and the Chi-square test or Fisher exact test for the comparison of the categorical variables. In order to determine independent risk factors associated with LEAD, a multivariable logistic regression model including backward stepwise procedure was performed. The level of significance for all the statistical analyses was set at P < 0.05 and interactions were checked. Some variables as age and sex or “traditional CV risk factors” (current smoking, diabetes, elevated plasma cholesterol level) were forced in the multivariable model estimating the association between LEAD and clinical HIV stages.
Results
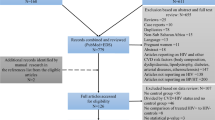
Three hundred consecutive HIV-infected outpatients (81.2% women), in four centres in Bujumbura (43% rural subjects) were included in the study. No subject declined participation. The flow chart is presented in Fig. 1.
The prevalence of low ABI (≤ 0.9) was 17.33% (n = 52) while 6.9% (n = 13) had incompressible arteries. The mean age of LEAD subjects was 49.5 ± 7.1 years. There was no significant age difference between patients with- or without LEAD (P = 0.14). General characteristics of the population are presented in Table 1. Only 6 subjects were active smokers (2.7%), no past smokers were registered. Possibly due to the small size of the sample, no significant difference between LEAD and no LEAD subjects was identified.
Patients with- and without LEAD had similar other CV associated risk factors and comorbidities. In order to identify factors associated with LEAD, 13 patients with ABI ≥ 1.40 were excluded from our analysis. Characteristics of excluded subjects were similar to those of the study population.
The mean CD4 count was 430.7 cells/mL and 8.4% (n = 24) of the subjects had detectable HIV RNA viral loads. The presumed duration of HIV infection was 9.91 years and the duration of ART 7.53 years. HIV clinical stages weren’t similar in both population (P = 0.04) (Table 2).
The prevalence of LEAD was increasing with the latest clinical HIV-stages and was higher in patients aged 50 or more (Fig. 2).
The HIV clinical stage IV (OR 3.41, 95% CI 1.04–13.46, P = 0.04) was associated with LEAD even after adjustment to all CV risk factors, CV morbidities and HIV infection characteristic. Results are summarised in Table 3. After multivariable analysis, factors associated with LEAD were hypertension (OR = 2.42; 95% CI 1.10–5.80; P = 0.04), and stage IV of HIV infection (OR = 4.92, 95% CI 1.19–20.36; P = 0.03). The association between LEAD and current smoking or HIV load did not reach statistical significance (P = 0.09).
Discussion
The major finding of our study is the high prevalence of LEAD in mid-aged HIV-infected outpatients, especially at the latest HIV clinical stages. To our knowledge, this is the first study on a large sample of HIV infected outpatients in Central Africa that accurately establish a clear relationship between clinical HIV stages and LEAD prevalence. Also, our study is the first one dealing with urban and rural HIV outpatients on the African continent.
Previous studies of LEAD among HIV-infected patients have reported prevalence ranging from 1 to more than 10% (Table 4). The prevalence of LEAD in the general population has been estimated between 2 and 4% in two large studies in young adults in USA24,25. In a cross-sectional hospital-based study in black Africans, the LEAD prevalence was estimated at 6.9%14. A recent Nigerian study reported no difference of LEAD prevalence between virologically-suppressed individuals and controls19. Both studies defined LEAD as an ABI < 0.9 and had methodologic bias. In our study, the prevalence of LEAD defined by an ABI ≤ 0.9 was 17.3% for a mean age of 49 ± 7.1 years. This prevalence is higher than expected in HIV mid aged patients and is comparable to the prevalence of LEAD in African elderly (> 70 years) general population26. These very different results reported in the literature, are due to the limited samples, the selection bias (hospital recruitment, exclusively asymptomatic subjects, young age, occidental population) and heterogenous LEAD diagnosis methods. Also, beyond HIV infection, Caucasian and African populations are difficult to compare. Prior studies have consistently demonstrated higher prevalence of LEAD among African Americans or African native subjects as compared to Caucasians26,27,28. This racial difference in LEAD prevalence remains independent and is only modestly attenuated by adjustment for traditional CVD risk markers as age, diabetes, smoking, plasma lipids and hypertension29,30. As many of these factors are very common in HIV patients receiving antiretroviral therapy a high prevalence of LEAD might be expected in PLWH31,32. Nevertheless, correlation between CV risk factors and LEAD in PLWH is inconsequent and varies from no association at all to different correlations, between hypertension, age, diabetes and/or smoking and LEAD, from one country to another. This might be due not only to the selection bias but also to different lifestyle factors. As tobacco is less common in African women, the lack of association between LEAD and smoking in our study is surprising but not unique in the literature. Four other studies, specifically undertaken in the elderly general population in Central Africa, also failed to find this association26,27,33, 34.
Among the HIV related factors, only HIV clinical stage IV was associated with LEAD (OR = 4.92, 95% CI 1.19–20.36; P = 0.03). This association has also been described, in hospital Spanish and Cameroon populations. These studies had either important LEAD diagnosis bias14 or exclusively focused on asymptomatic LEAD subjects17. Our results accurately analysed the association between HIV clinical stage and LEAD in urban and rural HIV outpatients and indicate that the severity of HIV infection could influence the arteries injury.
