Abstract
The purpose of this study was to compare the changes in DXA values including trabecular bone score (TBS) and bone mineral density (BMD) of lumbar spine (LS) and femur according to the hormone therapies including tamoxifen (TMXF) treatment with or without gonadotropin releasing hormone analog (GnRH analog) in women with breast cancer. We enrolled 119 women with breast cancer who had undergone breast-conserving surgery or mastectomy followed by TMXF treatment for postmenopausal women (TMXF group, n = 63, 52.9%) or by combination therapy of TMXF combined with GnRH analog for premenopausal women (TMXF + GnRH group, n = 56, 47.1%) from December 2013 to December 2017. The median follow-up period was 13 months (interquartile range [IQR], 12.0–14.75) for TMXF group and 13.5 months (IQR, 12.00–16.00) for TMXF + GnRH group, respectively. Patients did not receive bone-modifying therapy. The baseline dual-energy X-ray absorptiometry (DXA) scan before breast cancer surgery and follow-up DXA during hormone therapy. Comparing the first and follow-up DXA results, BMD in LS were significantly decreased in both TMXF (P < 0.001, mean difference: − 0.06) and TMXF + GnRH (P < 0.001, mean difference: − 0.09) groups. BMD values of femoral neck (P = 0.0011, mean difference: − 0.01) and total femur (P < 0.001, mean difference: − 0.03) was significantly changed between the baseline and follow-up DXA in TMXF + RnRH group. In the TMX group, a significant changed occurred in the BMD in total femur (P < 0.001, mean difference: − 0.030) but not the BMD of femoral neck (P = 0.095, mean difference: − 0.007). Regarding TBS, no significant change was found in the TMXF (P = 0.574, mean difference: − 0.004) group, whereas there was a significant decrease in TBS in the TMXF + GnRH (P < 0.001, mean difference: − 0.02) group during follow-up. TBS is more sensitive in reflecting the bone microarchitecture changes by TMXF or GnRH agonist in breast cancer patients than BMD. This finding demonstrates that TBS can be a useful parameter to detect bone microarchitectural changes in clinical applications.
Similar content being viewed by others
Introduction
Hormone-receptor positive cancers are the most common type of breast cancer, accounting for 75% of all breast cancers1,2,3. The recommended treatment is adjuvant endocrine therapy with aromatase inhibitors or tamoxifen (TMXF), a selective estrogen receptor modulator. TMXF is less effective than aromatase inhibitors in hormone-receptor positive breast cancer4,5,6,7; however, it is used for patients with low risk or those who cannot tolerate aromatase inhibitor.
In premenopausal women, it has been reported that TMXF with ovarian function suppression moderately improved disease-free survival compared to TMXF alone8. From the perspective of bone health, ovarian function suppression using gonadotropin-releasing hormone analog (GnRH analog) has the potential to increase the osteoporotic fracture risk of patients because of its estrogen-depriving effect9,10,11. It has been reported that TMXF could increase bone mineral density (BMD) because of its partial estrogen-receptor agonistic effects on the skeleton2,3,12. However, a recent study reported that TMXF did not have a preventive effect on the risk of fracture in postmenopausal women, and increased the risk of fracture in premenopausal women13. In premenopausal women, TMXF competes with estradiol for estrogen-receptor binding sites as a partial antagonist, leading to net bone loss13.
Qualitative parameters of bone, such as trabecular bone score (TBS), could predict osteoporotic fractures independent of BMD acquired from dual-energy X-ray absorptiometry (DXA)14. In a breast cancer prevention study, aromatase inhibitor therapy for 2 years compared to placebo was associated with loss of volumetric BMD and cortical thickness at a radius of − 4.3% and − 6.8%, respectively, whereas bone loss by DXA was < 2% at all sites15. In a study about the effect of endocrine treatment on densitometric results, results were discordant between the change in BMD and TBS in a TMXF group and an aromatase inhibitor group of postmenopausal women with breast cancer16.
Studies regarding the effect of combination therapy with TMXF and GNRH analog on bone changes are lacking. We used BMD and TBS to investigate how these endocrine treatments affect bone microarchitecture in breast cancer patients.
Therefore, we compared the changes in both TBS and BMD to evaluate the effect of TMXF treatment in postmenopausal women or combination therapy with TMXF and GnRH analog in premenopausal women with breast cancer.
