Abstract
Dystonia is conceptualized as a network disorder involving basal ganglia, thalamus, sensorimotor cortex and the cerebellum. The cerebellum has been implicated in dystonia pathophysiology, but studies testing cerebellar function in dystonia patients have provided equivocal results. This study aimed to further elucidate motor network deficits in cervical dystonia with special interest in the role of the cerebellum. To this end we investigated motor learning tasks, that differ in their dependence on cerebellar and basal ganglia functioning. In 18 cervical dystonia patients and 18 age matched healthy controls we measured implicit motor sequence learning using a 12-item serial reaction time task mostly targeting basal ganglia circuitry and motor adaptation and eyeblink conditioning as markers of cerebellar functioning. ANOVA showed that motor sequence learning was overall impaired in cervical dystonia (p = 0.01). Moreover, unlike healthy controls, patients did not show a learning effect in the first part of the experiment. Visuomotor adaptation and eyeblink conditioning were normal. In conclusion, these data lend support to the notion that motor learning deficits in cervical dystonia relate to basal ganglia-thalamo-cortical loops rather than being a result of defective cerebellar circuitry.
Similar content being viewed by others
Introduction
Dystonia is a movement disorder characterized by abnormal involuntary movements often repetitive and patterned1. Pathophysiologic concepts of dystonia have evolved from the hypothesis of dystonia representing a prototype basal ganglia disorder to the view of dystonia as a sensorimotor network disorder involving the basal ganglia, cerebellum, thalamus and sensorimotor cortex2. Neuro-functionally, dystonia is characterized by reduced inhibition, abnormal sensorimotor integration, maladaptive synaptic plasticity as well as reduced thalamic gating and dysfunctions of thalamo-striatal and cortico-striatal networks3,4,5,6. Synaptic plasticity is the neuronal mechanism underlying learning processes in general. Motor learning in dystonia patients has shown to be associated with reduced plasticity in inhibitory networks7, thus rendering motor learning paradigms within the sensorimotor system a useful tool in dystonia research.
Motor learning is often studied using variants of two main learning paradigms: Motor sequence learning (MSL) and motor adaptation (MA). Theoretical models propose two distinct brain networks to be involved in motor learning: the cerebello-thalamo-cortical (CTC) loop is considered to provide an internal forward model based on sensory states and cortical motor commands, which is then compared to the actual motor outcome. The basal ganglia-thalamo-cortical loop (BGTC) is predominantly engaged in action selection and probabilistic reward-based learning8,9. MSL and MA rely on shared cortical and subcortical motor network components involving BGTC and CTC networks, but MA has a greater demand on cerebellar computing10. Cerebellar pathology leads to deficits in MSL11 and MA12, while in patients with predominant basal ganglia pathology, MSL is impaired13 but MA is typically not affected12. A very different approach to cerebellum-dependent motor learning mechanisms is the investigation of classical eyeblink conditioning, which is typically impaired in patients with cerebellar lesions14 and relies basically on cerebellum and brainstem circuits15,16.
A special role of the cerebellum in the pathophysiology of dystonia has been a matter of debate17. It has been hypothesized that the cerebellum modulates striatal activity and dystonia may arise from disruptions in a cerebellum-basal ganglia network18,19. Evidence comes from animal studies20,21, and indirectly also from studies of patients with cerebellar lesions22. Importantly, dystonia can be a feature of neurodegenerative disorders of the cerebellum18. In idiopathic cervical dystonia (CD), both microstructural abnormalities of the cerebellum23 and abnormalities in functional activity and connectivity of the cerebellum were found23. Reduced cerebellar connectivity has also been implicated by transcranial magnetic stimulation24. Previous studies of motor learning designed to assess deficits in cerebellar functioning in dystonia yielded equivocal results. While MA was found normal across different types of dystonia in most (though not all) studies25,26,27,28,29, the investigation of eyeblink conditioning found cerebellar learning to be either impaired30,31,32 or normal33,34. Abnormal sequence learning was reported in DYT-TOR1A35, but was found intact in CD25 and writer’s cramp36,37. However, interpretation of the latter results in the context of involved brain networks is difficult because the applied tasks deviated substantially from the classical serial reaction time task (SRTT)38.