The role of the HIV infection as a LEAD risk factor was also prospectively established on over 90,000 participants (29,291 HIV infected subjects and 62,996 sex- and age-matched controls) in the Veterans Aging Cohort Study35. The study had nevertheless been designed for the analyse of the role of alcohol use and abuse in determining clinical outcomes in Americans. Also, no LEAD prevalence in PLWH was reported.
After adjusting for age, sex, race and cardiovascular risk factors including LDL cholesterol, triglycerides, diabetes, smoking, hypertension, obesity, impaired kidney function, hepatitis C infection, and alcohol or cocaine abuse, the investigators found an increased risk of LEAD in subjects with CD4 cell counts below 500 cells/mm3. This increased risk was also reported with lower levels of CD4 counts, even after adjusting to the absence of detectable viral load (which would indicate the use of effective antiretroviral treatment).
The authors concluded that among a population of HIV-positive military veterans with a high prevalence of risk factors for CVD, immunodeficiency (CD4 cell count below 500 cells/mm3) or a detectable viral load raised the risk of developing LEAD even more. Our study failed to prove this association, probably due to the limited study population (300 subjects).
Compared to previous cross sectional studies on LEAD prevalence in PLWH, our study included the largest outpatient sample. As HIV centres in Bujumbura capital deal with the regional population, our results are representative for the rural and urban HIV subjects. The limitations of our study are due to the transversal design. Other limitation of this study is the lack of a control group in order to evaluate the risk-excess of LEAD in HIV patients as compared to healthy counterparts. As inclusions were conducted in HIV-care centres, the access to non-diseased population was not feasible. Nevertheless, the increasing LEAD-risk excess with increasing HIV disease severity is an important finding of our study.
Conclusion
In summary, the prevalence rate of LEAD in our study was very high in mid aged HIV-infected patients and strongly correlated with the latest HIV clinical stage, independently from all other CV risk factors or morbidities. Our findings underline that PLWH are at high CV risk and also point out the need for LEAD screening in HIV-infected patients.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Smith, C. J. et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): A multicohort collaboration. Lancet (London, England) 384, 241–248. https://doi.org/10.1016/s0140-6736(14)60604-8 (2014).
Carr, A. et al. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: A cohort study. Lancet (London, England) 353, 2093–2099. https://doi.org/10.1016/s0140-6736(98)08468-2 (1999).
Santos, J., Palacios, R., Gonzalez, M., Ruiz, J. & Marquez, M. Atherogenic lipid profile and cardiovascular risk factors in HIV-infected patients (Netar Study). Int. J. STD AIDS 16, 677–680 (2005).
Periard, D. et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation 100, 700–705 (1999).
Hsue, P. Y. et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS (London, England) 23, 1059–1067. https://doi.org/10.1097/QAD.0b013e32832b514b (2009).
Morlat, P. et al. Causes of death among HIV-infected patients in France in 2010 (national survey): trends since 2000. AIDS (London, England) 28, 1181–1191. https://doi.org/10.1097/qad.0000000000000222 (2014).
Sackoff, J. E., Hanna, D. B., Pfeiffer, M. R. & Torian, L. V. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann. Intern. Med. 145, 397–406 (2006).
Currier, J. S. et al. Coronary heart disease in HIV-infected individuals. J. Acquir. Immune Defic. Syndr. 1999(33), 506–512 (2003).
Klein, D., Hurley, L. B., Quesenberry, C. P. Jr. & Sidney, S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection?. J. Acquir. Immune Defic. Syndr. 1999(30), 471–477 (2002).
Lang, S. et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS (London, England) 24, 1228–1230. https://doi.org/10.1097/QAD.0b013e328339192f (2010).
Obel, N. et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: A population-based cohort study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 44, 1625–1631. https://doi.org/10.1086/518285 (2007).
Islam, F. M., Wu, J., Jansson, J. & Wilson, D. P. Relative risk of cardiovascular disease among people living with HIV: A systematic review and meta-analysis. HIV Med. 13, 453–468. https://doi.org/10.1111/j.1468-1293.2012.00996.x (2012).
Aurpibul, L. et al. Peripheral artery disease in HIV-infected older adults on antiretroviral treatment in Thailand. HIV Med. https://doi.org/10.1111/hiv.12671 (2018).
Kamdem, F. et al. Prevalence and risk factors of peripheral artery disease in black Africans with HIV infection: A cross-sectional hospital-based study. Vasc. Health Risk Manag. 14, 401–408. https://doi.org/10.2147/vhrm.S165960 (2018).
Kwiatkowska, W. et al. Peripheral arterial disease and ankle-brachial index abnormalites in young and middle-aged HIV-positive patients in lower Silesia, Poland. PLoS ONE 9, e113857. https://doi.org/10.1371/journal.pone.0113857 (2014).
Qaqa, A. Y. et al. Epidemiologic aspects of abnormal ankle brachial index in the HIV infected population. Int. Angiol. J. Int. Union Angiol. 31, 227–233 (2012).