Materials and methods
Patients
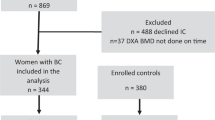
The study is a retrospective cross-sectional study. We recruited 119 women with breast cancer who received breast-conserving surgery or mastectomy for the treatment of breast cancer, followed by adjuvant endocrine therapy either by monotherapy of TMXF or combination therapy of TMXF and GNRH analogs between December 2013 and December 2017. All patients were followed-up for a median period of 13.00 months (interquartile range [IQR], 12.00–15.00). The median follow-up period was 13.00 months (IQR, 12.00–14.75) for TMXF group and 13.50 months (IQR, 12.00–16.00) ) for TMXF + GnRH group, respectively. Patients were eligible for the study if they met the following criteria: patients being treated with TMXF only for postmenopausal women (TMXF group), or with TMXF combined with GnRH for premenopausal women (TMXF + GnRH group), for more than 6 months, and who had no bone modifying therapy at any time. Prior chemotherapy was not considered for exclusion because the proportion of the patients was not different between postmenopausal and premenopausal status.
This study was approved by the Institutional Review Board of Pusan National University Hospital, which waived the requirement for written consent (IRB No. 1801-003-062). All methods were performed in accordance with relevant guidelines and regulations.
DXA
Both sites of lumbar spine (LS) and femur were evaluated using DXA scan images (Lunar Prodigy; GE Medical Systems, Milwaukee, WI, USA) and analyzed using Encore software (ver. 13.0; GE Healthcare, Madison, WI, USA). Baseline DXA scans were performed prior to breast cancer surgery, and follow-up studies were performed during endocrine therapy. We adopted the baseline DXA and the first follow-up DXA images for the analysis. The BMD (g/cm2) was obtained in LS, femoral neck (FN) and total femur (TF). TBS of the LS was derived from average values of L1–L4. A daily calibration and quality assurance test were performed, and the coefficient of variation for precise measurement of the BMD of the LS was 0.34%. The least significant change (LSC) at 95% confidence level were within 0.003 g/cm2 for BMD of LS, 0.048 g/cm2 for BMD of FN, 0.033 g/cm2 for BMD of TF and within 0.053 for TBS. All patients were evaluated with T-scores for the diagnosis, because at the time of the acquisition of DXA, all patients were already in perimenopausal or menopausal status due to physiologic causes or GnRH therapy. The T-score was defined according to the number of standard deviations (SDs) from the mean BMD of a reference group from the general population and matched for sex at 25–35 years of age. T-scores ≤ − 2.5 SDs from the reference mean were defined as osteoporosis; T-scores between − 2.5 and − 1.0 SDs from the reference mean were defined as osteopenia; T-scores ≥ − 1.0 SD from the reference mean were defined as normal. The TBS of the LS was extracted from the DXA file using iNsight software (ver. 3.0.2.0; Medimaps, Pessac, France). We used the following TBS cutoffs proposed by an international working group of TBS users for postmenopausal women: normal for TBS values ≥ 1.35, degraded microarchitecture for values between 1.20 and 1.35, and more degraded for values ≤ 1.2017.
Statistical analyses
Values of all non-normally distributed variables are expressed as medians and interquartile ranges (IQR; 25–75%). A Mann–Whitney U-test was used to compare continuous variables for the two groups. For comparing the categorical data of the groups, the chi-squared test was used. A paired t-test was used to compare the average differences in values between the baseline DXA and the follow-up DXA. The statistical analyses were performed using the MedCalc software (version 16.4.3; MedCalc, Mariakerke, Belgium) and RStudio (version 1.2b; RStudio, MA, USA). A P value less than 0.05 was regarded as indicative of significance.