This study aimed to examine in detail the deficits in cerebellar-associated motor learning in CD using well established tasks that rely to different extents on the integrity of CTC and BGTC networks. We investigated MSL via the classical SRTT, MA was probed with an established protocol of cerebellum-dependent visuomotor adaptation39. Further, eyeblink conditioning was tested, representing a classical cerebellar-learning task that is largely independent of the previously named networks15,16.
Results
Motor sequence learning
During the SRTT participants had to react to visually presented cues by pressing the corresponding button on the computer keyboard as fast as possible. After an introductory simple task, participants completed three identical sessions comprising 12 repetitions of a 12-item sequence (144 trials) followed by two blocks of 12 pseudo-randomly presented cues each (24 trials). There was a baseline difference in reaction times between groups, i.e. performance of the control group was faster during the simple task (t = 3.53, p < 0.001). Sequence learning was assessed as the reaction time advantage for sequentially repeated (RTSEQ) relative to pseudo-randomly presented cues (RTRAN), ΔRT = RTRAN − RTSEQ (Fig. 1A), with positive values indicating learning. ANOVA with SESSION (1, 2, 3) as within-subject factor and GROUP as between-subject factor showed a main effect of GROUP (F(1,34) = 7.1, p = 0.01), indicating superior learning in the healthy control (HC) group (ΔRT across all sessions was 66.6 ms ± 7.9 ms (SE) in HC and 33.8 ms ± 8.3 ms in CD patients). A main effect of SESSION (F(2,68) = 8.1, p < 0.001) indicated differences in the amplitude of learning effects between sessions. Post-hoc tests revealed that sequence learning was stronger in session 2 and 3 compared to session 1 (all p < 0.05 Bonferroni corrected). The GROUP X SESSION interaction was not significant.
Motor sequence learning. (A) Performance in the motor sequence learning task is shown as median reaction time (RT) ± SE for runs of 12 trials. Each block consisted of 12 × 12 sequence trials (SEQ) and 2 × 12 random trials (RAN). Grey bars indicate data points used to characterize sequence learning as ΔRT = RTRAN − RTSEQ. (B) ANOVA showed reduced sequence learning in cervical dystonia (CD) in general, and pairwise t-tests demonstrated no learning at all in session 1 in CD. *Significant learning (p < 0.05, Bonferroni corrected).
To further scrutinize these learning effects, we assessed whether ΔRT was statistically different from 0 (i.e., whether RT differed between the sequence and random condition) using Bonferroni corrected paired one-sample t-tests, separately for each group and session. Learning effects were found for all sessions in HC (all p ≤ 0.01, Bonferroni corrected). In contrast, CD patients did not show evidence for learning in session 1 (p = 1) but only in later sessions (session 2 and 3: p < 0.01, see Fig. 1B).
A repeated measures ANOVA of error rates with CONDITION (sequence, random) as within subject factor and GROUP as between subject factor showed no main effect of GROUP, but a significant effect of CONDITION (F(1,34) = 11.1, p = 0.002), reflecting higher error rates in random vs. sequence blocks in both tasks (4% vs. 2%).
Motor adaptation
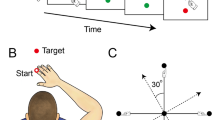
In the visuomotor adaptation task, participants performed fast center-out hand movements to one of eight radially arranged targets. Movements were recorded by a pen on a digitizing tablet, with visual feedback shown on a computer screen. A perturbation of the visual feedback was gradually introduced over 96 trials to a maximum of 30°, maintained for 64 trials (“plateau”) and suddenly removed (“extinction”). Adaptation was measured as the (automatic) adjustment of hand movement trajectories to rotated visuomotor feedback (Fig. 2A). For two CD patients, no data on MA was available. General measures of motor performance (mean movement duration per trial, maximum velocity, maximum pen pressure) were compared with two-sample t-tests and did not differ between groups. To assess MA between groups, we conducted t-tests of the amount of compensatory rotation in degrees at the last two runs of the plateau phase (adaptation; t = − 0.53, p = 0.61) and at the first two runs of the extinction phase (aftereffect; t = − 1.05; p = 0.30), indicating that adaptation did not differ systematically between groups (Fig. 2B).