Canalejo, E., Cabello, N., Perales, I., Allodi, S. & Sánchez-Purificación, A. Asymptomatic peripheral arterial disease detected by the ankle-brachial index in HIV-infected patients: Prevalence and associated risk factors. Enferm. Infecc. Microbiol. Clin. 29, 672–678. https://doi.org/10.1016/j.eimc.2011.07.006 (2011).
Knudsen, A., Malmberg, C. A., Kjaer, A. & Lebech, A. M. Low prevalence of peripheral arterial disease in a cross-sectional study of Danish HIV-infected patients. Infect. Dis. (London, England) 47, 776–782. https://doi.org/10.3109/23744235.2015.1061204 (2015).
Agu, C. E., Uchendu, I. K., Nsonwu, A. C., Okwuosa, C. N. & Achukwu, P. U. Prevalence and associated risk factors of peripheral artery disease in virologically suppressed HIV-infected individuals on antiretroviral therapy in Kwara state, Nigeria: A cross sectional study. BMC Public Health 19, 1143. https://doi.org/10.1186/s12889-019-7496-4 (2019).
in Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach (ed nd) (World Health Organization Copyright (c) World Health Organization 2016., 2016).
Williams, B. et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 39, 3021–3104. https://doi.org/10.1093/eurheartj/ehy339 (2018).
Aboyans, V. et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation 126, 2890–2909. https://doi.org/10.1161/CIR.0b013e318276fbcb (2012).
in Guidelines on Post-Exposure Prophylaxis for HIV and the Use of Co-Trimoxazole Prophylaxis for HIV-Related Infections Among Adults, Adolescents and Children: Recommendations for a Public Health Approach: December 2014 supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection (World Health Organization Copyright © World Health Organization 2014., 2014).
Murabito, J. M. et al. Prevalence and clinical correlates of peripheral arterial disease in the Framingham Offspring Study. Am. Heart J. 143, 961–965 (2002).
Zheng, Z. J. et al. Lower extremity arterial disease assessed by ankle-brachial index in a middle-aged population of African Americans and whites: the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Prev. Med. 29, 42–49. https://doi.org/10.1016/j.amepre.2005.07.019 (2005).
Desormais, I. et al. Prevalence of peripheral artery disease in the elderly population in urban and rural areas of Central Africa: The EPIDEMCA study. Eur. J. Prev. Cardiol. 22, 1462–1472. https://doi.org/10.1177/2047487314557945 (2015).
Guerchet, M. et al. Epidemiology of peripheral artery disease in elder general population of two cities of Central Africa: Bangui and Brazzaville. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 44, 164–169. https://doi.org/10.1016/j.ejvs.2012.05.019 (2012).
Kullo, I. J. et al. Ethnic differences in peripheral arterial disease in the NHLBI Genetic Epidemiology Network of Arteriopathy (GENOA) study. Vasc. Med. (London, England) 8, 237–242. https://doi.org/10.1191/1358863x03vm511oa (2003).
Allison, M. A. et al. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Am. Coll. Cardiol. 48, 1190–1197. https://doi.org/10.1016/j.jacc.2006.05.049 (2006).
Aboyans, V. et al. ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. https://doi.org/10.1093/eurheartj/ehx095 (2017).
Grinspoon, S. & Carr, A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N. Engl. J. Med. 352, 48–62. https://doi.org/10.1056/NEJMra041811 (2005).
Kamin, D. S. & Grinspoon, S. K. Cardiovascular disease in HIV-positive patients. AIDS (London, England) 19, 641–652 (2005).
Samba, H. et al. Ankle Brachial Index (ABI) predicts 2-year mortality risk among older adults in the Republic of Congo: The EPIDEMCA-FU study. Atherosclerosis 286, 121–127. https://doi.org/10.1016/j.atherosclerosis.2019.05.013 (2019).
Amidou, S. A. et al. Epidemiology of lower extremity artery disease in a rural setting in Benin, West Africa: The TAHES study. Int. J. Cardiol. 267, 198–201. https://doi.org/10.1016/j.ijcard.2018.05.099 (2018).
Beckman, J. A. et al. Association of human immunodeficiency virus infection and risk of peripheral artery disease. Circulation 138, 255–265. https://doi.org/10.1161/circulationaha.117.032647 (2018).
Acknowledgements
The authors are indebted to the study participants for their contribution to the project: the National Referral Center on HIV/AIDS in Burundi, as well as both University of Burundi and University of Limoges for their moral support.
Author information
Authors and Affiliations
Contributions
Authors I.D., D.H, T.N, P.L., V.A., PM.P., designed the study, directed its implementation, including quality assurance and control, prepared its analytic strategy. Authors I.D. and D.H. did the statistical analysis and drafted the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Desormais, I., Harimenshi, D., Niyongabo, T. et al. HIV clinical stages and lower extremity arterial disease among HIV infected outpatients in Burundi. Sci Rep 11, 8296 (2021). https://doi.org/10.1038/s41598-021-87862-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-87862-z
This article is cited by
-
The Global Epidemiological Transition in Cardiovascular Diseases: Unrecognised Impact of Endemic Infections on Peripheral Artery Disease
Journal of Epidemiology and Global Health (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.