Results
Baseline characteristics
A total of 119 women were enrolled; 63 (52.9%) were included in the TMXF group and 56 (47.1%) were included in the TMXF + GnRH group. Tamoplex was used as TMXF therapy with the dose of 20 mg once a day or 10 mg twice a day. Zoladex was added as a GnRH therapy for all patients in TMXF + GnRH group. For GnRH therapy, 3.6 mg of Zoladex was administered subcutaneously every 28 days. The average duration of use of TMXF was 57.5 months with interquartile range [IQR] of 51–59 months. The median duration of use of GnRH was 40.50 months with IQR of 26–54 months. The age (median age: 48 years; IQR, 44–77] for TMXF group versus 40 years; IQR, 30–49] for TMXF + GnRH group, P < 0.001) was significantly different between the two groups. Other characteristics including BMI, height, weight, stage of the breast cancer, hormonal status, type of operation and chemotherapy were not significantly different between two groups. The proportion of TMXF group who received radiotherapy was higher than that of TMXF + GnRH group (82.5% vs. 57.1%, P = 0.003). The median follow-up period was 14 months (IQR, 12.0–15.0) for all patients and there was no significant difference between the two groups (median: 13.00 months [IQR, 12.00–14.75) versus 13.50 months [12.00–16.00], P = 0.246). Demographic information and baseline characteristics are shown in Table 1.
Difference in BMD and TBS between pre-and postmenopausal women
Prior to breast cancer surgery, baseline BMD values were not significantly different in LS, FN, and TF (median LS, 1.17 [IQR, 1.04–1.31] versus 1.19 [IQR, 1.10–1.29], P = 0.399; median FN, 0.89 [IQR, 0.83–0.97] versus 0.88 [IQR, 0.84–0.97], P = 0.97; median TF, 0.97 [IQR, 0.88–1.06] versus 0.98 [IQR, 0.89–1.03], P = 0.62). In the baseline study, TBS was significantly lower in the TMXF group than in the TMXF + GnRH group (1.37 [IQR, 1.32–1.44] versus 1.41 [IQR, 1.37–1.46], P = 0.036). The follow-up DXA results showed similar values of both BMD and TBS comparing the two groups of patients (median LS BMD, 1.10 [IQR, 0.96–1.21] vs. 1.09 [IQR, 1.02–1.20], P = 0.796; median FN BMD, 0.89. [IQR, 0.80–0.96] vs. 0.97 [IQR, 0.83–0.93], P = 0.89; median TF, 0.93 [IQR, 0.85–1.04] vs. 0.95 [ 0.86–1.00], P = 0.49; median TBS, 1.38 [IQR, 1.32–1.43] vs. 1.39 [IQR, 1.36–1.43], P = 0.435; Fig. 1 & Table 2).
Difference in bone mineral density (BMD, g/cm2) and trabecular bone score (TBS) between TMXF group and TMXF + GnRH group in baseline and follow-up dual energy X-ray absorptiometry (DXA). There was no significant difference in BMD values including lumbar spine, femur neck, and total hip between two groups at baseline (A,E,G) and follow-up dual energy X-ray absorptiometry (DXA) (B,F,H). TBS was significantly lower in TMXF group at the baseline study (C), however, the difference changed as insignificant in follow-up DXA (D).
Changes of BMD and TBS between the 1st and 2nd DXA images
Comparing the 1st and the 2nd DXA results, LS BMD values were significantly decreased in both the TMXF (P < 0.01, mean difference: − 0.06) and TMXF + GnRH groups (P < 0.01, mean difference: − 0.09) (Fig. 2A,B). TBS showed no significant change between two scans in the TMXF (P = 0.57, mean difference: − 0.004) group, whereas there was significant decrease of TBS in the TMXF + GnRH (P < 0.01, mean difference: − 0.02) group during follow-up (Fig. 2C,D). BMD values of femur also showed significant decreasing trend during follow up in both groups, except for FN BMD in the TMXF (P = 0.095, mean difference: − 0.007) group. (Fig. 2E–H).
The changes of bone mineral density (BMD, g/cm2) and trabecular bone score (TBS) during follow up. Lumbar spine BMD values were significantly decreased in both TMXF group and TMXF + GnRH group (A,B). TBS did not significantly change in TMXF group (C), whereas it was significantly decreased in TMXF + GnRH group (D). Femur BMD were significantly decreased during follow up in both TMXF and TMXF + GnRH Group (F–H, except for femur neck BMD in the TMXF group (E).
Using diagnostic criteria of T-scores of LS BMD, the proportion of osteoporosis and osteopenia was higher in the TMXF group; however, there was no statistical significance between the two groups at both baseline (P = 0.40) and follow-up (P = 0.06) DXA scans. Regarding TBS, a significantly higher proportion of the TMXF patients showed a more degraded state compared with the TMXF + GnRH patients at baseline (37: 21: 5 vs. 48: 8: 0, P = 0.003). However, the there was no significant difference at the follow-up DXA scan (P = 0.166). The diagnosis of femur did not show difference between two groups at both baseline and follow-up DXA scans. (Table 2 and Fig. 3).
