Abstract
Inflammatory bowel disease (IBD) is a chronic, recurrent inflammatory disease of the gastrointestinal (GI) tract. Ulcerative colitis (UC) is a type of IBD. Pregnane X Receptor (PXR) is a member of the nuclear receptor superfamily. In order to deepen understanding and exploration of the molecular mechanism of regulation roles of PXR on UC, biological informatics analysis was performed. First, 878 overlapping differentially expressed genes (DEGs) between UC and normal samples were obtained from the Gene Expression Omnibus (GEO) database (GSE59071 and GSE38713) by using the "limma" R language package. Then WGCNA analysis was performed by 878 DEGs to obtain co-expression modules that were positively and negatively correlated with clinical traits. GSEA analysis of PXR results obtained the signal pathways enriched in the PXR high and low expression group and the active genes of each signal pathway. Then the association of PXR with genes that are both active in high expression group and negatively related to diseases (gene set 1), or both active in low expression group and negatively related to diseases (gene set 2) was analyzed by String database. Finally, carboxylesterase 2 (CES2), ATP binding cassette subfamily G member 2 (ABCG2), phosphoenolpyruvate carboxykinase (PCK1), PPARG coactivator 1 alpha (PPARGC1A), cytochrome P450 family 2 subfamily B member 6 (CYP2B6) from gene set 1 and C-X-C motif chemokine ligand 8 (CXCL8) from gene set 2 were screened out. After the above analysis and reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) verification, we speculated that PXR may exert a protective role on UC by promoting CES2, ABCG2, PCK1, PPARGC1A, CYP2B6 expression and inhibiting CXCL8 expression in their corresponding signal pathway in intestinal tissue.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD) is a chronic, recurrent inflammatory disease of the gastrointestinal (GI) tract1,2. Ulcerative colitis (UC) is known as one type of IBD3. The incidence of UC is increasing not only in Western countries, but also in developing countries. Lesions in UC are mostly located in colon and rectum, which can further spread to the whole colon and attack repeatedly4. The clinical features of UC mainly manifest as diarrhea, abdominal pain, mucus defection, bloody stool and weight loss, etc. The etiology of UC is not yet very clear. At present, it is believed that the intricate coordination between genetics, environment, and immunity affects the occurrence and development of diseases5,6. In the past 50 years, many advances have been made in the treatment of UC. Biological agents, biosimilars, and antibiotics et al7,8, all have certain therapeutic effects on UC. Among them, rifaximin is an oral antibiotic that is virtually unabsorbed and only acts locally in the intestinal tract, and is thus with low systemic response. Many studies have confirmed its therapeutic effect and safety9,10.
Rifaximin is an agonist of nuclear receptor (NR) Pregnane X receptor (PXR)11. NR is a type of ligand activated transcription factor. It regulates gene expression by inhibiting the binding of repressor proteins and recruiting co-activators. In recent years, more and more studies have shown that NRs play an important role in maintaining intestinal nutrient absorption and transport, intestinal barrier function, and intestinal immunity12. PXR is a member of the NR superfamily and is highly expressed in liver and intestinal tissues. In recent years, it has been reported that PXR can inhibit the development of UC by regulating the xenobiotics metabolism, anti-inflammatory, and maintaining the barrier function of intestinal epithelial cells13,14, indicating that using PXR as a drug target to treat UC has certain application prospects. However, the pathological process of UC is very complicated. Metabolic abnormalities15, inflammation, immune response, cell proliferation, and apoptosis et al. are all involved in UC16,17. With the increase in research on PXR, the physiological role of PXR has also been better understood. In addition to acting as a sensor for exogenous substances detoxification, drug metabolism, and drug interactions by regulating the expression of enzyme encoding drug metabolism or drug transporter genes, PXR also plays an important regulatory role in glycolipid metabolism18, inflammation, cell proliferation, apoptosis, cell migration, and immune regulation19,20,21. However, whether PXR could regulate UC through these roles is not completely clear. Therefore, deeply understanding and exploration of the molecular mechanism of regulation roles of PXR on UC are of great significance for the treatment of UC as PXR being a drug target in the future.
Bioinformatics can effectively analyze gene chip data. As the latest bioinformatics research method, weighted gene co-expression network analysis (WGCNA) is a system biology method that uses gene expression data to construct a scale-free network. It can cluster genes with similar expression patterns into the same gene module. By associating modules with external sample traits, the relationship between modules and phenotypes could be explored22. Gene Set Enrichment Analyses (GSEA) could reveal the biological signal pathway by enriching genes into related gene sets23.