Motor adaptation. (A) Schematic course of the experiment indicating difference between visible movements of the cursor on screen and invisible movement of the hand on the digitizing tablet. (B) Adaptation to a gradually introduced visuomotor perturbation did not differ between cervical dystonia patients and healthy controls. Points represent adaptation (mean ± SE of eight consecutive trials), grey lines indicate the applied perturbation.
Classical eyeblink conditioning
Eyeblink conditioning refers to pavlovian conditioning of the corneal reflex (Fig. 3A–C). Over ten blocks of ten trials each an air puff was applied to the right eye eliciting a forceful closing of the eyelid. The air puff was preceded by a loud tone as the conditioning stimulus, and conditioning was measured as the percentage of trials per block in which the lid was closed before onset of the air puff. The conditioning phase (100 trials) was followed by an extinction phase (30 trials) in which the tone was presented alone. Repeated measures ANOVA of occurrence of conditioned responses (CR) in the conditioning phase with BLOCK as within-subject factor and GROUP as between-subject factor showed no main effect of GROUP and no interaction, but a significant effect of BLOCK (F(9,306) = 28.6, p < 0.001), indicating that conditioning of the blink reflex to a tone was achieved in both groups, which is reflected by increasing mean values for CR over blocks (Fig. 3D). The same analysis of the extinction phase revealed no main effect of GROUP or BLOCK. The spontaneous blink rate did not differ between groups (HC18/min, CD 25/min, t(29) = 1.4, p = 0.17). To obtain a measure of learning for correlational analysis we collapsed occurrence of CR over block 1 to 5. Mean CR did not differ between groups (t(33) = 0.4, p = 0.68) and did not correlate with the spontaneous blink rate.
Classical eyeblink conditioning. (A) Schematic course of the experiment: In the conditioning phase a tone as the conditioning stimulus (CS; 550 ms, 88 dB), is paired with an air puff as the unconditioned stimulus (US; 100 ms, 110 kPa). In the extinction phase the CS is presented alone. (B) Example surface EMG activity of the orbicularis oculi muscle over the course of the conditioning phase of a participant that shows conditioning and (C) of a participant that does not exhibit a conditioned response to air puff stimulation. (D) Conditioning of the blink reflex is acquired in patients with cervical dystonia to the same extent as in healthy controls. Points represent percentage + SE of conditioned responses of the blink reflex per runs of ten trials.
Correlation to clinical parameters and between experiments
Pearson’s correlations with Bonferroni correction for multiple testing did not show any association between measures of motor learning (MSL: mean ΔRT across all sessions; MA: adaptation in degree at the end of plateau; eyeblink conditioning: mean percentage of conditioned responses in block 1–5) and clinical parameters (age at onset, TWSTRS, TRS) or demographic factors (sex, age). Performance measures were not found to be significantly correlated across experiments.
Discussion
In this study, we compared performance in motor learning tasks involving different networks including the cerebellum between patients with sporadic isolated CD and HC. The main finding of this study is that MSL was impaired in CD patients whereas MA and eyeblink conditioning were normal. Our findings support the view that dystonia is a network disorder involving impaired basal ganglia function. On the other hand, the assumption of a cerebellar dysfunction per se in CD could not be corroborated.
The finding of intact visuomotor adaptation in the MA task agrees with previous studies in CD25,26 and writer’s cramp40, and also monogenic dystonias such as DYT-TOR1A27 and DYT-SGCE28. Also, adaptation of gait during split-belt treadmill walking has been found to be normal in CD, but not in patients with writer’s cramp or blepharospasm29. Adaptation to catching balls of different weight has been found to be altered in CD patients with dystonic tremor but not in CD per se41. Studies on eyeblink conditioning reported reduced conditioning in CD using a different experimental approach with electrical stimulation of the supraorbital nerve instead of air puff30. However, this deficit was later attributed to the presence of dystonic tremor rather than dystonia per se by the same group33. In DYT-SGCE, a type of dystonia with myoclonus as the hallmark clinical feature eyeblink conditioning was found to be reduced32 or the extinction of eyeblink conditioning was altered42 indicating prominent cerebellar dysfunction in these patients and suggesting that myoclonus might be a sign of abnormal cerebellar activity. In patients with DYT-TOR1A and DYT-THAP1 no deficits in eyeblink conditioning have been found34. Taken together, evidence from these experiments does not support the assumption of prominent cerebellar dysfunction as a hallmark feature of dystonia per se. Instead, cerebellar abnormalities might rather be related to other clinical features associated with dystonia including tremor or myoclonus in the case of DYT-SCGE. Although tremor was present in the majority of dystonia subjects also in this study, tremor severity as measured by the TRS (mean 2.1, range 0–7) was considerably lower compared to studies reporting eyeblink deficits in tremulous dystonia33.