The diagnostic proportion of bone mineral density (BMD, g/cm2) and trabecular bone score (TBS) during follow up. The proportion of osteoporosis and osteopenia of lumbar spine BMD was higher in the TMXF group, but not significant (A,B). Regarding TBS, a significantly higher proportion of the TMXF group are degraded or more degraded status compared with the TMXF + GnRH group at baseline (C), but the significance disappeared at the follow-up (D). The diagnostic proportion of femur are not difference between two groups at both baseline and follow-up (E–H).
Discussion
In the present study, BMD was not significantly different between the TXMF group and the TMXF + GnRH group at the baseline DXA, regardless of their age or menopause status. BMD values also showed synchronized decrease, with no distinction between treatment regimens. Although BMD decreased significantly, the amount of the variance of value was within LSC, which could not affect the change of diagnosis of osteoporosis or osteopenia. On the other hand, TBS was significantly different between the two groups at the baseline DXA, but decreased in the TMXF + GnRH group during follow-up. The results imply that GnRH analogs caused more deterioration in bone in terms of bone quality rather than bone mass, after median 14 months of treatment.
Both GnRH analogs and the postmenopausal state may decrease bone mass and degrade microarchitecture because of the estrogen-depriving effect. Estrogen deficiency reduces bone mass, due to its effects of increased bone resorption and increased osteoclastic activity. Also, estrogen deficiency decreases new bone formation due to decreased osteoblastic life3. Estrogen inhibits bone resorption by inducing cumulative changes in multiple estrogen-dependent regulatory factors: tumor necrosis factor-α, transforming growth factor-β, and osteoprotegerin (OPG)/receptor activator of nuclear factor kappa-B ligand (RANKL)/RANK system10.
The annual BMD percent change in the women who are perimenopausal is greater than that in the women who were postmenopausal18,19,20,21. A sigmoid pattern of bone loss across menopause has begun 2–3 years before the last menses and ended 3–4 years after the last menses20. In the current study, we also found that the decrease of BMD and TBS in LS was greater in the TMXF + GnRH group compared to the TMXF group. In addition, our data shows that the mean change in LS BMD in the TMXF + GnRH group was greater than that of FN and BMD, this is consistent with previous studies that the estrogen deprivation has greater effect on LS bone loss than FN bone loss19,20 We presumed that those changes would imply that the deterioration bone status is initiated from the area where the trabecular bone is dominant in young patients with GnRH therapy, which has similar effect on bone status of perimenopausal change.
In premenopausal women, relative rates of bone loss during endocrine therapy were more marked than in postmenopausal women22. A previous study reported that TBS is negatively correlated with age23. In our study, the baseline TBS of the TMXF group was significantly lower than that of the TMXF + GnRH group before the latter used GnRH analogs; we suspected that this was because of their greater age. However, after use of GnRH analogs, the TBS of the TMXF + GnRH group showed a greater decrease than that of TMXF group. Previous controlled studies also reported that the differences in annual bone loss rate were 1.4% with TMXF, but 5.6% with TMXF and GnRH analog in premenopausal women22. In skeletal site-specific response to ovariectomy in a rat model, the trabecular structure shifted from a plate-like to a more vulnerable rod-like shape, which explains the poor bone quality and high fracture risk in patients with ovarian function suppression11.
Several clinical studies on breast cancer patients reported that TMXF might have a positive effect on bone metabolism2,24,25,26. In a prospective study with 44 postmenopausal women with hormone-receptor positive breast cancer, BMD was minimally increased in the LS and FN after 12 months of treatment with TMXF, with a significant decrease in osteocalcin levels2. Postmenopausal women showed that TMXF increases areal BMD and reduces fracture risk compared to placebo (relative risk 0.68, 95% confidence interval 0.51–0.92)16. Experimental studies on ovariectomized rat models also reported that TMXF had a protective effect for bone3,27,28,29. After ovariectomy, TMXF administration in rats induced collagen fibers to increase in the periphery of the compact bone, osteoblastic activity increased, matrix released, osteocyte differentiated, and osteoclast cells decreased3.