In this study, WGCNA was used to cluster DGEs into co-expression modules and correlate module with clinical traits. Then GSEA was performed to obtain the signaling pathways and corresponding active genes enriched in PXR high and low expression group. Based on the above analysis, the protein–protein interaction (PPI) network was carried out to screen and identify genes that are interacted and functionally associated with PXR. At last, reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) was performed to verify the results of bioinfomatics analysis. The research provides data support for exploring the molecular mechanism of PXR regulating UC.
Methods
Data information
The gene expression profile of GSE59071 and GSE38713 was obtained from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). GSE59071, the platform is the [HuGene-1_0-st] Affymetrix Human Gene 1.0 ST Array, which includes 74 UC samples and 11 normal. GSE38713, the platform is the [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array, consists of 15 UC samples and 13 normal samples.
Data preprocessing and DEGs analysis
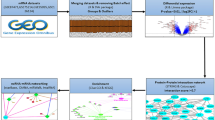
The research was designed according to the flow chart (Fig. 1). There were a total of 20,741 probes in GSE59071 datasets and 21,753 probes in GSE38713 datasets, which are all annotated by the Affymetrix annotation file. Log2 transformation and normalization were performed for processing data. Based on the initial data, we utilized the "limma" R package in R to screen the DEGs between UC samples and normal samples. Genes with adjusted P-value (adj. P) < 0.05 and |log Fold Change| (|log FC|) > 0.9 were considered as DEGs.
WGCNA analysis
We performed WGCNA analysis by WGCNA R package in R. WGCNA is a systematic biological method for constructing scale-free networks using gene expression data. Firstly the expression data profile of the overlapping DEGs in GSE59071 was tested to see whether they were suitable samples and genes. And then the similarity matrix was established according to Pearson’s correlation. Thirdly, the adjacency matrix was constructed to obtain a scale-free network from the similarity matrix by computing a correlation raised to a soft threshold power β between every pair of genes. Fourthly, the adjacency matrix was transformed into a topological overlap matrix (TOM) and the corresponding dissimilarity (dissTOM) was then calculated. Hierarchical clustering based on dissTOM was carried out to get a systematic clustering tree. With the parameters of the minimum number of base 30, the dynamic tree cut algorithm groups genes into modules. After that, according to the cutoff value of 0.25, module eigengenes (MEs) were clustered in order to merge very similar modules into a new one. We also calculated the correlation between the clinical traits and ME in each module.
GSEA analysis
GSEA is a computational approach to identify significantly enriched or depleted groups of genes23,24. We used GSEA 4.0.3 software for our analysis. The median expression value of PXR was used as the cut-off value. 74 UC samples from the GSE59071 GEO database were divided into high expression group and low expression group. GSEA was conducted to analyze biological pathways associated with PXR. Gene size ≥ 5, |normalized enrichment score (NES)|> 1.0, and the top 20 pathway were used as the cut-off criteria.
Protein interaction and functional association of PXR
STRING (version 11.0) database (https://string-db.org)25 was used to build the PPI network (score > 0.4) and the protein that interacted and associated with PXR was screened out. Cytoscape software was used for visualization26.
Identification of PXR target genes
Pearson’s correlation coefficients (R-values) between the expression levels of PXR and the other genes were analyzed by the "Pearson" method in R Studio. JASPAR (http://JASPAR.genereg.net/) database contains a curated, non-redundant set of profiles, derived from published and experimentally defined transcription factor binding sites for eukaryotes27. The PXR binding site on the target gene promoter was predicted by the JASPAR database.
Cell culture and adenovirus infection
HCT116, epethelial-like cell isolated from colon carcinoma, were cultured in DMEM containing 10% FBS with penicillin (100 U/ml) and streptomycin (100 U/ml) at 37 °C in a 5% CO2 incubator. In order to increase the expression level of PXR in HCT116, adenovirus PXR (Ad-PXR) was used to infect HCT116 for 24 h.
RT-qPCR
Total RNA was isolated using TRIzol (Sangon), converted to complementary DNA (cDNA) by HiScript III 1st Strand cDNA Synthesis Kit (Vazyme). RT-qPCR was performed by using ChamQ® Universal SYBR qPCR Master Mix (Vazyme). Primer sequences were described in supplementary Table 1. Amplification was carried out with an initial step at 95 °C for 30 s, followed by 40 cycles of amplification (95 °C for 10 s, 60 °C for 30 s) by using a CFX96 qPCR system (Bio-Rad). GAPDH was used as an internal control, and data were expressed as the ratio of target mRNA to GAPDH mRNA. All results were representative of at least three independent experiments.