MSL deficits in CD patients in this study were present using a 12-item implicit SRTT task, and deficits were most pronounced in the early stages of the experiment. The cerebellar contribution to MSL encompasses formation of an internal model, error reduction, fine tuning of motor components and maintenance of stimulus–response mappings43. The proposed role of the basal ganglia is the formation of associations between individual stimuli and movements, i.e. sequence learning9. This view has recently been corroborated by a meta-analysis of functional imaging data identifying the anterior striatum and globus pallidus internus (GPi) as the structures responsible for the act of sequence learning44. A theoretical model proposes, that learning an implicit sequence can be separated in an early learning phase in which an associative circuit including the anterior-medial striatum and associative parts of the cerebellum, is engaged in in encoding the sequential component of the sequence, resulting in quick reductions of reaction times. Over the course of the experiment activity shifts to sensorimotor circuits, characterized by a shift of neural activity within the striatum to dorso-lateral parts and decreasing cerebellar activity9. The finding of a deficit in acquisition of the sequence (reflected by the lack of a sequence vs. random advantage) in the early learning stage might hence be interpreted as an impairment in transition from associative to sensorimotor circuits9. In this context it is of special interest that although performance was normal, reduced activity in the anterior putamen and GPi was observed in writer’s cramp patients during MSL36. Also, in X-linked dystonia parkinsonism, a hereditary neurodegenerative disease that is characterized by an initial phase of rapidly generalizing dystonia and a later phase dominated by parkinsonism, neuroimaging found degeneration of the anterior putamen and GPi as a hallmark feature of the dystonic phase45. It has to be born in mind though that the BGTC and CTC circuits are interconnected through a di-synaptic connection from the dentate nucleus to the striatum46, and it has been shown that during early sequence learning the putamen negatively modulates cerebellar activity47. The finding of impaired baseline performance in the CD group might indicate abnormal functioning of a sequence learning-independent motor network including the cerebellum10. Altered functional and anatomical connectivity between the cerebellum and the basal ganglia has been found in CD and writer’s cramp23,48. Moreover, a two-hit model including both the cerebellum and basal ganglia has been proposed for dystonia pathophysiology supported by findings in animal models of dystonia49.
The results of the present study contrast earlier findings of intact sequence learning in CD25 and writer’s cramp36. These discrepancies might result from the use of shorter sequences of five to eight items. Patients with Parkinson’s disease did not show sequence learning in a 12-item sequence, but were able to learn an 8- or 10-item sequence11, thus implicating deficits in extracting more and more complex sequences as a result of reduced basal ganglia functioning13. On the other hand, MSL was impaired in manifesting and non-manifesting DYT-TOR1A (but not DYT-THAP1) mutation carriers when learning an 8-item sequence, accompanied by reduced CTC tract integrity35. In these patients, dystonia tends to generalize, so that impairments in MSL even when shorter sequences are used might indicate more widespread or profound abnormalities in BGTC and CTC in these patients compared to patients with focal or segmental dystonia as studied here. This is to say that the threshold for MSL deficits to occur may be higher in the latter.
As pointed out above, previous studies indicated that concomitant features such as tremor rather than dystonia per se have been found to be related to deficits in cerebellar dependent learning paradigms33,41. The present study was not designed to assess the influence of tremor so that conclusions in this regard are limited. However, although tremor was present in most of our patients tremor rating was usually low. Moreover, we did not find changes in motor learning tasks that were previously associated with tremor33,41. We therefore consider it unlikely that our results are confounded by the presence of tremor.
In conclusion, we found deficits in MSL in CD patients whereas motor learning in classical cerebellar learning tasks including MA and eyeblink conditioning was normal. The finding of abnormal basal ganglia related motor learning in cervical dystonia is in keeping with the concept of dystonia as network disorder involving BGTC networks. Although a potential role of the cerebellum in the pathophysiology of dystonia is supported by numerous studies, our findings of deficits in sequence learning do not support the assumption of CD being associated with predominant cerebellar motor control dysfunction.