As osteoporosis is characterized by both loss of bone mass and architectural deterioration, BMD alone has a limitation for predicting fracture risk. For the evaluation of bone microarchitecture of LS, TBS, a novel gray-level texture measurement, was developed to be extracted from DXA images30. Higher scores of TBS correlate with fracture-resistant microarchitectures, whereas lower scores reflect fracture-susceptible microarchitecture, despite identical BMD31. Previous studies reported inconsistent results about BMD and TBS. Our previous study reported that after thyroid-stimulating hormone therapy, although the average areal BMD of patients was not significantly changed, the mean TBS significantly decreased32. The other study reported that after bisphosphonate treatment, BMD decreased and TBS increased during a 3-year follow up period33.
Previous studies also reported the sensitivity of TBS in predicting fracture risk in other endocrine diseases. The prevalence of vertebral fracture is higher in patients with primary aldosteronism34,35. Plasma aldosterone concentrations were inversely correlated with the TBS (P = 0.028) but not with bone mass in women36. TBS is more sensitive for predicting osteoporotic fracture than BMD in diabetes37. For patients with breast cancer, consistently, our study showed increased TBS in some TMXF group as the positive effect of TMXF.
Our study had several strengths. We investigated and compared both bone quality and bone density in the premenopausal women who received combination treatment with TMXF and GnRH analogs and the postmenopausal women treated with only TMXF. The numbers of enrolled subjects were relatively larger than in previous studies16,38.
Our study also had some limitations. Because of its retrospective design, bone turnover markers were excluded because they were not routinely measured in our practical clinical setting. The follow-up period for our study was also relatively short. However, it is known that bone loss is most marked in the first year of endocrine therapy for breast cancer and is particularly evident at the LS39. Finally, there were no data available on fragility fractures during follow-up because no clinical data regarding fracture history were available. More prospective studies of TBS and the correlation with the risk of fracture are needed to confirm our findings.
The present study found that TMXF had a positive effect on bone microarchitecture for postmenopausal breast cancer patients. On the other hand, estrogen deprivation by GnRH analogs reduced bone microarchitecture in premenopausal breast cancer patients, even those who were receiving combination therapy with TMXF. The results of our study showed that TBS had changed more sensitively compared with BMD. Therefore, it may be helpful to consider the possibility of using TBS as a more sensitive measure of bone microarchitecture, especially for premenopausal patients receiving hormone therapy with GnRH analogs.
Change history
07 August 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-38676-8
References
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752. https://doi.org/10.1038/35021093 (2000).
Zidan, J., Keidar, Z., Basher, W. & Israel, O. Effects of tamoxifen on bone mineral density and metabolism in postmenopausal women with early-stage breast cancer. Med. Oncol. 21, 117–121. https://doi.org/10.1385/MO:21:2:117 (2004).
Baloglu, M. & Gokalp Ozkorkmaz, E. Biochemical and immunohistochemical investigations on bone formation and remodelling in ovariectomized rats with tamoxifen citrate administration. Folia Morphol. (Warsz) https://doi.org/10.5603/FM.a2019.0035 (2019).
Arimidex, T. A. O. I. C. T. G. et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 9, 45–53. https://doi.org/10.1016/S1470-2045(07)70385-6 (2008).
Coates, A. S. et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J. Clin. Oncol. 25, 486–492. https://doi.org/10.1200/JCO.2006.08.8617 (2007).
Coombes, R. C. et al. Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 369, 559–570. https://doi.org/10.1016/S0140-6736(07)60200-1 (2007).
Early Breast Cancer Trialists’ Collaborative, G. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386, 1341–1352. https://doi.org/10.1016/S0140-6736(15)61074-1 (2015).
Francis, P. A. et al. Adjuvant ovarian suppression in premenopausal breast cancer. N. Engl. J. Med. 372, 436–446. https://doi.org/10.1056/NEJMoa1412379 (2015).
Frenkel, B. et al. Regulation of adult bone turnover by sex steroids. J. Cell. Physiol. 224, 305–310. https://doi.org/10.1002/jcp.22159 (2010).
Riggs, B. L. The mechanisms of estrogen regulation of bone resorption. J. Clin. Invest. 106, 1203–1204. https://doi.org/10.1172/JCI11468 (2000).