Statistical analysis
All data were expressed as mean ± SE. Student’s t test was performed to determine statistical differences between groups. P < 0.05 was considered statistically significant.
Result
PXR expression in various UC and health samples
PXR mRNA expression level in various UC and health samples was assessed. A statistically significant reduction of PXR expression level was found in UC patients compared with control subjects (Fig. 2A,B), indicating that PXR may have a protective role in UC development.
Identification the differentially expressed genes. (A) The expression level of PXR in GSE59071 and GSE38713 datasets. **P < 0.01. (B, C) The volcanoplot of the DEGs between UC patients and control subjects in GSE59071 and GSE38713 datasets. (D) The Venn plot for selection of the overlapping DEGs in GSE59071 and GSE38713 datasets, overlapping DEGs = 878.
Identification of DEGs
After data processing, a total 1,447 DEGs (including 549 down-regulated and 898 up-regulated) and 1,657 DEGs (including 689 down-regulated and 968 up-regulated) were identified in GSE59071 and GSE38713 datasets respectively. The volcano plot of DEGs in GSE59071 and GSE38713 was shown in Fig. 2C and D. The 878 overlapping DEGs between the two datasets were obtained by intersection analysis (Fig. 2E).
WGCNA analysis
WGCNA analysis was performed using the 878 overlapping DEGs in GSE59071. As shown in Fig. 3A, the value of R2 > 0.9, indicating that the scale-free network was successfully constructed. According to the cutoff value of 0.25, DEGs were clustered into two co-expression modules (Fig. 3B,C). The grey module represented a gene set that was not assigned to any of the modules. After combining clinical traits, the brown module (r = −0.72, P < 7.0e−15) was negatively correlated with the disease present. The yellow module (r = 0.65, P < 2.0e−11) was positively correlated with the disease present (Fig. 3D).
The WGCNA of 878 overlapping DEGs between the UC group and the control group. (A) Scale-free networks checking, R2 > 0.9. (B Module clustering dendrogram. The module is merged with the cutoff value of 0.25. (C) Module clustering dendrogram. Each branch in the figure represents one gene, and every color below represents one co-expression module. (D) The correlation between each module with UC disease present.
GSEA associated with PXR
To investigate the possible function and mechanism of PXR in regulating UC, we used the expression matrix of all DEGs for PXR GSEA analysis. The top 20 signal pathways and active genes enriched in high and low expression samples were shown in Table 1 and Table 2 respectively. Among them, signal pathways related to drug and energy metabolism like metabolism of xenobiotics by cytochrome P450 and glycolysis gluconegenesis et al. were enriched in PXR high expression group (Fig. 4A,B), signal pathways related to inflammatory like toll-like receptor signaling pathway, cytokine-cytokine receptor interaction et al. were enriched in PXR low groups (Fig. 4C,D).
Six genes were screened out that can interact and functionally associate with PXR
In order to screen the genes that can interact and functionally associate with PXR, intersection and further PPI analysis were performed. First two overlapping gene sets named gene set 1 and gene set 2 were obtained by intersection analysis. The genes in the brown module that are negatively correlated with the disease intersected with the active genes enriched in the signal pathway of the high expression group constructed gene set 1. As shown in Fig. 5A, there are 94 overlapping genes in this set. The genes in the yellow module that are positively related to the disease crossed with the active genes enriched in the signal pathways of low expression group constructed gene set 2. As shown in Fig. 5B, there are 41 overlapping genes in this set. Further PPI analysis with PXR was performed on the two gene sets (Fig. 5C,D). Carboxylesterase 2 (CES2), ATP binding cassette subfamily G member 2 (ABCG2), phosphoenolpyruvate carboxykinase (PCK1), PPARG coactivator 1 alpha (PPARGC1A), cytochrome P450 family 2 subfamily B member 6 (CYP2B6) from gene set 1 and C-X-C Motif chemokine ligand 8 (CXCL8) from gene set 2 that were predicted to interact and functionally associate with PXR were screened out (Fig. 5E). The gene symbol name of these six genes and its signaling pathways they participate in were listed in Table 3.
Intersection analysis and protein–protein interaction network with PXR. (A) Gene set 1 was constructed by the intersection between the active genes in high expression group after GSEA and the genes in module that are negatively correlated with disease after WGCNA. (B) Gene set 2 was constructed by the intersection between the active genes in low expression group after GSEA and the genes in module that are positively correlated with disease after WGCNA. (C) PPI-network of 94 overlapping gene in gene set 1. (D) PPI-network of 41 overlapping gene in gene set 2. (E) CES2, ABCG2, PCK1, PPARGC1A and CYP2B6 from gene set 1 and CXCL8 from gene set 2 were predicted to interact and functionally associate with PXR.