Methods
Participants
For this study we recruited 18 CD patients at least ten weeks after their last botulinum toxin injection and 18 age-matched HC. For only nine CD patients and eight HC data on eyeblink conditioning was available while the remaining either refused participation (dry eyes, use of contact lenses) or showed inconsistent response to the air puff stimulation upon analysis. Hence, another nine patients and ten healthy controls were recruited resulting in 18 participants each (Table 1). Severity of dystonia and tremor was assessed with the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) and the Fahn–Tolosa–Marin Tremor Rating Scale (TRS) based on a standardized video protocol. Genetic testing by gene panel analysis has been performed in 20/27 patients. No pathogenic variants were detected in the TOR1A, THAP1, GNAL, SGCE or GCH1 genes.
The study was approved by the ethics committee of the University of Lübeck (No 17-369). All participants gave written informed consent, and all experiments were performed in accordance to the declaration of Helsinki.
Serial reaction time task
Four black squares were presented in a horizontal array on a computer screen using Presentation software (Neurobehavioral Systems Inc, Berkeley, USA). When one of the squares turned blue (stimulus), participants were requested to press the corresponding target button on the computer keyboard with the middle or index finger of the left or right hand as quickly as possible. Correct responses were indicated by a change of the stimulus in size and color. Stimuli were presented with a fixed response-stimulus interval of 400 ms. Participants were informed that the items were presented in a repetitive manner but were not told the sequence. Reaction time (RT) was defined as the period from presentation of the stimulus to button presses.
After a practice block that repeated a simple sequence (4–3–2–1–4–3–2–1–4–3–2–1) six times, the main task consisted of three identical sessions, each containing 12 runs of a fixed 12-item sequence (1–2–1–4–2–3-4–1–3–2–4–3) followed by 2 × 12 pseudo-randomly presented stimuli (Fig. 1A).
For statistical analysis, the median RT of each run was calculated. For each session, sequence learning was defined as the difference in mean RT of the last two sequence blocks and the subsequent 2 × 12 pseudo-randomly presented trials, expressed as ΔRT (RTRAN − RTSEQ), with positive values indicating a sequence-vs.-random advantage, i.e., learning.
Motor adaptation
A MA paradigm was programmed in Matlab (The MathWorks, Natick, MA, USA), closely following a previous study39. Participants were instructed to draw straight lines from a central starting point “shooting” trough a target in one of eight possible positions arrayed around the starting point at a distance of 70 mm. Targets were equally distributed every 45°. Movements were performed with a hand-held pen on a digitizing tablet (Wacom Intuos Pro L, Wacom, Kazo, Japan) and visualized on a screen. Participants wore special goggles to prevent visual feedback from the moving hand. Targets were presented in pseudo-random order, with each direction appearing once in every block of eight consecutive trials. Over the course of the experiment, a counter-clockwise (visuo-motor) perturbation of the cursor movement displayed on the screen in relation to the actual hand movement trajectory on the tablet was introduced (Fig. 2A).
The cursor was visible during the entire experiment. In case of a hit, the target changed its color to green, otherwise a red dot marked the position where the cursor crossed the (invisible) circle connecting the target positions. When participants hit the target in less than 500 ms, they were rewarded with a pleasant bell sound. If movement durations exceeded 500 ms, a pre-recorded voice (“faster, please!”) was played. Trials with a movement duration > 1000 ms led to immediate repetition of the previous and current trial.
After a practice block, the experiment started with 6 × 8 trials with veridical feedback (baseline condition). In the subsequent perturbation condition, the perturbation was increased in steps of 0.31° degree per trial over 96 (12 × 8) trials to a maximum deviation of 30° degrees counter-clockwise. The perturbation was kept at 30° for another 8 × 8 trials in the plateau condition to allow for further adaptation. Then, in the extinction condition, the perturbation was removed abruptly and kept a veridical feedback over 96 (12 × 8) trials.