Liu, X. L., Li, C. L., Lu, W. W., Cai, W. X. & Zheng, L. W. Skeletal site-specific response to ovariectomy in a rat model: change in bone density and microarchitecture. Clin. Oral. Implants Res. 26, 392–398. https://doi.org/10.1111/clr.12360 (2015).
Reid, D. M. et al. Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat. Rev. 34(Suppl 1), S3-18. https://doi.org/10.1016/j.ctrv.2008.03.007 (2008).
Kyvernitakis, I., Kostev, K. & Hadji, P. The tamoxifen paradox-influence of adjuvant tamoxifen on fracture risk in pre- and postmenopausal women with breast cancer. Osteoporos. Int. 29, 2557–2564. https://doi.org/10.1007/s00198-018-4642-2 (2018).
Hans, D., Goertzen, A. L., Krieg, M. A. & Leslie, W. D. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J. Bone Miner. Res. 26, 2762–2769. https://doi.org/10.1002/jbmr.499 (2011).
Cheung, A. M. et al. Bone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: a nested substudy of the MAP.3 randomised controlled trial. Lancet Oncol. 13, 275–284. https://doi.org/10.1016/S1470-2045(11)70389-8 (2012).
Kalder, M. et al. Effects of Exemestane and Tamoxifen treatment on bone texture analysis assessed by TBS in comparison with bone mineral density assessed by DXA in women with breast cancer. J. Clin. Densitom. 17, 66–71. https://doi.org/10.1016/j.jocd.2013.03.003 (2014).
Silva, B. C. & Bilezikian, J. P. Trabecular bone score: perspectives of an imaging technology coming of age. Arq. Bras. Endocrinol. Metabol. 58, 493–503 (2014).
Falch, J. A. & Sandvik, L. Perimenopausal appendicular bone loss: a 10-year prospective study. Bone 11, 425–428. https://doi.org/10.1016/8756-3282(90)90138-o (1990).
Guthrie, J. R. et al. A prospective study of bone loss in menopausal Australian-born women. Osteoporos Int 8, 282–290. https://doi.org/10.1007/s001980050066 (1998).
Recker, R., Lappe, J., Davies, K. & Heaney, R. Characterization of perimenopausal bone loss: a prospective study. J. Bone Miner. Res. 15, 1965–1973. https://doi.org/10.1359/jbmr.2000.15.10.1965 (2000).
Finkelstein, J. S. et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J. Clin. Endocrinol. Metab. 93, 861–868. https://doi.org/10.1210/jc.2007-1876 (2008).
Ramchand, S. K., Cheung, Y. M. & Grossmann, M. Bone health in women with breast cancer. Climacteric 22, 589–595. https://doi.org/10.1080/13697137.2019.1580257 (2019).
Shin, Y. H., Gong, H. S., Lee, K. J. & Baek, G. H. Older age and higher body mass index are associated with a more degraded trabecular bone score compared to bone mineral density. J. Clin. Densitom. 22, 266–271. https://doi.org/10.1016/j.jocd.2017.06.006 (2019).
Kristensen, B. et al. Tamoxifen and bone metabolism in postmenopausal low-risk breast cancer patients: a randomized study. J. Clin. Oncol. 12, 992–997. https://doi.org/10.1200/JCO.1994.12.5.992 (1994).
Grey, A. B. et al. The effect of the antiestrogen tamoxifen on bone mineral density in normal late postmenopausal women. Am. J. Med. 99, 636–641. https://doi.org/10.1016/s0002-9343(99)80251-4 (1995).
Marttunen, M. B., Hietanen, P., Tiitinen, A. & Ylikorkala, O. Comparison of effects of tamoxifen and toremifene on bone biochemistry and bone mineral density in postmenopausal breast cancer patients. J. Clin. Endocrinol. Metab. 83, 1158–1162. https://doi.org/10.1210/jcem.83.4.4688 (1998).
Frolik, C. A., Bryant, H. U., Black, E. C., Magee, D. E. & Chandrasekhar, S. Time-dependent changes in biochemical bone markers and serum cholesterol in ovariectomized rats: effects of raloxifene HCl, tamoxifen, estrogen, and alendronate. Bone 18, 621–627. https://doi.org/10.1016/8756-3282(96)00085-3 (1996).
Turner, R. T., Wakley, G. K., Hannon, K. S. & Bell, N. H. Tamoxifen inhibits osteoclast-mediated resorption of trabecular bone in ovarian hormone-deficient rats. Endocrinology 122, 1146–1150. https://doi.org/10.1210/endo-122-3-1146 (1988).