The six genes were regulated by PXR
The expression level of CES2, ABCG2, PCK1, PPARGC1 and CYP2B6 from gene set 1 were lower in disease than in control group, while the CXCL8 from gene set 2 were higher in disease than in control group (Fig. 6A,B). In order to predict whether the five genes from set 1 are the target genes of PXR, the Pearson’s correlation coefficient between PXR and the five genes expression level was calculated. As shown in supplementary Fig. 1A–J, the Pearson’s correlation coefficient between PXR and the five genes expression level was all greater than 0.5 in two datasets. JASPAR analysis showed that there is at least one PXR binding site on the promoter of these genes. Table 4 showed the predicted sequences that PXR bind on gene promoters with the highest scores. All of these analyses showed CES2, ABCG2, PCK1, PPARGC1A, and CYP2B6 are all possibly PXR target genes. To further verify the bioinformatics analysis results, we examined the expressions levels of CES2, ABCG2, PCK1, PPARGC1A, CYP2B6 and CXCL8 in HCT116 cells. As shown in Fig. 6C and D, overexpression of PXR significantly increased messenger RNA (mRNA) level of CES2, ABCG2, PCK1, PPARGC1A, CYP2B6, and reduced the up-regulated expression of CLX8 induced by lipopolysaccharide (LPS).
CES2, ABCG2, PCK1, PPARGC1A, CYP2B6 and CXCL8 were regulated by PXR. (A) The expression level of CES2, ABCG2, PCK1, PPARGC1A and CYP2B6 in GSE59071 and GSE38713 datasets. (B) The expression level of CXCL8 in GSE59071 and GSE38713 datasets. (C) PXR increased the expression of CES2, ABCG2, PCK1, PPARGC1A and CYP2B6 in HCT116 cells. HCT116 cells were infected with Ad-PXR or mock infection for 24 h. The mRNA levels of CES2, ABCG2, PCK1, PPARGC1A and CYP2B6 were quantified by RT-qPCR. (D) PXR reduced the up-regulated expression of CLX8 induced by LPS. HCT116 cells were infected with Ad-PXR or mock for 24 h before exposure to LPS (1 μg/ml) for 12 h, the mRNA levels of CXCL8 were quantified by RT-qPCR. Data shown are as mean ± S.E. of at least three independent experiments. *P < 0.05 versus control; **P < 0.01 versus control.
Discussion
More and more documents showed that the activation of PXR has a protective role on UC. Antibiotic rifaximin, a PXR agonist was used clinically for the treatment of UC9. In order to deeply explore the molecular mechanism of PXR on UC regulation, integrated bioinformatics methods were used in this study.
We first used two GEO database (GSE59071 and GSE38713) to statistically analyze the expression level of PXR in the health and UC groups. The results showed that the expression level of PXR was significantly lower in UC compared to health groups, which was consistent with Langmann and other authors’ reports28,29, indicating PXR is negatively related to the disease. WGCNA can cluster genes with co-expression patterns into the same module and associate the modules with clinical traits. After WGCNA analysis by using 878 DEGs, the brown module that is positively related to disease and yellow module that is negatively related to the disease was identified. Then single PXR gene of GSEA was performed to obtain the signal pathway enriched in PXR high expression or low expression group. As PXR expression level is low in UC disease and has a protective role on UC, PXR may protect UC by positively regulating the signal pathway enriched in the high expression group or negatively regulating the signal pathway enriched in the low expression group. Based on analysis of WGCNA and GSEA, we speculated that if PXR can interact or functionally associated with genes that are both active in the high expression group and negatively related to diseases, then PXR may inhibit UC by promoting these genes expression. Similarly, if PXR can interact or functionally associated with genes that are both active in the low expression group and positively related to diseases, then PXR may play a role in protecting UC by inhibiting these gene expressions. As protein interaction analysis based on the string database includes not only the direct (physical) interaction but also indirect (functional) association, the PPI network with PXR was performed by using these two gene sets (gene set 1 and gene set 2). Finally, five genes (CES2, ABCG2, PCK1, PPARGC1A, CYP2B6) from set 1 and one gene (CXCL8) from set 2 were screened out (Fig. 5E). In this way, PXR can play a protective effect on UC by promoting CES2, ABCG2, PCK1, PPARGC1A, CYP2B6 expression, or inhibiting CXCL8 gene expression. Furthermore, the results of RT-qPCR further confirmed that PXR indeed increased the expression level of CES2, ABCG2, PCK1, PPARGC1A, CYP2B6, and inhibited the expression of CXCL8 induced by LPS.