In order to minimize the effect of corrective movements, the movement direction was assessed in the “ballistic” phase at 50% of the start-target distance. Adaptation was calculated per trial as the angular difference between the actual movement direction and the respective target angle. For statistical analysis, the mean adaptation per block of eight consecutive trials (i.e., one trial per movement direction) was calculated.
Classical eyeblink conditioning
Eyeblink conditioning was performed following an established protocol32 using air puff stimulation. In conditioning trials, air puffs (100 ms, 110 kPa) as unconditioned stimuli (US) were preceded by a tone (1 kHz, 440 ms) as the conditioning stimulus (CS) (Fig. 3A). After familiarization to the experimental setting (trials 1-20), the conditioning phase started with 10 × 10 conditioning trials (trials 21-120) with paired stimuli (US + CS), followed by 3 × 10 extinction trials (trials 121-150) where the CS was presented alone. A conditioned response (CR) was defined as a blink with onset at least 150 ms after the CS and onset before the US. Blinks were recorded by surface electromyography (EMG) of the orbicularis oculi muscle (Fig. 3B,C). The EMG signals were amplified and filtered (20 Hz and 2 kHz) with a D360 amplifier (Digitimer Limited) and digitized sampled at 5 kHz, digitized using a laboratory interface (Micro 1401; Cambridge Electronics Design, Cambridge, U.K.), and recorded and stored on a personal computer using SIGNAL 6 software (Cambridge Electronic Devices, Cambridge, U.K.). Single trial data were rectified, and smoothed by a low-pass filter (cut-off frequency of 0.06 Hz).
Blinks were automatically identified when having a minimum integral of 0.1 mV x ms and minimum amplitude of 0.001 mV after baseline correction, as well as a minimum duration of 50 ms. Blinks were visually inspected and manually corrected if necessary. Occurrence of CR per block of ten trials was expressed as percentage for further analysis. Trials had a duration of 20 s with onset of the CS after 10.3 s. The spontaneous blink rate was assessed in the first ten seconds of each trial.
Statistical analysis
Statistical analyses were performed using repeated measures ANOVA or t-test. Post-hoc t-tests for statistically significant main effects or interactions were corrected for multiple comparisons (Bonferroni-Holm). Relation of learning between experiments or correlation to clinical parameters was assessed using Pearson’s correlation coefficient. Analysis was conducted in R. A value of p < 0.05 was considered statistically significant.
Original data will be made available upon justified request.
References
Albanese, A. et al. Phenomenology and classification of dystonia: A consensus update. Mov. Disord. 28, 863–873 (2013).
Jinnah, H. A., Neychev, V. & Hess, E. J. The Anatomical basis for dystonia: The motor network model. Tremor Other Hyperkinet. Mov. (N. Y.) 7, 506 (2017).
Hallett, M. Neurophysiology of dystonia: The role of inhibition. Neurobiol. Dis. 42, 177–184 (2011).
Quartarone, A. & Pisani, A. Abnormal plasticity in dystonia: Disruption of synaptic homeostasis. Neurobiol. Dis. 42, 162–170 (2011).
Ding, J. B., Guzman, J. N., Peterson, J. D., Goldberg, J. A. & Surmeier, D. J. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron 67, 294–307 (2010).
Sciamanna, G. et al. Cholinergic dysfunction alters synaptic integration between thalamostriatal and corticostriatal inputs in DYT1 dystonia. J. Neurosci. 32, 11991–12004 (2012).
Meunier, S., Russmann, H., Shamim, E., Lamy, J.-C. & Hallett, M. Plasticity of cortical inhibition in dystonia is impaired after motor learning and paired-associative stimulation. Eur. J. Neurosci. 35, 975–986 (2012).
Doyon, J. et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res. 199, 61–75 (2009).
Penhune, V. B. & Steele, C. J. Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav. Brain Res. 226, 579–591 (2012).
Hardwick, R. M., Rottschy, C., Miall, R. C. & Eickhoff, S. B. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage 67, 283–297 (2013).
Pascual-Leone, A. et al. Procedural learning in Parkinson’s disease and cerebellar degeneration. Ann. Neurol. 34, 594–602 (1993).
Smith, M. A. & Shadmehr, R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J. Neurophysiol. 93, 2809–2821 (2005).