Turner, R. T., Wakley, G. K., Hannon, K. S. & Bell, N. H. Tamoxifen prevents the skeletal effects of ovarian hormone deficiency in rats. J. Bone Miner. Res. 2, 449–456. https://doi.org/10.1002/jbmr.5650020513 (1987).
Shevroja, E. et al. Use of trabecular bone score (TBS) as a complementary approach to dual-energy X-ray absorptiometry (DXA) for fracture risk assessment in clinical practice. J. Clin. Densitom. 20, 334–345. https://doi.org/10.1016/j.jocd.2017.06.019 (2017).
Pothuaud, L. et al. Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: a preliminary spine BMD-matched, case-control study. J. Clin. Densitom. 12, 170–176. https://doi.org/10.1016/j.jocd.2008.11.006 (2009).
Kim, E. H. et al. Effects of thyrotropin suppression on bone health in menopausal women with total thyroidectomy. J. Bone Metab. 26, 31–38. https://doi.org/10.11005/jbm.2019.26.1.31 (2019).
Shin, M. S., Cho, E. H. & Kim, H. Y. Longitudinal change in trabecular bone score during and after treatment of osteoporosis in postmenopausal Korean women. J. Bone. Metab. 24, 117–124. https://doi.org/10.11005/jbm.2017.24.2.117 (2017).
Notsu, M., Yamauchi, M., Yamamoto, M., Nawata, K. & Sugimoto, T. Primary aldosteronism as a risk factor for vertebral fracture. J. Clin. Endocrinol. Metab. 102, 1237–1243. https://doi.org/10.1210/jc.2016-3206 (2017).
Salcuni, A. S. et al. Bone involvement in aldosteronism. J. Bone Miner. Res. 27, 2217–2222. https://doi.org/10.1002/jbmr.1660 (2012).
Kim, B. J. et al. Lower trabecular bone score in patients with primary aldosteronism: human skeletal deterioration by aldosterone excess. J. Clin. Endocrinol. Metab. 103, 615–621. https://doi.org/10.1210/jc.2017-02043 (2018).
Leslie, W. D., Aubry-Rozier, B., Lamy, O., Hans, D. & Manitoba Bone Density, P. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab 98, 602–609. doi:https://doi.org/10.1210/jc.2012-3118 (2013).
Ramchand, S. K. et al. Premenopausal women with early breast cancer treated with estradiol suppression have severely deteriorated bone microstructure. Bone 103, 131–135. https://doi.org/10.1016/j.bone.2017.06.024 (2017).
Hong, A. R. et al. Long-term effect of aromatase inhibitors on bone microarchitecture and macroarchitecture in non-osteoporotic postmenopausal women with breast cancer. Osteoporos. Int. 28, 1413–1422. https://doi.org/10.1007/s00198-016-3899-6 (2017).
Acknowledgements
Eun Heui Kim was supported by research funding from Korean Society of Bone and Mineral Research. Keunyoung Kim was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI18C2383). This work was supported by clinical research grant from Pusan National University Hospital 2020.
Author information
Authors and Affiliations
Contributions
E.H.K. searched the literature databases and co-wrote the original draft. Y.K.J. conducted conceptualization, supervision, and validation. K.J.P., T.W.K., and K.E.K. conducted investigation and data curation. S.J.K. conducted supervision. I.J.K. conducted conceptualization, project administration, and supervision. K.Y.K. conducted conceptualization, investigation, methodology, data curation, formal analysis, project administration, supervision, validation, review, and editing, and co-wrote the original draft. All authors interpreted the results, reviewed, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article, Affiliation 2 was incorrectly given as ‘Department of Nuclear Medicine and Biomedical Research Institute, Pusan National University Hospital, Busan, Republic of Korea’. The correct affiliation is: Department of Nuclear Medicine and Biomedical Research Institute, Pusan National University Hospital and School of Medicine, Pusan National University, Busan, Republic of Korea.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, E.H., Jeon, Y.K., Pak, K. et al. Effect of tamoxifen with or without gonadotropin-releasing hormone analog on DXA values in women with breast cancer. Sci Rep 11, 3407 (2021). https://doi.org/10.1038/s41598-021-82824-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82824-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.