The five genes (CES2, ABCG2, PCK1, PPARGC1A, CYP2B6) are all distributed in pathways related to metabolism, such as glucose and lipid metabolism and drug metabolism. PCK1 is a key enzyme regulating liver gluconeogenesis. It has been reported that activation of PXR in mice can inhibit PCK1 expression by inhibiting FOXO1 (forkhead box protein O1), the main transcription factor of PCK1, or by competitively binding to peroxisome proliferator-activated receptor-gamma coactivator 1α (PPARGC1A) with hepatocyte nuclear factor 4 alpha (HNF4α)30. However, the regulation of PCK1 expression in human showed the opposite result. PXR up-regulates the expression of PCK1 through the unique human PXR-SGK2 signaling pathway31. Our results showed that the expression level of PCK1 in UC patient samples is down-regulated as PXR, and Pearson's correlation coefficient and JASPAR analysis both showed that PCK1 maybe the target gene of PXR. Therefore, it is speculated that PXR may directly initiate PCK1 transcription and up-regulates its expression to promote gluconeogenesis in colon tissue. Further literature mining suggested that UC disease showed lower nutritional levels and excessive energy consumption32. At the same time, lipid metabolism and tricarboxylic acid cycle metabolism levels in UC disease decreased, resulting in the loss of energy homeostasis33,34,35. Studies have reported that in addition to liver and kidney, gluconeogenesis genes are indeed expressed in intestinal tissues36,37, and intestinal gluconeogenesis can promote glucose and energy balance36. Therefore, we speculated that PXR may protect UC by promoting the expression of PCK1 and increasing the level of gluconeogenesis in intestinal tissues.
Studies have shown that PPARGC1A is highly expressed in intestinal epithelial cells, but its expression is low in UC diseases38, which is consistent with the expression level of the GEO dataset (GSE59071 and GSE38713). Lack of PPARGC1A in mouse intestinal epithelial cells aggravates the occurrence of colitis38, showing the protective effect of PPARGC1A on diseases. Studies have reported that the dysfunction of mitochondrial oxidative phosphorylation plays an important role in the occurrence of UC39. Literature mining results showed that PPARGC1A can maintain mitochondrial functions, such as inhibiting oxidative stress, controlling the balance of ROS levels and inhibiting inflammation by promoting mitochondrial biogenesis in intestinal epithelial cells38. What’s more, mitochondria are of great significance in maintaining the function of the electron transport chain and generating ATP energy. UC disease has low energy characteristics32, which is obviously not helpful for the formation of tight junction that requires energy in intestinal epithelial cells40. Damage to tight junction will lead to the invasion of pathogens and aggravate the occurrence of UC41. Therefore, PPARGC1A may ensure the integrity of the physical barrier of intestinal epithelial cells through mitochondrial biogenesis38. Combined with the results of our study, we speculated that PXR could play an important protective role in mitochondrial biogenesis by up-regulating the expression of PPARGC1A.
It is widely believed that detoxification and biotransformation play an important role in protecting intestinal epithelial cells42. The deletion of detoxification enzyme genes is an important event leading to the initiation and development of UC disease43. GEO database data shows that CES2, CYP2B6, and ABCG2 detoxification genes are down-regulated in disease samples (Fig. 6 A,B). As the main transcription factor of detoxification genes, we speculated that PXR can play an anti-UC effect by regulating expression of detoxification genes like CES2, CYP2B6, and ABCG2 in intestinal tissue. Although the literature has shown that PXR can regulate the expression of CYP2B6 and ABCG2, the regulation role is not occurred in the intestinal tissues44,45. Therefore, our results revealed new targets for PXR to regulate detoxification genes in intestinal tissues.
The abnormal immune response is the main pathological change when UC occurs, mucosal inflammation caused by immune cell infiltration is the main histopathological feature of UC28,46,47. CXCL8 is a member of the CXC chemokine family. It can induce neutrophils to accumulate to the diseased colon mucosa through its receptors, causing tissue infiltration47,48, stimulating neutrophils degranulation, and inducing respiratory bursts. The resulting large amount of toxic substances trigger an intestinal inflammatory response, leading to the destruction of the intestinal barrier and tissue damage49. As a pattern recognition receptor for pathogens, TLR (Toll-like receptor) can sense the stimulation of external pathogens and activate NF-κB to produce inflammatory factors and trigger an inflammatory response. Studies have reported that TLR4 of intestinal epithelial cells was stimulated by pathogenic bacteria to up-regulate the expression of CXCL8 via NF-κB50, thereby increasing the infiltration of neutrophils, aggravating inflammation, and causing damage to intestinal tissue51. In addition, the necrosis of intestinal epithelial cells and the subsequent release of DAMPs (damage-associated molecular patterns) caused by intestinal tissue damage can also further activate TLR2 and trigger the occurrence of inflammation52,53,54. Numerous studies have shown that PXR inhibits the up-regulated expression of CXCL8 by inhibiting NF-κB50,55. Our combined analysis of WGCNA and PXR GSEA showed that PXR can protect UC by inhibiting the up-regulated expression of CXCL8.