Clark, G. M., Lum, J. A. & Ullman, M. T. A meta-analysis and meta-regression of serial reaction time task performance in Parkinson’s disease. Neuropsychology 28, 945–958 (2014).
Gerwig, M. et al. Comparison of eyeblink conditioning in patients with superior and posterior inferior cerebellar lesions. Brain 126, 71–94 (2003).
Kotani, S., Kawahara, S. & Kirino, Y. Classical eyeblink conditioning in decerebrate guinea pigs. Eur. J. Neurosci. 15, 1267–1270 (2002).
De Zeeuw, C. I. & Yeo, C. H. Time and tide in cerebellar memory formation. Curr. Opin. Neurobiol. 15, 667–674 (2005).
Prudente, C. N., Hess, E. J. & Jinnah, H. A. Dystonia as a network disorder: What is the role of the cerebellum?. Neuroscience 260, 23–35 (2014).
Schirinzi, T., Sciamanna, G., Mercuri, N. B. & Pisani, A. Dystonia as a network disorder: A concept in evolution. Curr. Opin. Neurol. 31, 498–503 (2018).
Brüggemann, N. Contemporary functional neuroanatomy and pathophysiology of dystonia. J. Neural Transm. https://doi.org/10.1007/s00702-021-02299-y (2021).
Pizoli, C. E., Jinnah, H. A., Billingsley, M. L. & Hess, E. J. Abnormal cerebellar signaling induces dystonia in mice. J. Neurosci. 22, 7825–7833 (2002).
Fremont, R., Tewari, A., Angueyra, C. & Khodakhah, K. A role for cerebellum in the hereditary dystonia DYT1. Elife https://doi.org/10.7554/eLife.22775 (2017).
Corp, D. T. et al. Network localization of cervical dystonia based on causal brain lesions. Brain 142, 1660–1674 (2019).
Filip, P. et al. Disruption in cerebellar and basal ganglia networks during a visuospatial task in cervical dystonia. Mov. Disord. 32, 757–768 (2017).
Porcacchia, P. et al. Abnormal cerebellar connectivity and plasticity in isolated cervical dystonia. PLoS ONE 14, e0211367 (2019).
Katschnig-Winter, P. et al. Motor sequence learning and motor adaptation in primary cervical dystonia. J. Clin. Neurosci. 21, 934–938 (2014).
Sadnicka, A. et al. Normal motor adaptation in cervical dystonia: A fundamental cerebellar computation is intact. Cerebellum 13, 558–567 (2014).
Sadnicka, A. et al. High motor variability in DYT1 dystonia is associated with impaired visuomotor adaptation. Sci. Rep. 8, 3653 (2018).
Sadnicka, A. et al. Delineating cerebellar mechanisms in DYT11 myoclonus-dystonia. Mov. Disord. 33, 1956 (2018).
Hoffland, B. S. et al. A gait paradigm reveals different patterns of abnormal cerebellar motor learning in primary focal dystonias. Cerebellum 13, 760–766 (2014).
Teo, J. T., van de Warrenburg, B. P., Schneider, S. A., Rothwell, J. C. & Bhatia, K. P. Neurophysiological evidence for cerebellar dysfunction in primary focal dystonia. J. Neurol. Neurosurg. Psychiatry 80, 80–83 (2009).
Kojovic, M. et al. Secondary and primary dystonia: Pathophysiological differences. Brain 136, 2038–2049 (2013).
Weissbach, A. et al. Alcohol improves cerebellar learning deficit in myoclonus-dystonia: A clinical and electrophysiological investigation. Ann. Neurol. 82, 543–553 (2017).
Antelmi, E. et al. Impaired eye blink classical conditioning distinguishes dystonic patients with and without tremor. Parkinsonism Relat. Disord. 31, 23–27 (2016).
Sadnicka, A. et al. All in the blink of an eye: New insight into cerebellar and brainstem function in DYT1 and DYT6 dystonia. Eur. J. Neurol. 22, 762–767 (2015).
Carbon, M. et al. Impaired sequence learning in dystonia mutation carriers: A genotypic effect. Brain 134, 1416–1427 (2011).
Zeuner, K. E. et al. Increased volume and impaired function: The role of the basal ganglia in writer’s cramp. Brain Behav. 5, e00301 (2015).