Conclusion
After integrated bioinformatics analysis and RT-qPCR test, we thought that PXR may protect UC by promoting intestinal gluconeogenesis, maintaining the intestinal mitochondrial function, promoting detoxification through upregulation of CES2, ABCG2, PCK1, PPARGC1A, CYP2B6 gene expression, and inhibiting the expression of the inflammatory factor CXCL8 (Fig. 7).
Data availability
All authors make sure that all data and materials support published claims and comply with field standards.
References
Xavier, R. J. & Podolsky, D. K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434. https://doi.org/10.1038/nature06005 (2007).
Park, S. J., Kim, W. H. & Cheon, J. H. Clinical characteristics and treatment of inflammatory bowel disease: a comparison of Eastern and Western perspectives. World J. Gastroenterol. 20, 11525–11537. https://doi.org/10.3748/wjg.v20.i33.11525 (2014).
Khaki-Khatibi, F. et al. Calprotectin in inflammatory bowel disease. Clinica chimica acta; Int. J. Clin. Chem. 510, 556–565. https://doi.org/10.1016/j.cca.2020.08.025 (2020).
Kucharzik, T., Koletzko, S., Kannengiesser, K. & Dignass, A. Ulcerative colitis-diagnostic and therapeutic algorithms. Deutsches Arzteblatt Int. 117, 564–574. https://doi.org/10.3238/arztebl.2020.0564 (2020).
Niu, W. et al. Polysaccharides from natural resources exhibit great potential in the treatment of ulcerative colitis: a review. Carbohydrate Polym. 254, 117189. https://doi.org/10.1016/j.carbpol.2020.117189 (2021).
Macpherson, A. J. & Harris, N. L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 4, 478–485. https://doi.org/10.1038/nri1373 (2004).
Danese, S. et al. ECCO position statement on the use of biosimilars for inflammatory bowel disease-an update. J. Crohns. Colitis 11, 26–34. https://doi.org/10.1093/ecco-jcc/jjw198 (2017).
Fiocchi, C. & Iliopoulos, D. What’s new in IBD therapy: An “omics network” approach. Pharmacol. Res. 159, 104886. https://doi.org/10.1016/j.phrs.2020.104886 (2020).
Sartor, R. B. Review article: the potential mechanisms of action of rifaximin in the management of inflammatory bowel diseases. Aliment. Pharmacol. Ther. 43(Suppl 1), 27–36. https://doi.org/10.1111/apt.13436 (2016).
Guslandi, M. Rifaximin in the treatment of inflammatory bowel disease. World J. Gastroenterol. 17, 4643–4646. https://doi.org/10.3748/wjg.v17.i42.4643 (2011).
Terc, J., Hansen, A., Alston, L. & Hirota, S. A. Pregnane X receptor agonists enhance intestinal epithelial wound healing and repair of the intestinal barrier following the induction of experimental colitis. Eur. J. Pharm. Sci. 55, 12–19. https://doi.org/10.1016/j.ejps.2014.01.007 (2014).
Ning, L., Lou, X., Zhang, F. & Xu, G. Nuclear receptors in the pathogenesis and management of inflammatory bowel disease. Med. Inflamm. 2019, 2624941. https://doi.org/10.1155/2019/2624941 (2019).
Garg, A. et al. Pregnane X receptor activation attenuates inflammation-associated intestinal epithelial barrier dysfunction by inhibiting cytokine-induced myosin light-chain kinase expression and c-Jun N-terminal kinase 1/2 activation. J. Pharmacol. Exp. Ther. 359, 91–101. https://doi.org/10.1124/jpet.116.234096 (2016).
Zhang, G. et al. Patchouli alcohol activates PXR and suppresses the NF-κB-mediated intestinal inflammatory. J. Ethnopharmacol. 248, 112302. https://doi.org/10.1016/j.jep.2019.112302 (2020).
Garcia, M. et al. Nuclear receptor metabolism of bile acids and xenobiotics: a coordinated detoxification system with impact on health and diseases. Int. J. Mol. Sci. https://doi.org/10.3390/ijms19113630 (2018).
Sairenji, T., Collins, K. L. & Evans, D. V. An update on inflammatory bowel disease. Prim. Care 44, 673–692. https://doi.org/10.1016/j.pop.2017.07.010 (2017).
Guan, Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J. Immunol. Res. 2019, 7247238. https://doi.org/10.1155/2019/7247238 (2019).
Chen, K. et al. The role of xenobiotic receptors on hepatic glycolipid metabolism. Curr. Drug Metab. 20, 29–35. https://doi.org/10.2174/1389200219666180918152241 (2019).
Huang, K. et al. Targeting the PXR-TLR4 signaling pathway to reduce intestinal inflammation in an experimental model of necrotizing enterocolitis. Pediatr. Res. 83, 1031–1040. https://doi.org/10.1038/pr.2018.14 (2018).
Ouyang, N. et al. Pregnane X receptor suppresses proliferation and tumourigenicity of colon cancer cells. Br. J. Cancer 102, 1753–1761. https://doi.org/10.1038/sj.bjc.6605677 (2010).
Oladimeji, P. O. & Chen, T. PXR: more than just a master xenobiotic receptor. Mol. Pharmacol. 93, 119–127. https://doi.org/10.1124/mol.117.110155 (2018).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinf. 9, 559. https://doi.org/10.1186/1471-2105-9-559 (2008).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550. https://doi.org/10.1073/pnas.0506580102 (2005).
Debrabant, B. The null hypothesis of GSEA, and a novel statistical model for competitive gene set analysis. Bioinformatics (Oxford, England) 33, 1271–1277. https://doi.org/10.1093/bioinformatics/btw803 (2017).
Szklarczyk, D. et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447-452. https://doi.org/10.1093/nar/gku1003 (2015).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. https://doi.org/10.1101/gr.1239303 (2003).
Khan, A. et al. JASPAR 2018: update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 46, D260–D266. https://doi.org/10.1093/nar/gkx1126 (2018).
Dring, M. M. et al. The pregnane X receptor locus is associated with susceptibility to inflammatory bowel disease. Gastroenterology 130, 341–348. https://doi.org/10.1053/j.gastro.2005.12.008 (2006).
Langmann, T. et al. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology 127, 26–40. https://doi.org/10.1053/j.gastro.2004.04.019 (2004).
Pavek, P. Pregnane X receptor (PXR)-mediated gene repression and cross-talk of PXR with other nuclear receptors via coactivator interactions. Front. Pharmacol. 7, 456. https://doi.org/10.3389/fphar.2016.00456 (2016).
Mackowiak, B., Hodge, J., Stern, S. & Wang, H. The roles of xenobiotic receptors: beyond chemical disposition. Drug Metab. Dispos. 46, 1361–1371. https://doi.org/10.1124/dmd.118.081042 (2018).
Balestrieri, P. et al. Nutritional aspects in inflammatory bowel diseases. Nutrients https://doi.org/10.3390/nu12020372 (2020).
Sasaki, M. et al. Energy expenditure in Japanese patients with severe or moderate ulcerative colitis. J. Clin. Biochem. Nutr. 47, 32–36. https://doi.org/10.3164/jcbn.10-07 (2010).
Takaoka, A. et al. Comparison of energy metabolism and nutritional status of hospitalized patients with Crohn’s disease and those with ulcerative colitis. J. Clin Biochem. Nutr. 56, 208–214. https://doi.org/10.3164/jcbn.14-95 (2015).
Yamamoto, R., Muroi, K. & Imaishi, H. Serum derived from ulcerative colitis mouse changes the metabolism of the fluorescent substrate by P450 depending on the degree of disease progression. Chemico-Biol. Interact. 290, 88–98. https://doi.org/10.1016/j.cbi.2018.05.012 (2018).
Vily-Petit, J. et al. Intestinal gluconeogenesis prevents obesity-linked liver steatosis and non-alcoholic fatty liver disease. Gut https://doi.org/10.1136/gutjnl-2019-319745 (2020).
Zhan, K., Yang, T. Y., Chen, Y., Jiang, M. C. & Zhao, G. Q. Propionate enhances the expression of key genes involved in the gluconeogenic pathway in bovine intestinal epithelial cells. J. Dairy Sci. 103, 5514–5524. https://doi.org/10.3168/jds.2019-17309 (2020).
Cunningham, K. E. et al. Peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC1α) protects against experimental murine colitis. J. Biol. Chem. 291, 10184–10200. https://doi.org/10.1074/jbc.M115.688812 (2016).
Ferramosca, A. et al. Prunus mahaleb fruit extract prevents chemically induced colitis and enhances mitochondrial oxidative metabolism via the activation of the Nrf2 pathway. Mol. Nutr. Food Res. 63, e1900350. https://doi.org/10.1002/mnfr.201900350 (2019).