Gallea, C. et al. Increased cortico-striatal connectivity during motor practice contributes to the consolidation of motor memory in writer’s cramp patients. Neuroimage Clin. 8, 180–192 (2015).
Robertson, E. M. The serial reaction time task: Implicit motor skill learning?. J. Neurosci. 27, 10073–10075 (2007).
Galea, J. M., Vazquez, A., Pasricha, N., de Xivry, J. J. & Celnik, P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: The motor cortex retains what the cerebellum learns. Cereb. Cortex 21, 1761–1770 (2011).
Hubsch, C. et al. Defective cerebellar control of cortical plasticity in writer’s cramp. Brain 136, 2050–2062 (2013).
Avanzino, L. et al. Adaptation of feedforward movement control is abnormal in patients with cervical dystonia and tremor. Clin. Neurophysiol. 129, 319–326 (2018).
Popa, T. et al. The neurophysiological features of myoclonus-dystonia and differentiation from other dystonias. JAMA Neurol. 71, 612 (2014).
Tzvi, E., Munte, T. F. & Kramer, U. M. Delineating the cortico-striatal-cerebellar network in implicit motor sequence learning. Neuroimage 94, 222–230 (2014).
Janacsek, K. et al. Sequence learning in the human brain: A functional neuroanatomical meta-analysis of serial reaction time studies. Neuroimage 207, 116387 (2020).
Hanssen, H. et al. Imaging gradual neurodegeneration in a basal ganglia model disease. Ann. Neurol. 86, 517 (2019).
Bostan, A. C., Dum, R. P. & Strick, P. L. Functional anatomy of basal ganglia circuits with the cerebral cortex and the cerebellum. Curr. Concepts Movem. Disord. Manage. 33, 50–61 (2018).
Tzvi, E., Stoldt, A., Witt, K. & Krämer, U. M. Striatal–cerebellar networks mediate consolidation in a motor sequence learning task: An fMRI study using dynamic causal modelling. Neuroimage 122, 52–64 (2015).
Rothkirch, I. et al. Dynamic causal modeling revealed dysfunctional effective connectivity in both, the cortico-basal-ganglia and the cerebello-cortical motor network in writers’ cramp. NeuroImage Clin. 18, 149–159 (2018).
Neychev, V. K., Fan, X., Mitev, V. I., Hess, E. J. & Jinnah, H. A. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain 131, 2499–2509 (2008).
Acknowledgements
This work has been supported by the Federal Ministry of Education and Research (BMBF; Grant No 01GM1514A) to Sebastian Loens, Tobias Bäumer and Alexander Münchau. It has also been supported by the German Research Foundation (DFG; SFB 936 and WE5919/2-1) to Alexander Münchau and Anne Weissbach and the Else Kröner-Fresenius Foundation (2018_A55) to Anne Weissbach. Sebastian Loens, Anne Weissbach, Alexander Münchau and Tobias Bäumer are members of the European Reference Network for Rare Neurological Diseases—Project ID No 739510.
Funding
Open Access funding enabled and organized by Projekt DEAL. Supported by a grant from the Federal Ministry of Education and Research (BMBF; Grant No 01GM1514A). The funding source was not involved in conceptualization of the study, data acquisition, interpretation of the results or publication of the manuscript.
Author information
Authors and Affiliations
Contributions
S.L., J.V., A.M. and T.B. designed and coordinated the study. S.L., V.H., A.K. and A.W. collected the data. J.V. and E.T. programmed the motor learning paradigms. S.L., J.V., A.M. and T.B. interpreted the data. S.L. drafted the manuscript. J.V., J.J., E.T., A.W., A.M. and T.B. critically revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.M. receives honoraria from Ipsen and Merz Pharmaceuticals. He is member of the advisory boards of Merz Pharmaceuticals. T.B. receives honoraria from Merz Pharmaceuticals, Allergan and Ipsen Pharma. He is member of the advisory boards of Merz Pharmaceuticals, Allergan and Ipsen Pharma and receives consultancies from Merz Pharmaceuticals and Allergan. The other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loens, S., Verrel, J., Herrmann, VM. et al. Motor learning deficits in cervical dystonia point to defective basal ganglia circuitry. Sci Rep 11, 7332 (2021). https://doi.org/10.1038/s41598-021-86513-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86513-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.