Olivier, S. et al. AMPK activation promotes tight junction assembly in intestinal epithelial Caco-2 cells. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20205171 (2019).
Su, L. et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology 145, 407–415. https://doi.org/10.1053/j.gastro.2013.04.011 (2013).
Ding, Y. P. et al. Integrative network-based analysis of colonic detoxification gene expression in ulcerative colitis according to smoking status. J. Crohn’s Colitis 11, 474–484. https://doi.org/10.1093/ecco-jcc/jjw179 (2017).
Mencarelli, A. et al. Pregnane-X-receptor mediates the anti-inflammatory activities of rifaximin on detoxification pathways in intestinal epithelial cells. Biochem. Pharmacol. 80, 1700–1707. https://doi.org/10.1016/j.bcp.2010.08.022 (2010).
Malekshah, O. M., Lage, H., Bahrami, A. R., Afshari, J. T. & Behravan, J. PXR and NF-κB correlate with the inducing effects of IL-1β and TNF-α on ABCG2 expression in breast cancer cell lines. Eur. J. Pharm. Sci. 47, 474–480. https://doi.org/10.1016/j.ejps.2012.06.011 (2012).
Goodwin, B., Moore, L. B., Stoltz, C. M., McKee, D. D. & Kliewer, S. A. Regulation of the human CYP2B6 gene by the nuclear pregnane X receptor. Mol. Pharmacol. 60, 427–431 (2001).
Maloy, K. J. & Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474, 298–306. https://doi.org/10.1038/nature10208 (2011).
Muthas, D. et al. Neutrophils in ulcerative colitis: a review of selected biomarkers and their potential therapeutic implications. Scand. J. Gastroenterol. 52, 125–135. https://doi.org/10.1080/00365521.2016.1235224 (2017).
Cifarelli, V. et al. CD36 deficiency impairs the small intestinal barrier and induces subclinical inflammation in mice. Cell. Mol. Gastroenterol. Hepatol. 3, 82–98. https://doi.org/10.1016/j.jcmgh.2016.09.001 (2017).
Rugtveit, J. et al. Respiratory burst of intestinal macrophages in inflammatory bowel disease is mainly caused by CD14+L1+ monocyte derived cells. Gut 37, 367–373. https://doi.org/10.1136/gut.37.3.367 (1995).
Mencarelli, A. et al. Inhibition of NF-κB by a PXR-dependent pathway mediates counter-regulatory activities of rifaximin on innate immunity in intestinal epithelial cells. Eur. J. Pharmacol. 668, 317–324. https://doi.org/10.1016/j.ejphar.2011.06.058 (2011).
Signs, S. A. et al. Stromal miR-20a controls paracrine CXCL8 secretion in colitis and colon cancer. Oncotarget 9, 13048–13059. https://doi.org/10.18632/oncotarget.24495 (2018).
Yu, M. et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock (Augusta, Ga.) 26, 174–179. https://doi.org/10.1097/01.shk.0000225404.51320.82 (2006).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107. https://doi.org/10.1038/nature08780 (2010).
Medzhitov, R. Inflammation 2010: new adventures of an old flame. Cell 140, 771–776. https://doi.org/10.1016/j.cell.2010.03.006 (2010).
Dogan, B., Fu, J., Zhang, S., Scherl, E. J. & Simpson, K. W. Rifaximin decreases virulence of Crohn’s disease-associated Escherichia coli and epithelial inflammatory responses. J. Antibiot. 71, 485–494. https://doi.org/10.1038/s41429-017-0022-y (2018).
Acknowledgements
We thank Nanping Wang for providing PXR adenovirus and helpful suggestion on bioinformatical analysis.
Funding
This study was funded by Liaoning province natural science and technology plan guidance project (2019-ZD-0301) and doctoral scientific research foundation of Liaoning Normal University (BS2020L002).
Author information
Authors and Affiliations
Contributions
Y.C. and N.C. contributed to the study conception and design, and revised the manuscript. Methodology and bioinformatics analysis were performed by H.G. and Y.C. The first draft of the manuscript was written by H.G. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, H., Chi, Y. & Chi, N. Bioinformatis analysis reveals possible molecular mechanism of PXR on regulating ulcerative colitis. Sci Rep 11, 5428 (2021). https://doi.org/10.1038/s41598-021-83742-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-83742-8
This article is cited by
-
Exploring the molecular mechanism of comorbidity of autism spectrum disorder and inflammatory bowel disease by combining multiple data sets
Journal of Translational Medicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.